Early Degenerative Changes in a Spontaneous Osteoarthritis Model Assessed by Nanoindentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Nanoindentation
2.3. Histological Assessment of Osteoarthritis
2.4. Immunohistochemistry
2.5. Statistical Analaysis
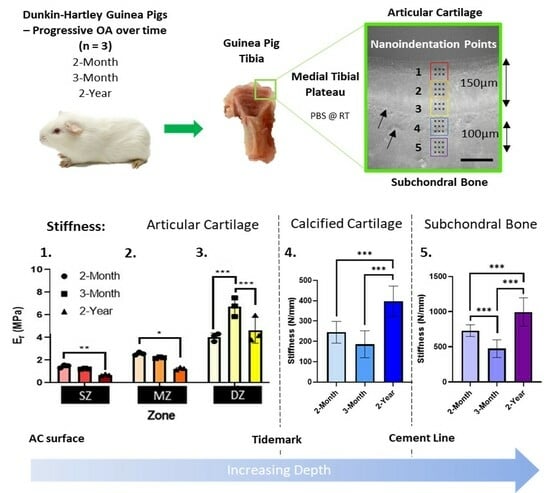
3. Results
3.1. Age-Associated Degeneration of Dunkin-Hartley Guinea Pig Joints
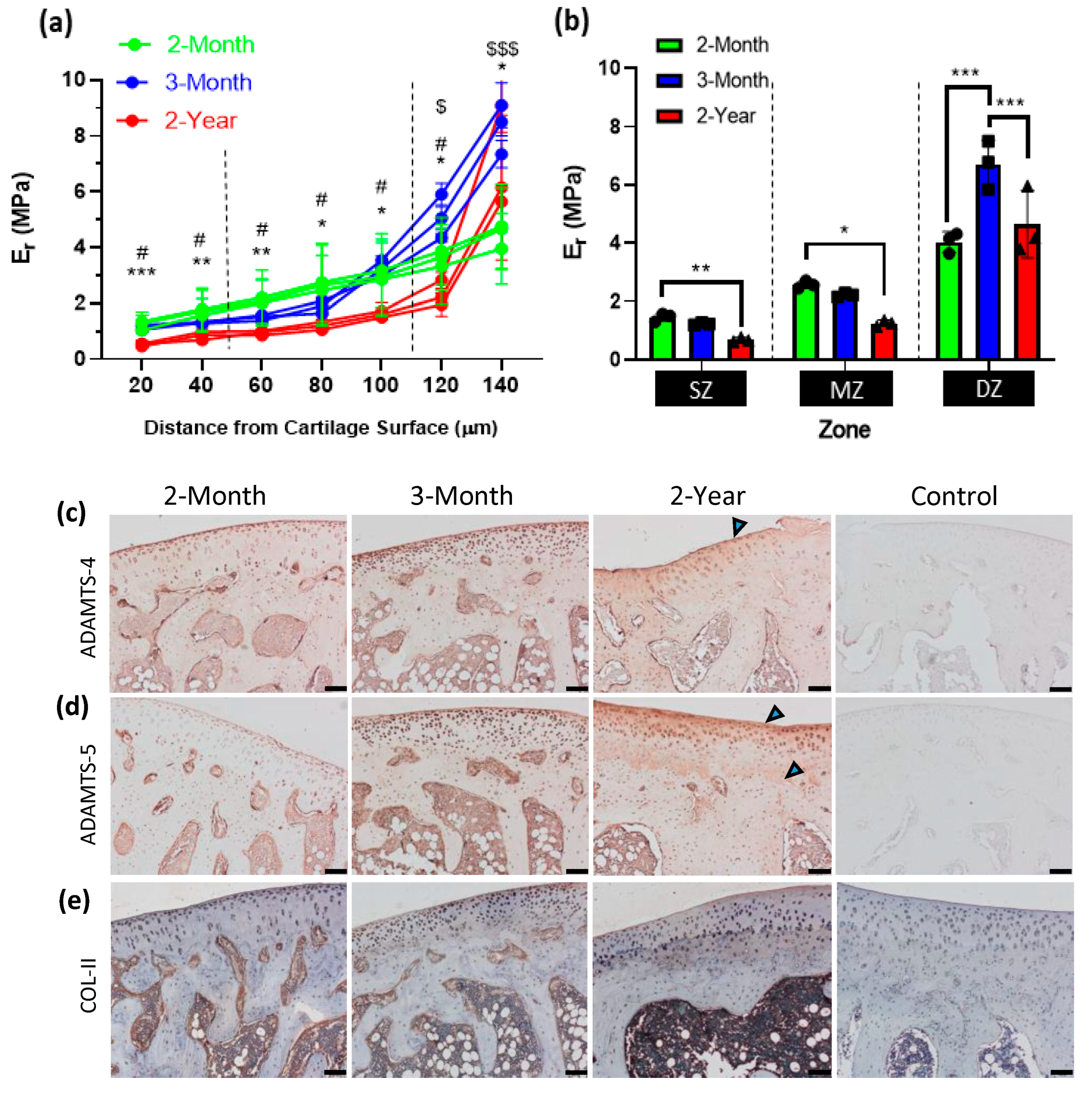
3.2. Mechanical Alterations to Articular Cartilage during Osteoarthritis Developmen
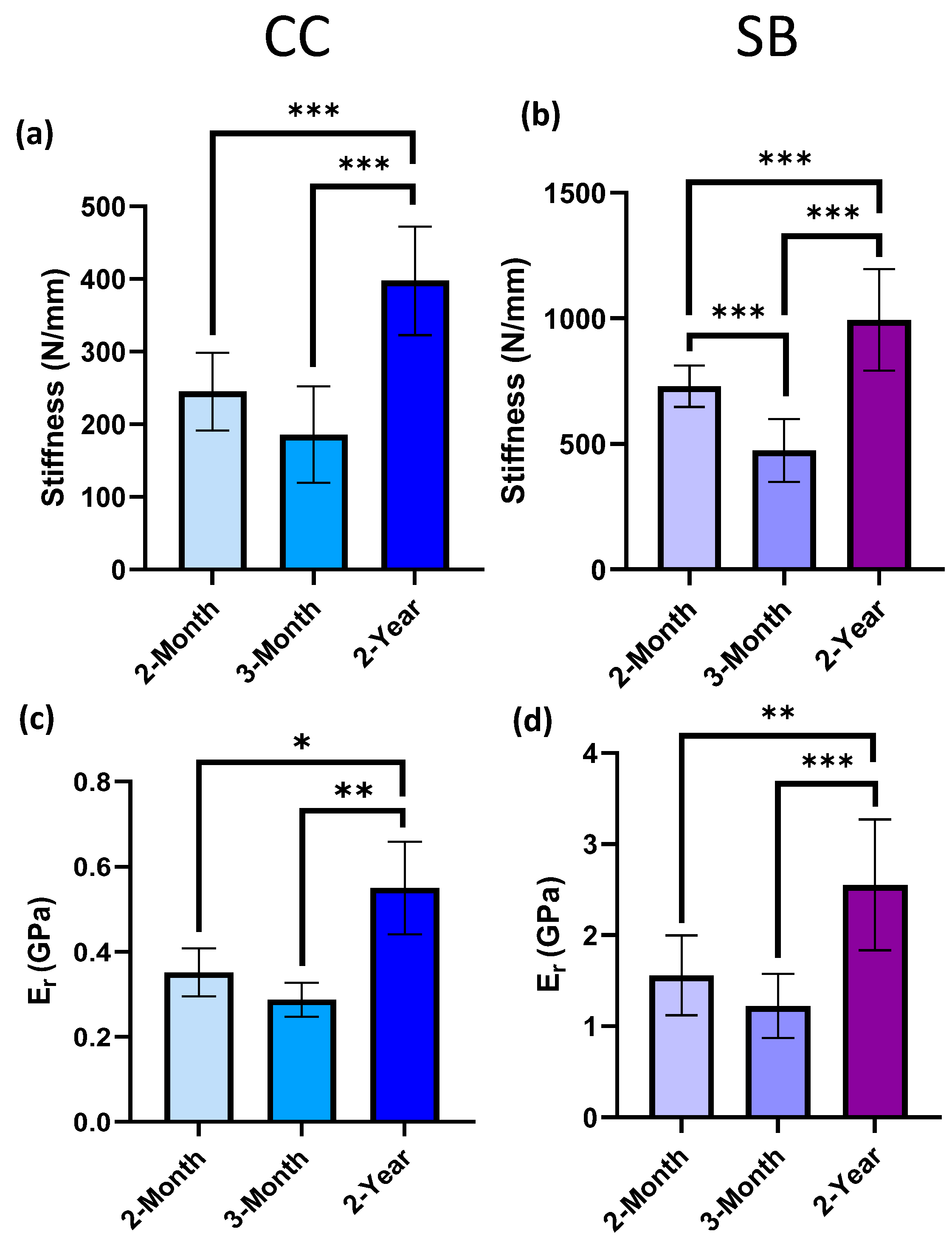
3.3. Mechanical Alterations to Mineralized Regions of Calcified Cartilage and Subchondral Bone
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [PubMed]
- Ratzlaff, C.; Ashbeck, E.; Guermazi, A.; Roemer, F.; Duryea, J.; Kwoh, C. A quantitative metric for knee osteoarthritis: Reference values of joint space loss. Osteoarthr. Cartil. 2018, 26, 1215–1224. [Google Scholar] [CrossRef]
- Crisco, J.; Morton, A.; Moore, D.; Kahan, L.; Ladd, A.; Weiss, A.-P. Osteophyte growth in early thumb carpometacarpal osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1315–1323. [Google Scholar] [CrossRef]
- Pottenger, L.A.; Phillips, F.M.; Draganich, L.F. The effect of marginal osteophytes on reduction of varus-valgus instability in osteoarthritic knees. Arthritis Rheum. 1990, 33, 853–858. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Hunter, D.J.; Gerstenfeld, L.; Bishop, G.; Davis, A.D.; Mason, Z.D.; Einhorn, T.A.; Maciewicz, R.A.; Newham, P.; Foster, M.; Jackson, S.; et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res. Ther. 2009, 11, R11. [Google Scholar] [CrossRef]
- Baker, K.; Grainger, A.; Niu, J.; Clancy, M.; Guermazi, A.; Crema, M.; Hughes, L.; Buckwalter, J.; Wooley, A.; Nevitt, M.; et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 2010, 69, 1779–1783. [Google Scholar] [CrossRef]
- Berenbaum, F.; Meng, Q.-J. The brain–joint axis in osteoarthritis: Nerves, circadian clocks and beyond. Nat. Rev. Rheumatol. 2016, 12, 508–516. [Google Scholar] [CrossRef]
- Walsh, D.; Verghese, P.; Cook, G.; McWilliams, D.; Mapp, P.; Ashraf, S.; Wilson, D. Lymphatic vessels in osteoarthritic human knees. Osteoarthr. Cartil. 2012, 20, 405–412. [Google Scholar] [CrossRef]
- Øiestad, B.; Juhl, C.; Eitzen, I.; Thorlund, J. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 171–177. [Google Scholar] [CrossRef]
- Slemenda, C.; Heilman, D.K.; Brandt, K.D.; Katz, B.P.; Mazzuca, S.A.; Braunstein, E.M.; Byrd, D. Reduced quadriceps strength relative to body weight: A risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998, 41, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Mascarenhas, R.; Saltzman, B.M.; Rollins, M.; Bach, B.R.; Macdonald, P. The Relationship between Anterior Cruciate Ligament Injury and Osteoarthritis of the Knee. Adv. Orthop. 2015, 2015, 928301. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Englund, M.; Roemer, F.W.; Hayashi, D.; Crema, M.D.; Guermazi, A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat. Rev. Rheumatol. 2012, 8, 412–419. [Google Scholar] [CrossRef]
- Bettica, P.; Cline, G.; Hart, D.J.; Meyer, J.; Spector, T.D. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: Longitudinal results from the Chingford study. Arthritis Rheum. 2002, 46, 3178–3184. [Google Scholar] [CrossRef]
- Aho, O.-M.; Finnilä, M.; Thevenot, J.; Saarakkala, S.; Lehenkari, P. Subchondral bone histology and grading in osteoarthritis. PLoS ONE 2017, 12, e0173726. [Google Scholar] [CrossRef]
- Bobinac, D.; Spanjol, J.; Zoricic, S.; Maric, I. Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone 2003, 32, 284–290. [Google Scholar] [CrossRef]
