A Thermally Stable Recombinant Human Fibronectin Peptide-Fused Protein (rhFN3C) for Faster Aphthous Ulcer (AU) Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Materials
2.1.1. Reagents and Solvents
2.1.2. Cell Cultures
2.2. Methods
2.2.1. Expression of Recombinant Fibronectin Peptide-Fused Protein in E. coli
2.2.2. Screening and Expression of Recombinant Fibronectin Peptide-Fused Protein
2.2.3. Purification and Characterization of Recombinant Fibronectin Peptide-Fused Protein
2.2.4. Thermal Stability of rhFN3C
2.2.5. rhFN3C Cytotoxicity against L929 Cells
2.2.6. Scratch Test to Detect the Pro-Migratory Effect of rhFN3C on HaCaT Cells
2.2.7. Crystal Violet Cell Adhesion Assay to Test the Promoting Adhesion Effect of rhFN3C on BALB/c 3T3 and HaCaT Cells
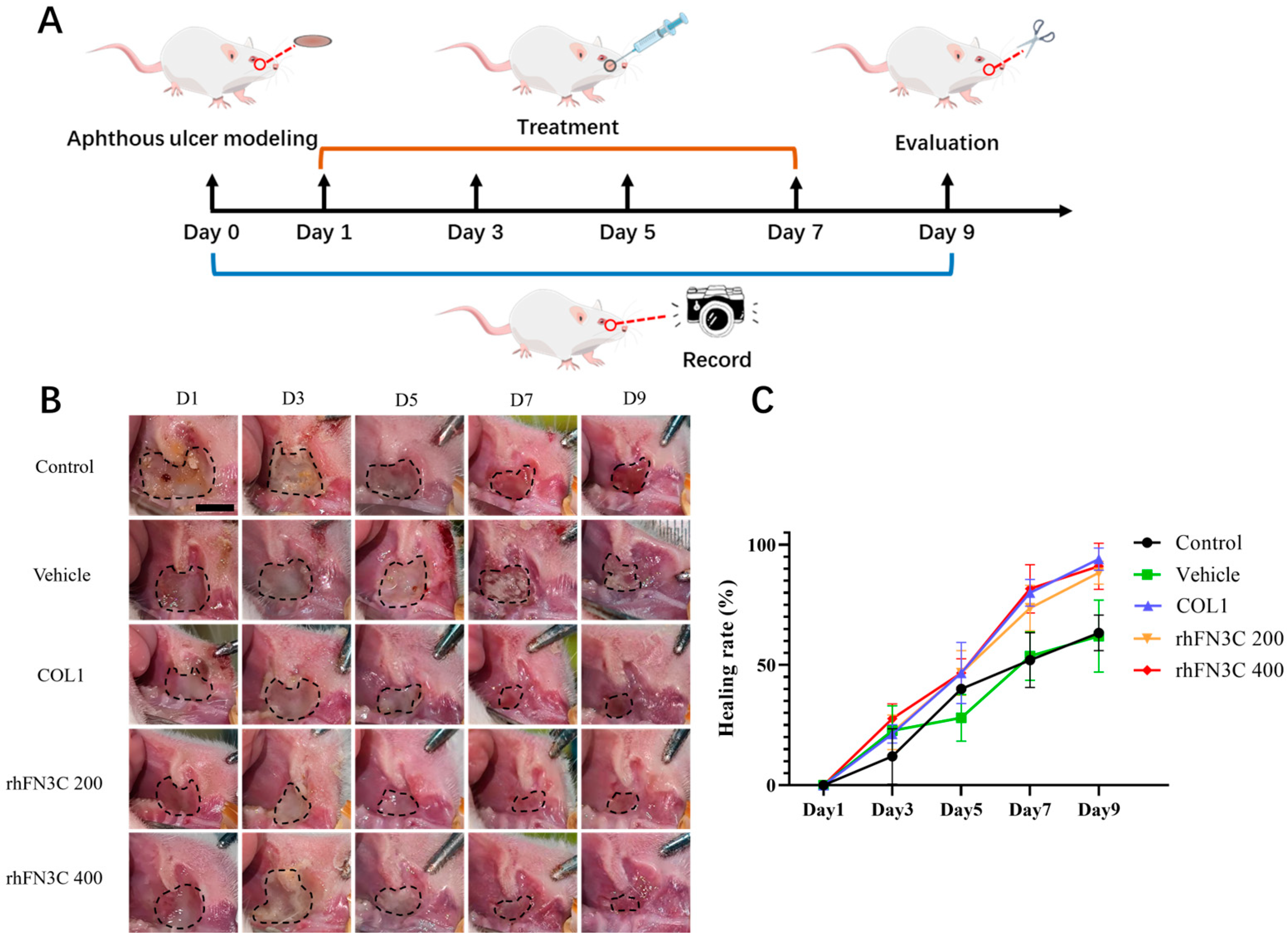
2.2.8. Therapeutic Effect of rhFN3C on Aphthous Ulcer Healing
2.2.9. Statistical Analysis
3. Results
3.1. Construction, Expression, and Identification of rhFN3C
3.2. Thermal Stability of rhFN3C
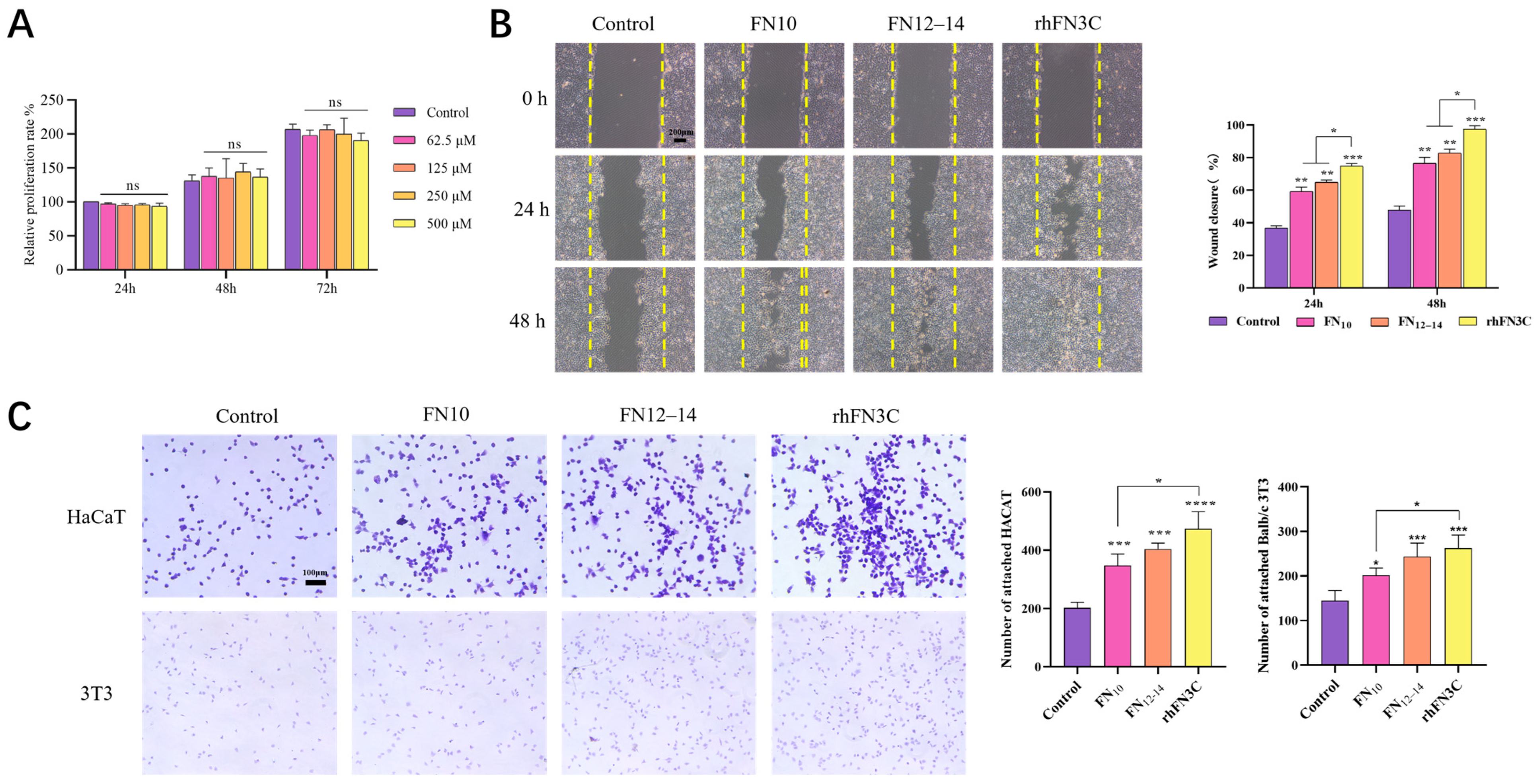
3.3. Cell Viability Assessment after Exposure to rhFN3C
3.3.1. rhFN3C Cytotoxicity on L929 Cells
3.3.2. Scratch Test to Detect the Promigratory Effect of rhFN3C on HaCaT Cells
3.3.3. Crystal Violet Cell Adhesion Assay to Test the Promoting Adhesion Effect of rhFN3C on BALB/c 3T3 and HaCaT Cells
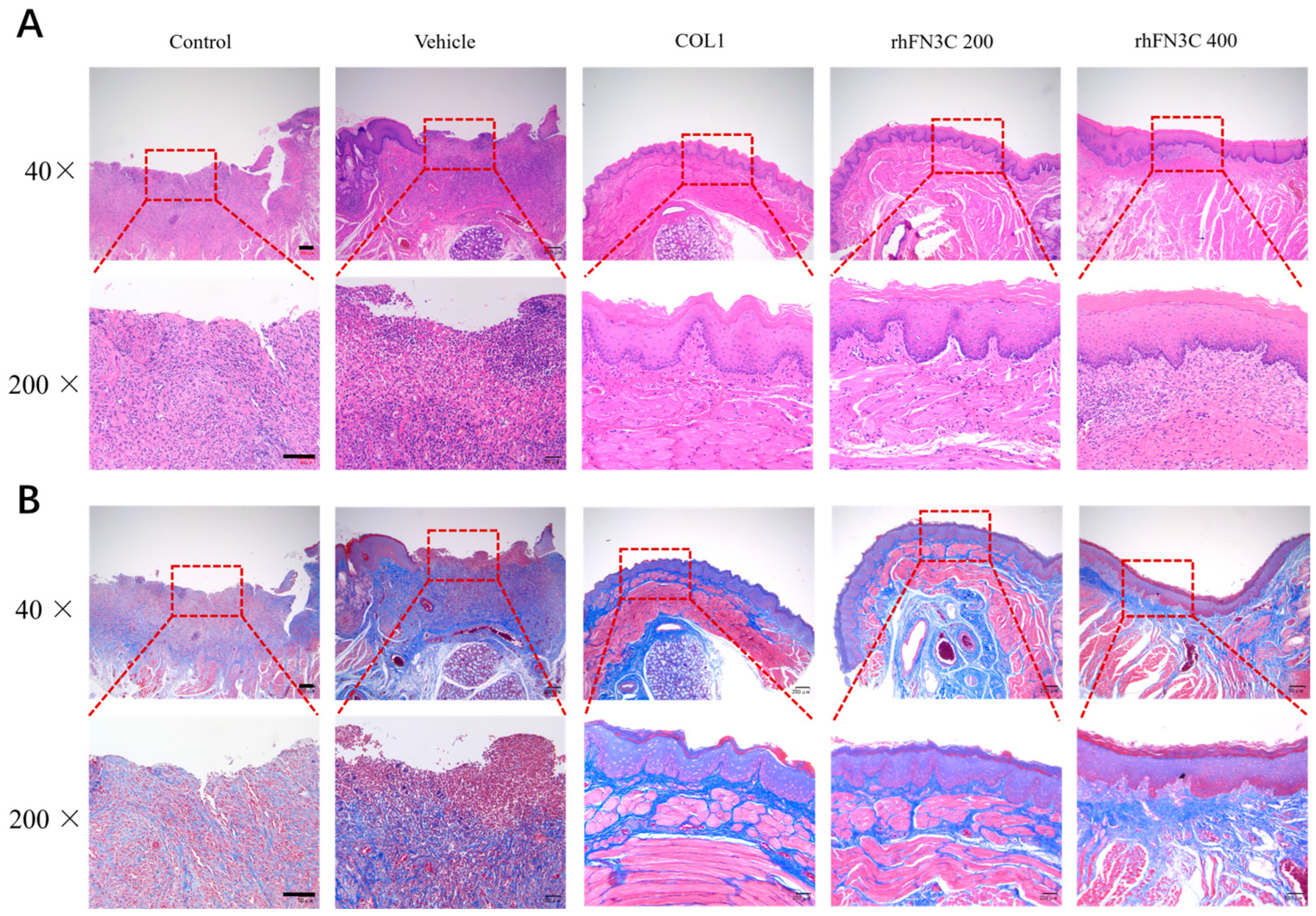
3.4. rhFN3C Promoted Aphthous Ulcer Healing in SD Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. CA Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Lee, C.M.; Saunders, D.P.; Curtis, A.; Dunlap, N.; Nangia, C.; Lee, A.S.; Gordon, S.M.; Kovoor, P.; Arevalo-Araujo, R.; et al. Phase IIb, randomized, double-blind trial of GC4419 versus placebo to reduce severe oral mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. J. Clin. Oncol. 2019, 37, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
- Dudding, T.; Haworth, S.; Lind, P.A.; Sathirapongsasuti, J.F.; Tung, J.Y.; Mitchell, R.; Colodro-Conde, L.; Medland, S.E.; Gordon, S.; Elsworth, B.; et al. Genome wide analysis for mouth ulcers identifies associations at immune regulatory loci. Nat. Commun. 2019, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.G.; Cohen, D.M.; Clark, A.N. Ulcerated lesions of the oral mucosa: Clinical and histologic review. Head Neck Pathol. 2019, 13, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lin, W.; Wu, Y.; Li, Q.; Zhou, X.; Li, H.; Xiao, Q.; Wang, Y.; Shao, B.; Yuan, Q. CBD promotes oral ulcer healing via inhibiting CMPK2-mediated inflammasome. J. Dent. Res. 2022, 101, 206–215. [Google Scholar] [CrossRef]
- Liao, Y.-F.; Gotwals, P.J.; Koteliansky, V.E.; Sheppard, D.; Van De Water, L. The EIIIA segment of fibronectin is a ligand for integrins α9β1 and α4β1providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 2002, 277, 14467–14474. [Google Scholar] [CrossRef]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
- Maione, A.G.; Smith, A.; Kashpur, O.; Yanez, V.; Knight, E.; Mooney, D.J.; Veves, A.; Tomic-Canic, M.; Garlick, J.A. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen. 2016, 24, 630–643. [Google Scholar] [CrossRef]
- Kanta, J.; Zavadakova, A. Role of fibronectin in chronic venous diseases: A review. Vasc. Med. 2020, 25, 588–597. [Google Scholar] [CrossRef]
- Moroz, A.; Deffune, E. Platelet-rich plasma and chronic wounds: Remaining fibronectin may influence matrix remodeling and regeneration success. Cytotherapy 2013, 15, 1436–1439. [Google Scholar] [CrossRef]
- Schultz, G.S.; Ladwig, G.; Wysocki, A. Extracellular matrix: Review of its roles in acute and chronic wounds. World Wide Wounds 2005, 2005, 1–18. [Google Scholar]