- Fell, N.; Lawless, B.; Cox, S.; Cooke, M.; Eisenstein, N.; Shepherd, D.; Espino, D. The role of subchondral bone, and its histomorphology, on the dynamic viscoelasticity of cartilage, bone and osteochondral cores. Osteoarthr. Cartil. 2019, 27, 535–543. [Google Scholar] [CrossRef]
- Nevitt, M.C.; Zhang, Y.; Javaid, M.K.; Neogi, T.; Curtis, J.R.; Niu, J.; McCulloch, C.E.; Segal, N.A.; Felson, D.T. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: The MOST study. Ann. Rheum. Dis. 2010, 69, 163–168. [Google Scholar] [CrossRef]
- Lo, G.; Zhang, Y.; McLennan, C.; Niu, J.; Kiel, D.; McLean, R.; Aliabadi, P.; Felson, D.; Hunter, D. The ratio of medial to lateral tibial plateau bone mineral density and compartment-specific tibiofemoral osteoarthritis. Osteoarthr. Cartil. 2006, 14, 984–990. [Google Scholar] [CrossRef]
- Radin, E.L.; Martin, R.B.; Burr, D.B.; Caterson, B.; Boyd, R.D.; Goodwin, C. Effects of mechanical loading on the tissues of the rabbit knee. J. Orthop. Res. 1984, 2, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Peterfy, C.; Zaim, S.; Schoenharting, M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: A three-month longitudinal study. Arthritis Rheum. 2005, 52, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Wluka, A.E.; Hanna, F.; Davies-Tuck, M.; Wang, Y.; Bell, R.J.; Davis, S.R.; Adams, J.; Cicuttini, F.M. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann. Rheum. Dis. 2009, 68, 850–855. [Google Scholar] [CrossRef]
- Wang, Y.; Wluka, A.E.; Pelletier, J.-P.; Martel-Pelletier, J.; Abram, F.; Ding, C.; Cicuttini, F.M. Meniscal extrusion predicts increases in subchondral bone marrow lesions and bone cysts and expansion of subchondral bone in osteoarthritic knees. Rheumatology 2010, 49, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Dore, D.; Martens, A.; Quinn, S.; Ding, C.; Winzenberg, T.; Zhai, G.; Pelletier, J.-P.; Martel-Pelletier, J.; Abram, F.; Cicuttini, F.; et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: A prospective study in older adults. Thromb. Haemost. 2010, 12, R222. [Google Scholar] [CrossRef]
- Hunter, D.J.; Zhang, Y.; Niu, J.; Goggins, J.; Amin, S.; LaValley, M.P.; Guermazi, A.; Genant, H.; Gale, D.; Felson, D.T. Increase in bone marrow lesions associated with cartilage loss: A longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006, 54, 1529–1535. [Google Scholar] [CrossRef]
- Davis, S.; Karali, A.; Zekonyte, J.; Roldo, M.; Blunn, G. Development of a method to investigate strain distribution across the cartilage-bone interface in guinea pig model of spontaneous osteoarthritis using lab-based contrast enhanced X-ray-computed tomography and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2023, 144, 105999. [Google Scholar] [CrossRef]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 10. [Google Scholar] [CrossRef]
- Hargrave-Thomas, E.; van Sloun, F.; Dickinson, M.; Broom, N.; Thambyah, A. Multi-scalar mechanical testing of the calcified cartilage and subchondral bone comparing healthy vs early degenerative states. Osteoarthr. Cartil. 2015, 23, 1755–1762. [Google Scholar] [CrossRef]
- Mente, P.L.; Lewis, J.L. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J. Orthop. Res. 1994, 12, 637–647. [Google Scholar] [CrossRef]
- Buffinton, C.M.; Tong, K.J.; Blaho, R.A.; Buffinton, E.M.; Ebenstein, D.M. Comparison of mechanical testing methods for biomaterials: Pipette aspiration, nanoindentation, and macroscale testing. J. Mech. Behav. Biomed. Mater. 2015, 51, 367–379. [Google Scholar] [CrossRef]
- Ebenstein, D.M.; Pruitt, L.A. Nanoindentation of biological materials. Nano Today 2006, 1, 26–33. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Franke, O.; Göken, M.; Meyers, M.; Durst, K.; Hodge, A. Dynamic nanoindentation of articular porcine cartilage. Mater. Sci. Eng. C 2011, 31, 789–795. [Google Scholar] [CrossRef]
- Doyran, B.; Tong, W.; Li, Q.; Jia, H.; Zhang, X.; Chen, C.; Enomoto-Iwamoto, M.; Lu, X.; Qin, L.; Han, L. Nanoindentation modulus of murine cartilage: A sensitive indicator of the initiation and progression of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2017, 25, 108–117. [Google Scholar] [CrossRef]
- Ebenstein, D.M.; Kuo, A.; Rodrigo, J.J.; Reddi, A.H.; Ries, M.; Pruitt, L. A nanoindentation technique for functional evaluation of cartilage repair tissue. J. Mater. Res. 2004, 19, 273–281. [Google Scholar] [CrossRef]
- Li, C.; Pruitt, L.A.; King, K.B. Nanoindentation differentiates tissue-scale functional properties of native articular cartilage. J. Biomed. Mater. Res. Part A 2006, 78A, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Taffetani, M.; Gottardi, R.; Gastaldi, D.; Raiteri, R.; Vena, P. Poroelastic response of articular cartilage by nanoindentation creep tests at different characteristic lengths. Med. Eng. Phys. 2014, 36, 850–858. [Google Scholar] [CrossRef]
- Doube, M.; Firth, E.; Boyde, A.; Bushby, A. Combined nanoindentation testing and scanning electron microscopy of bone and articular calcified cartilage in an equine fracture predilection site. Eur. Cells Mater. 2010, 19, 242–251. [Google Scholar] [CrossRef]
- Manitta, L.; Fayolle, C.; Olive, L.; Berteau, J.-P. Nanoindentation of Subchondral Bone During Osteoarthritis Pathological Process Using Atomic Force Microscopy. Lect. Notes Comput. Vis. Biomech. 2020, 36, 505–517. [Google Scholar] [CrossRef]
- Zuo, Q.; Lu, S.; Du, Z.; Friis, T.; Yao, J.; Crawford, R.; Prasadam, I.; Xiao, Y. Characterization of nano-structural and nano-mechanical properties of osteoarthritic subchondral bone. BMC Musculoskelet. Disord. 2016, 17, 367. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Qi, H.J.; Ortiz, C. Effect of mineral content on the nanoindentation properties and nanoscale deformation mechanisms of bovine tibial cortical bone. J. Mater. Sci. Mater. Med. 2005, 16, 947–959. [Google Scholar] [CrossRef]
- Sun, L.; Chen, C.; Yin, L.; Tian, X.; Duan, X.; Xiong, R.; Guo, L.; Chen, K.; Wang, F.; Yang, L. Probing the Elasticity of Calcified Cartilage Zone Using Nano-Indentation. J. Biomater. Tissue Eng. 2017, 7, 556–560. [Google Scholar] [CrossRef]
- Yu, D.-G.; Ding, H.-F.; Mao, Y.-Q.; Liu, M.; Yu, B.; Zhao, X.; Wang, X.-Q.; Li, Y.; Liu, G.-W.; Nie, S.-B.; et al. Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol. Sin. 2013, 34, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Boi, M.; Marchiori, G.; Berni, M.; Gambardella, A.; Salamanna, F.; Visani, A.; Bianchi, M.; Fini, M.; Filardo, G. Nanoindentation: An advanced procedure to investigate osteochondral engineered tissues. J. Mech. Behav. Biomed. Mater. 2019, 96, 79–87. [Google Scholar] [CrossRef]
- Campbell, S.E.; Ferguson, V.L.; Hurley, D.C. Nanomechanical mapping of the osteochondral interface with contact resonance force microscopy and nanoindentation. Acta Biomater. 2012, 8, 4389–4396. [Google Scholar] [CrossRef]
- Gupta, H.; Schratter, S.; Tesch, W.; Roschger, P.; Berzlanovich, A.; Schoeberl, T.; Klaushofer, K.; Fratzl, P. Two different correlations between nanoindentation modulus and mineral content in the bone–cartilage interface. J. Struct. Biol. 2005, 149, 138–148. [Google Scholar] [CrossRef]