- Qiu, Z.; Kwon, A.-H.; Kamiyama, Y. Effects of plasma fibronectin on the healing of full-thickness skin wounds in streptozotocin-induced diabetic rats. J. Surg. Res. 2007, 138, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, H.; Deng, X.; Jin, Y.; Wang, N.; Chu, J. Acellular dermal matrix scaffolds coated with connective tissue growth factor accelerate diabetic wound healing by increasing fibronectin through PKC signalling pathway. J. Tissue Eng. Regen. Med. 2018, 12, e1461–e1473. [Google Scholar] [CrossRef] [PubMed]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, A.J.; Smith, M.L. Fibronectin, the extracellular glue. Matrix Biol. 2017, 60, 27–37. [Google Scholar] [CrossRef]
- Vasiliev, S.; Gorgidze, L.; Efremov, E.; Belinin, G.Y.; Moiseeva, T.; Al-Radi, L.; Sokolova, M.; Guria, G.; Zozulya, N.; Kokhno, A. Fibronectin: Structure, functions, clinical significance. Aterotromboz = Atherothrombosis 2022, 12, 138–158. [Google Scholar] [CrossRef]
- Prakash, P.; Kulkarni, P.P.; Lentz, S.R.; Chauhan, A.K. Cellular fibronectin containing extra domain A promotes arterial thrombosis in mice through platelet Toll-like receptor 4. Blood J. Am. Soc. Hematol. 2015, 125, 3164–3172. [Google Scholar] [CrossRef]
- Leiss, M.; Beckmann, K.; Girós, A.; Costell, M.; Fässler, R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr. Opin. Cell Biol. 2008, 20, 502–507. [Google Scholar] [CrossRef]
- Benito-Jardón, M.; Klapproth, S.; Gimeno-LLuch, I.; Petzold, T.; Bharadwaj, M.; Müller, D.J.; Zuchtriegel, G.; Reichel, C.A.; Costell, M. The fibronectin synergy site re-enforces cell adhesion and mediates a crosstalk between integrin classes. eLife 2017, 6, e22264. [Google Scholar] [CrossRef]