- Ferguson, V.L.; Bushby, A.J.; Boyde, A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J. Anat. 2003, 203, 191–202. [Google Scholar] [CrossRef]
- Mieloch, A.A.; Richter, M.; Trzeciak, T.; Giersig, M.; Rybka, J.D. Osteoarthritis Severely Decreases the Elasticity and Hardness of Knee Joint Cartilage: A Nanoindentation Study. J. Clin. Med. 2019, 8, 1865. [Google Scholar] [CrossRef]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. The effect of ageing and osteoarthritis on the mechanical properties of cartilage and bone in the human knee joint. Sci. Rep. 2018, 8, 5931. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Wu, C.-B.; Tang, B.; Wang, T.; Yan, C.-H.; Lu, W.; Pan, H.; Hu, Y.; Chiu, K.-Y. Collagen fibril stiffening in osteoarthritic cartilage of human beings revealed by atomic force microscopy. Osteoarthr. Cartil. 2012, 20, 916–922. [Google Scholar] [CrossRef]
- Ozcivici, E.; Ferreri, S.; Qin, Y.-X.; Judex, S. Determination of Bone’s Mechanical Matrix Properties by Nanoindentation. Methods Mol. Biol. 2008, 455, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Franke, O.; Durst, K.; Maier, V.; Göken, M.; Birkholz, T.; Schneider, H.; Hennig, F.; Gelse, K. Mechanical properties of hyaline and repair cartilage studied by nanoindentation. Acta Biomater. 2007, 3, 873–881. [Google Scholar] [CrossRef]
- Paietta, R.C.; Campbell, S.E.; Ferguson, V.L. Influences of spherical tip radius, contact depth, and contact area on nanoindentation properties of bone. J. Biomech. 2011, 44, 285–290. [Google Scholar] [CrossRef]
- Bushby, A.; Ferguson, V.; Boyde, A. Nanoindentation of bone: Comparison of specimens tested in liquid and embedded in polymethylmethacrylate. J. Mater. Res. 2004, 19, 249–259. [Google Scholar] [CrossRef]
- Hoffler, C.E.; Guo, X.E.; Zysset, P.K.; Goldstein, S.A. An Application of Nanoindentation Technique to Measure Bone Tissue Lamellae Properties. J. Biomech. Eng. 2005, 127, 1046–1053. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Swain, M.V. Influence of hydration on nanoindentation induced energy expenditure of dentin. J. Biomech. 2012, 45, 1679–1683. [Google Scholar] [CrossRef]
- Angker, L.; Nijhof, N.; Swain, M.V.; Kilpatrick, N.M. Influence of hydration and mechanical characterization of carious primary dentine using an ultra-micro indentation system (UMIS). Eur. J. Oral Sci. 2004, 112, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, K.J.; Nowak, T.E.; Blum, J.; Kuhn, S.; Welker, M.; Sternstein, W.; Mueller, L.P.; Rommens, P.M. Influence of formalin fixation on the biomechanical properties of human diaphyseal bone. Biomed Tech 2010, 55, 361–365. [Google Scholar] [CrossRef]
- Rouleau, L.; Tremblay, D.; Cartier, R.; Mongrain, R.; Leask, R.L. Regional variations in canine descending aortic tissue mechanical properties change with formalin fixation. Cardiovasc. Pathol. 2012, 21, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.J.; Khayyeri, H.; Guizar-Sicairos, M.; Isaksson, H. Effects of tissue fixation and dehydration on tendon collagen nanostructure. J. Struct. Biol. 2017, 199, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, K.W.; Gluzband, Y.A.; Kaku, M.; Ambia-Sobhan, H.; Shapses, S.A.; Yamauchi, M.; Spencer, R.G. Effects of formalin fixation and collagen cross-linking onT2 and magnetization transfer in bovine nasal cartilage. Magn. Reson. Med. 2007, 57, 1000–1011. [Google Scholar] [CrossRef]
- Chapman, J.A.; Tzaphlidou, M.; Meek, K.M.; Kadler, K.E. The collagen fibril—A model system for studying the staining and fixation of a protein. Electron Microsc. Rev. 1990, 3, 143–182. [Google Scholar] [CrossRef]
- Rodriguez-Florez, N.; Oyen, M.L.; Shefelbine, S.J. Insight into differences in nanoindentation properties of bone. J. Mech. Behav. Biomed. Mater. 2013, 18, 90–99. [Google Scholar] [CrossRef]
- Galli, M.; Comley, K.S.; Shean, T.A.; Oyen, M.L. Viscoelastic and poroelastic mechanical characterization of hydrated gels. J. Mater. Res. 2009, 24, 973–979. [Google Scholar] [CrossRef]
- Bembey, A.; Bushby, A.; Boyde, A.; Ferguson, V.; Oyen, M. Hydration effects on the micro-mechanical properties of bone. J. Mater. Res. 2006, 21, 1962–1968. [Google Scholar] [CrossRef]
- Anderson-MacKenzie, J.M.; Quasnichka, H.L.; Starr, R.L.; Lewis, E.J.; Billingham, M.E.; Bailey, A.J. Fundamental subchondral bone changes in spontaneous knee osteoarthritis. Int. J. Biochem. Cell Biol. 2005, 37, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.A.; Glasson, S.S.; Trubetskoy, O.V.; Haimes, H.B. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: Histologic, radiologic, and biochemical changes. Comp. Med. 1997, 47, 598–601. [Google Scholar]
- Musci, R.V.; Walsh, M.A.; Konopka, A.R.; Wolff, C.A.; Peelor, F.F.; Reiser, R.F.; Santangelo, K.S.; Hamilton, K.L. The Dunkin Hartley Guinea Pig Is a Model of Primary Osteoarthritis That Also Exhibits Early Onset Myofiber Remodeling That Resembles Human Musculoskeletal Aging. Front. Physiol. 2020, 11, 571372. [Google Scholar] [CrossRef]
- McDougall, J.J.; Andruski, B.; Schuelert, N.; Hallgrímsson, B.; Matyas, J.R. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain 2009, 141, 222–232. [Google Scholar] [CrossRef]
- Thomsen, J.; Straarup, T.; Danielsen, C.; Oxlund, H.; Brüel, A. Relationship between articular cartilage damage and subchondral bone properties and meniscal ossification in the Dunkin Hartley guinea pig model of osteoarthritis. Scand. J. Rheumatol. 2011, 40, 391–399. [Google Scholar] [CrossRef]
- Muraoka, T.; Hagino, H.; Okano, T.; Enokida, M.; Teshima, R. Role of subchondral bone in osteoarthritis development: A comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. 2007, 56, 3366–3374. [Google Scholar] [CrossRef]
- Bendele, A.; Mccomb, J.; Gould, T.; Mcabee, T.; Sennello, G.; Chlipala, E.; Guy, M. Animal Models of Arthritis: Relevance to Human Disease. Toxicol. Pathol. 1999, 27, 134–142. [Google Scholar] [CrossRef]
- Bendele, A.M.; Hulman, J.F. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988, 31, 561–565. [Google Scholar] [CrossRef]
- Heraud, F.; Héraud, A.; Harmand, M.-F. Apoptosis in normal and osteoarthritic human articular cartilage. Ann. Rheum. Dis. 2000, 59, 959–965. [Google Scholar] [CrossRef]
- Zamli, Z.; Brown, K.R.; Sharif, M. Subchondral Bone Plate Changes More Rapidly than Trabecular Bone in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1496. [Google Scholar] [CrossRef] [PubMed]
- Tonge, D.P.; Bardsley, R.G.; Parr, T.; Maciewicz, R.A.; Jones, S.W. Evidence of changes to skeletal muscle contractile properties during the initiation of disease in the ageing guinea pig model of osteoarthritis. Longev. Heal. 2013, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Changoor, A.; Fereydoonzad, L.; Yaroshinsky, A.; Buschmann, M.D. Effects of Refrigeration and Freezing on the Electromechanical and Biomechanical Properties of Articular Cartilage. J. Biomech. Eng. 2010, 132, 064502. [Google Scholar] [CrossRef] [PubMed]
- Swadener, J.G.; Rho, J.-Y.; Pharr, G.M. Effects of anisotropy on elastic moduli measured by nanoindentation in human tibial cortical bone. J. Biomed. Mater. Res. 2001, 57, 108–112. [Google Scholar] [CrossRef]
- Schmitz, N.; Laverty, S.; Kraus, V.B.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010, 18, S113–S116. [Google Scholar] [CrossRef]