- Asghari Sana, F.; Çapkın Yurtsever, M.; Kaynak Bayrak, G.; Tunçay, E.Ö.; Kiremitçi, A.S.; Gümüşderelioğlu, M. Spreading, proliferation and differentiation of human dental pulp stem cells on chitosan scaffolds immobilized with RGD or fibronectin. Cytotechnology 2017, 69, 617–630. [Google Scholar] [CrossRef]
- Luo, X.; Geng, D.; Zhang, Q.; Ye, T.; Zhang, Y.; Li, Z.; Huang, Y.; Xiang, Q. Recombinant expression a novel fibronectin—Collage fusion peptide modulating stem cell stemness via integrin β3. Appl. Microbiol. Biotechnol. 2022, 106, 3765–3776. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.T.; Zhong, X.; Seibel, N.; Arnolds, O.; Schöpel, M.; Stoll, R. Human melanoma inhibitory protein binds to the FN12–14 Hep II domain of fibronectin. Biointerphases 2017, 12, 02D415. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 2011, 3, 100ra89. [Google Scholar] [CrossRef] [PubMed]
- Bryksin, A.V.; Matsumura, I. Overlap extension PCR cloning: A simple and reliable way to create recombinant plasmids. BioTechniques 2010, 48, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef]
- Hua, C.; Zhu, Y.; Xu, W.; Ye, S.; Zhang, R.; Lu, L.; Jiang, S. Characterization by high-resolution crystal structure analysis of a triple-helix region of human collagen type III with potent cell adhesion activity. Biochem. Biophys. Res. Commun. 2019, 508, 1018–1023. [Google Scholar] [CrossRef]
- Nodai, T.; Hitomi, S.; Ono, K.; Masaki, C.; Harano, N.; Morii, A.; Sago-Ito, M.; Ujihara, I.; Hibino, T.; Terawaki, K.; et al. Endothelin-1 elicits TRP-mediated pain in an acid-induced oral ulcer model. J. Dent. Res. 2018, 97, 901–908. [Google Scholar] [CrossRef]