- Kraus, V.; Huebner, J.; DeGroot, J.; Bendele, A. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2010, 18, S35–S52. [Google Scholar] [CrossRef]
- Ren, P.; Niu, H.; Cen, H.; Jia, S.; Gong, H.; Fan, Y. Biochemical and Morphological Abnormalities of Subchondral Bone and Their Association with Cartilage Degeneration in Spontaneous Osteoarthritis. Calcif. Tissue Int. 2021, 109, 179–189. [Google Scholar] [CrossRef]
- Yan, J.-Y.; Tian, F.-M.; Wang, W.-Y.; Cheng, Y.; Xu, H.-F.; Song, H.-P.; Zhang, Y.-Z.; Zhang, L. Age Dependent Changes in Cartilage Matrix, Subchondral Bone Mass, and Estradiol Levels in Blood Serum, in Naturally Occurring Osteoarthritis in Guinea Pigs. Int. J. Mol. Sci. 2014, 15, 13578–13595. [Google Scholar] [CrossRef] [PubMed]
- Bendele, A.M.; White, S.L.; Hulman, J.F. Osteoarthrosis in guinea pigs: Histopathologic and scanning electron microscopic features. Lab. Anim. Sci. 1989, 39, 115–121. Available online: https://europepmc.org/article/MED/2709799 (accessed on 6 July 2023). [PubMed]
- Bendele, A.M.; Hulman, J.F. Effects of Body Weight Restriction on the Development and Progression of Spontaneous Osteoarthritis in Guinea Pigs. Arthritis Rheum. 1991, 34, 1180–1184. [Google Scholar] [CrossRef]
- Veronesi, F.; Salamanna, F.; Martini, L.; Fini, M. Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model. Int. J. Mol. Sci. 2022, 23, 7309. [Google Scholar] [CrossRef]
- Wang, T.; Wen, C.-Y.; Yan, C.-H.; Lu, W.-W.; Chiu, K.-Y. Spatial and temporal changes of subchondral bone proceed to microscopic articular cartilage degeneration in guinea pigs with spontaneous osteoarthritis. Osteoarthr. Cartil. 2013, 21, 574–581. [Google Scholar] [CrossRef]
- Tomkoria, S.; Patel, R.V.; Mao, J.J. Heterogeneous nanomechanical properties of superficial and zonal regions of articular cartilage of the rabbit proximal radius condyle by atomic force microscopy. Med. Eng. Phys. 2004, 26, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Tan, H.; Chen, G.; Guo, L.; Yang, L. Analysis of the Mineral Composition of the Human Calcified Cartilage Zone. Int. J. Med. Sci. 2012, 9, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bader, D.; Kempson, G. The Short-Term Compressive Properties of Adult Human Articular Cartilage. Bio-Med. Mater. Eng. 1994, 4, 245–256. [Google Scholar] [CrossRef]
- Moshtagh, P.R.; Pouran, B.; Korthagen, N.M.; Zadpoor, A.A.; Weinans, H. Guidelines for an optimized indentation protocol for measurement of cartilage stiffness: The effects of spatial variation and indentation parameters. J. Biomech. 2016, 49, 3602–3607. [Google Scholar] [CrossRef] [PubMed]
- Schinagl, R.M.; Gurskis, D.; Chen, A.C.; Sah, R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J. Orthop. Res. 1997, 15, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wahlquist, J.A.; DelRio, F.W.; Randolph, M.A.; Aziz, A.H.; Heveran, C.M.; Bryant, S.J.; Neu, C.P.; Ferguson, V.L. Indentation mapping revealed poroelastic, but not viscoelastic, properties spanning native zonal articular cartilage. Acta Biomater. 2017, 64, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef] [PubMed]
- Caterson, B.; Flannery, C.R.; Hughes, C.E.; Little, C.B. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000, 19, 333–344. [Google Scholar] [CrossRef]
- Cheung, K.S.C.; Hashimoto, K.; Yamada, N.; Roach, H.I. Expression of ADAMTS-4 by chondrocytes in the surface zone of human osteoarthritic cartilage is regulated by epigenetic DNA de-methylation. Rheumatol. Int. 2008, 29, 525–534. [Google Scholar] [CrossRef]