- Messadi, D.V.; Younai, F. Aphthous ulcers. Dermatol. Ther. 2010, 23, 281–290. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Mahdani, F.Y.; Ernawati, D.S.; Sarasati, A.; Rezkita, F. The macrophage responses during diabetic oral ulcer healing by liquid coconut shell smoke: An immunohistochemical analysis. Eur. J. Dent. 2020, 14, 410–414. [Google Scholar] [CrossRef]
- Hielscher, A.; Ellis, K.; Qiu, C.; Porterfield, J.; Gerecht, S. Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS ONE 2016, 11, e0147600. [Google Scholar] [CrossRef]
- Johnson, M.B.; Pang, B.; Gardner, D.J.; Niknam-Benia, S.; Soundarajan, V.; Bramos, A.; Perrault, D.P.; Banks, K.; Lee, G.K.; Baker, R.Y.; et al. Topical fibronectin improves wound healing of irradiated skin. Sci. Rep. 2017, 7, 3876. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-X.; Lin, C.; Lin, B.-B.; Wang, Z.-G.; Zhang, H.-Y.; Wu, F.-Z.; Cheng, Y.; Xiang, L.-J.; Guo, D.-J.; Luo, X.; et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS ONE 2013, 8, e59966. [Google Scholar] [CrossRef] [PubMed]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef]
- Roy, D.C.; Mooney, N.A.; Raeman, C.H.; Dalecki, D.; Hocking, D.C. Fibronectin matrix mimetics promote full-thickness wound repair in diabetic mice. Tissue Eng. Part A 2013, 19, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Glim, J.E.; Everts, V.; Niessen, F.B.; Ulrich, M.M.; Beelen, R.H. Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch. Oral Biol. 2014, 59, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudaki, G.; Creber, K.; Hamilton, D.W. Wound healing and fibrosis: A contrasting role for periostin in skin and the oral mucosa. Am. J. Physiol. Cell Physiol. 2020, 318, C1065–C1077. [Google Scholar] [CrossRef]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.; Niessen, F.B.; Gibbs, S. The bigger picture: Why oral mucosa heals better than skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef]