- Nia, H.T.; Gauci, S.J.; Azadi, M.; Hung, H.-H.; Frank, E.; Fosang, A.J.; Ortiz, C.; Grodzinsky, A.J. High-bandwidth AFM-based rheology is a sensitive indicator of early cartilage aggrecan degradation relevant to mouse models of osteoarthritis. J. Biomech. 2015, 48, 162–165. [Google Scholar] [CrossRef]
- Jakes, J.E.; Frihart, C.R.; Beecher, J.F.; Moon, R.J.; Resto, P.J.; Melgarejo, Z.H.; Suárez, O.M.; Baumgart, H.; Elmustafa, A.A.; Stone, D.S. Nanoindentation near the edge. J. Mater. Res. 2009, 24, 1016–1031. [Google Scholar] [CrossRef]
- Jakes, J.E.; Stone, D.S. The edge effect in nanoindentation. Philos. Mag. 2011, 91, 1387–1399. [Google Scholar] [CrossRef]
- Stolz, M.; Raiteri, R.; Daniels, A.; VanLandingham, M.R.; Baschong, W.; Aebi, U. Dynamic Elastic Modulus of Porcine Articular Cartilage Determined at Two Different Levels of Tissue Organization by Indentation-Type Atomic Force Microscopy. Biophys. J. 2004, 86, 3269–3283. [Google Scholar] [CrossRef]
- Loparic, M.; Wirz, D.; Daniels, A.; Raiteri, R.; VanLandingham, M.R.; Guex, G.; Martin, I.; Aebi, U.; Stolz, M. Micro- and Nanomechanical Analysis of Articular Cartilage by Indentation-Type Atomic Force Microscopy: Validation with a Gel-Microfiber Composite. Biophys. J. 2010, 98, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Bau, B.; Gebhard, P.M.; Haag, J.; Knorr, T.; Bartnik, E.; Aigner, T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002, 46, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F.; Pelletier, J.-P.; Hambor, J.; Cloutier, J.-M.; Martel-Pelletier, J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ. In vitro mimicking effect by transforming growth factor β. Arthritis Rheum. 1997, 40, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.G.; Magna, H.A.; Reeves, L.M.; Lopresti-Morrow, L.L.; Yocum, S.A.; Rosner, P.J.; Geoghegan, K.F.; Hambor, J.E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996, 97, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Zamli, Z.; Adams, M.A.; Tarlton, J.F.; Sharif, M. Increased Chondrocyte Apoptosis Is Associated with Progression of Osteoarthritis in Spontaneous Guinea Pig Models of the Disease. Int. J. Mol. Sci. 2013, 14, 17729–17743. [Google Scholar] [CrossRef]
- Chen, M.-H.; Wang, J.-L.; Wong, C.-Y.; Yao, C.-C.; Chen, Y.-J.; Jiang, C.-C. Relationship of chondrocyte apoptosis to matrix degradation and swelling potential of osteoarthritic cartilage. J. Formos. Med. Assoc. 2005, 104, 264–272. [Google Scholar]
- Halonen, K.S.; Mononen, M.E.; Jurvelin, J.S.; Töyräs, J.; Korhonen, R.K. Importance of depth-wise distribution of collagen and proteoglycans in articular cartilage—A 3D finite element study of stresses and strains in human knee joint. J. Biomech. 2013, 46, 1184–1192. [Google Scholar] [CrossRef]
- Huebner, J.; Williams, J.; Deberg, M.; Henrotin, Y.; Kraus, V. Collagen fibril disruption occurs early in primary guinea pig knee osteoarthritis. Osteoarthr. Cartil. 2010, 18, 397–405. [Google Scholar] [CrossRef]
- Finnilä, M.A.; Das Gupta, S.; Turunen, M.J.; Hellberg, I.; Turkiewicz, A.; Lutz-Bueno, V.; Jonsson, E.; Holler, M.; Ali, N.; Hughes, V.; et al. Mineral Crystal Thickness in Calcified Cartilage and Subchondral Bone in Healthy and Osteoarthritic Human Knees. J. Bone Miner. Res. 2022, 37, 1700–1710. [Google Scholar] [CrossRef]
- Carter, D.; Hayes, W.; Schurman, D. Fatigue life of compact bone—II. Effects of microstructure and density. J. Biomech. 1976, 9, 211–218. [Google Scholar] [CrossRef]
- Li, B.; Aspden, R.M. Composition and Mechanical Properties of Cancellous Bone from the Femoral Head of Patients with Osteoporosis or Osteoarthritis. J. Bone Miner. Res. 1997, 12, 641–651. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Yu, Y.E.; Zhang, X.; Watts, T.; Zhou, B.; Wang, J.; Wang, T.; Zhao, W.; Chiu, K.Y.; et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J. Bone Miner. Res. 2018, 33, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Simha, N.K.; Jin, H.; Hall, M.L.; Chiravarambath, S.; Lewis, J.L. Effect of Indenter Size on Elastic Modulus of Cartilage Measured by Indentation. J. Biomech. Eng. 2007, 129, 767–775. [Google Scholar] [CrossRef]
- Li, C.; Allen, J.; Alliston, T.; Pruitt, L.A. The use of polyacrylamide gels for mechanical calibration of cartilage—A combined nanoindentation and unconfined compression study. J. Mech. Behav. Biomed. Mater. 2011, 4, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Olesiak, S.E.; Oyen, M.L.; Ferguson, V.L. Viscous-elastic-plastic behavior of bone using Berkovich nanoindentation. Mech. Time-Dependent Mater. 2010, 14, 111–124. [Google Scholar] [CrossRef]
- Jin, C.; Ebenstein, D.M. Nanoindentation of compliant materials using Berkovich tips and flat tips. J. Mater. Res. 2017, 32, 435–450. [Google Scholar] [CrossRef]
- Szarko, M.; Muldrew, K.; Bertram, J.E. Freeze-thaw treatment effects on the dynamic mechanical properties of articular cartilage. BMC Musculoskelet. Disord. 2010, 11, 231. [Google Scholar] [CrossRef]
- Kiefer, G.N.; Sundby, K.; McAllister, D.; Shrive, N.G.; Frank, C.B.; Lam, T.; Schachar, N.S. The effect of cryopreservation on the biomechanical behavior of bovine articular cartilage. J. Orthop. Res. 1989, 7, 494–501. [Google Scholar] [CrossRef]
- Moore, A.; Burris, D. Tribological and material properties for cartilage of and throughout the bovine stifle: Support for the altered joint kinematics hypothesis of osteoarthritis. Osteoarthr. Cartil. 2015, 23, 161–169. [Google Scholar] [CrossRef]
- Peters, A.E.; Comerford, E.J.; Macaulay, S.; Bates, K.T.; Akhtar, R. Micromechanical properties of canine femoral articular cartilage following multiple freeze-thaw cycles. J. Mech. Behav. Biomed. Mater. 2017, 71, 114–121. [Google Scholar] [CrossRef]
- Oyen, M.L. Nanoindentation of hydrated materials and tissues. Curr. Opin. Solid State Mater. Sci. 2015, 19, 317–323. [Google Scholar] [CrossRef]
- Fang, T.-H.; Weng, C.-I.; Chang, J.-G. Molecular dynamics analysis of temperature effects on nanoindentation measurement. Mater. Sci. Eng. A 2003, 357, 7–12. [Google Scholar] [CrossRef]
- Xia, R.; Zhou, H.; Wu, R.; Wu, W.-P. Nanoindentation Investigation of Temperature Effects on the Mechanical Properties of Nafion® 117. Polymers 2016, 8, 344. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, S.; Zekonyte, J.; Karali, A.; Roldo, M.; Blunn, G. Early Degenerative Changes in a Spontaneous Osteoarthritis Model Assessed by Nanoindentation. Bioengineering 2023, 10, 995. https://doi.org/10.3390/bioengineering10090995
Davis S, Zekonyte J, Karali A, Roldo M, Blunn G. Early Degenerative Changes in a Spontaneous Osteoarthritis Model Assessed by Nanoindentation. Bioengineering. 2023; 10(9):995. https://doi.org/10.3390/bioengineering10090995
Chicago/Turabian StyleDavis, Sarah, Jurgita Zekonyte, Aikaterina Karali, Marta Roldo, and Gordon Blunn. 2023. "Early Degenerative Changes in a Spontaneous Osteoarthritis Model Assessed by Nanoindentation" Bioengineering 10, no. 9: 995. https://doi.org/10.3390/bioengineering10090995
APA StyleDavis, S., Zekonyte, J., Karali, A., Roldo, M., & Blunn, G. (2023). Early Degenerative Changes in a Spontaneous Osteoarthritis Model Assessed by Nanoindentation. Bioengineering, 10(9), 995. https://doi.org/10.3390/bioengineering10090995