- Núñez, M.A.G.; de Matos, F.R.; de Almeida Freitas, R.; Galvão, H.C. Immunohistochemical study of integrin α5β1, fibronectin, and Bcl-2 in normal oral mucosa, inflammatory fibroepithelial hyperplasia, oral epithelial dysplasia, and oral squamous cell carcinoma. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 354–361. [Google Scholar] [CrossRef]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular matrix revisited: Roles in tissue engineering. Int. Neurourol. J. 2016, 20, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Sgarioto, M.; Vigneron, P.; Patterson, J.; Malherbe, F.; Nagel, M.-D.; Egles, C. Collagen type I together with fibronectin provide a better support for endothelialization. Comptes Rendus Biol. 2012, 335, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Zhu, J.; Luo, X.; Jin, G.; Huang, Y.; Li, L. A Thermally Stable Recombinant Human Fibronectin Peptide-Fused Protein (rhFN3C) for Faster Aphthous Ulcer (AU) Healing. Bioengineering 2024, 11, 38. https://doi.org/10.3390/bioengineering11010038
Cai X, Zhu J, Luo X, Jin G, Huang Y, Li L. A Thermally Stable Recombinant Human Fibronectin Peptide-Fused Protein (rhFN3C) for Faster Aphthous Ulcer (AU) Healing. Bioengineering. 2024; 11(1):38. https://doi.org/10.3390/bioengineering11010038
Chicago/Turabian StyleCai, Xiang, Jiawen Zhu, Xin Luo, Guoguo Jin, Yadong Huang, and Lihua Li. 2024. "A Thermally Stable Recombinant Human Fibronectin Peptide-Fused Protein (rhFN3C) for Faster Aphthous Ulcer (AU) Healing" Bioengineering 11, no. 1: 38. https://doi.org/10.3390/bioengineering11010038
APA StyleCai, X., Zhu, J., Luo, X., Jin, G., Huang, Y., & Li, L. (2024). A Thermally Stable Recombinant Human Fibronectin Peptide-Fused Protein (rhFN3C) for Faster Aphthous Ulcer (AU) Healing. Bioengineering, 11(1), 38. https://doi.org/10.3390/bioengineering11010038






