Differences in Trochlear Morphology of a New Femoral Component Designed for Kinematic Alignment from a Mechanical Alignment Design
Abstract
1. Introduction
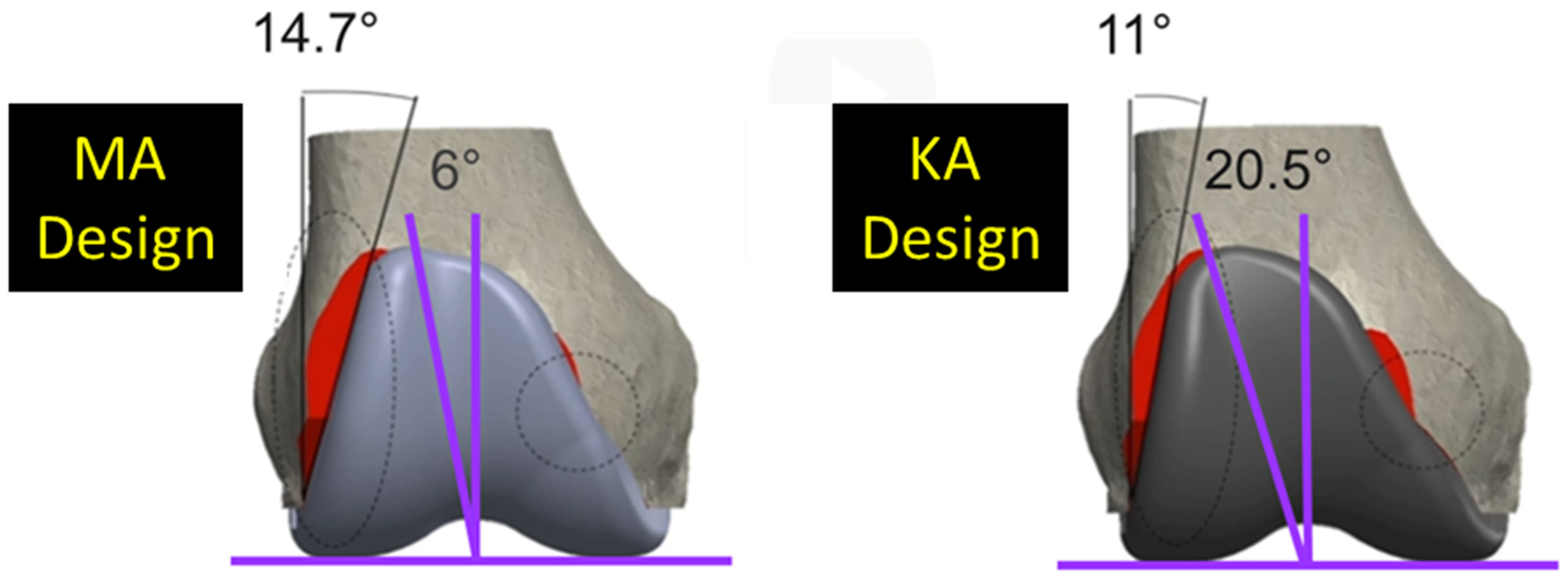
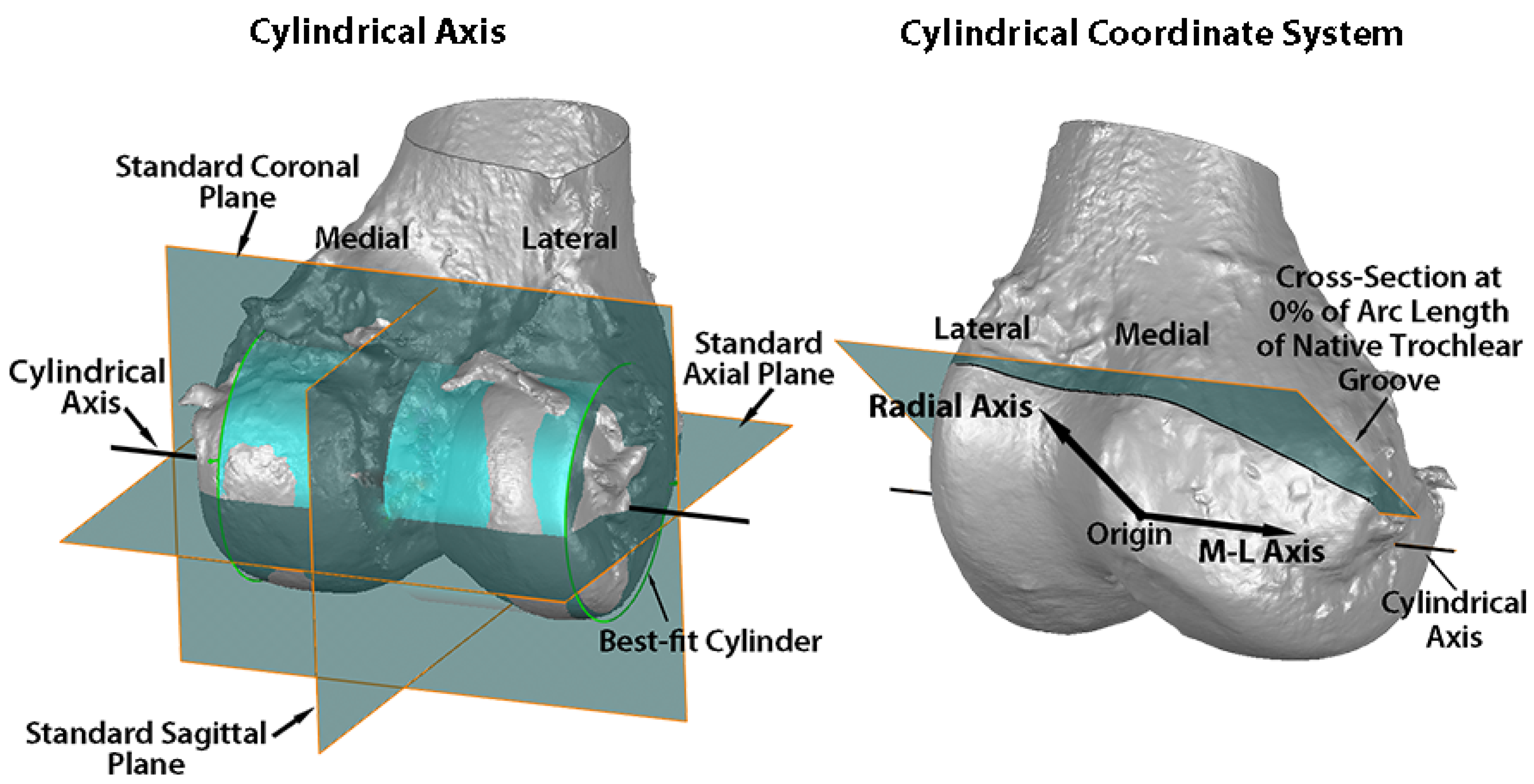
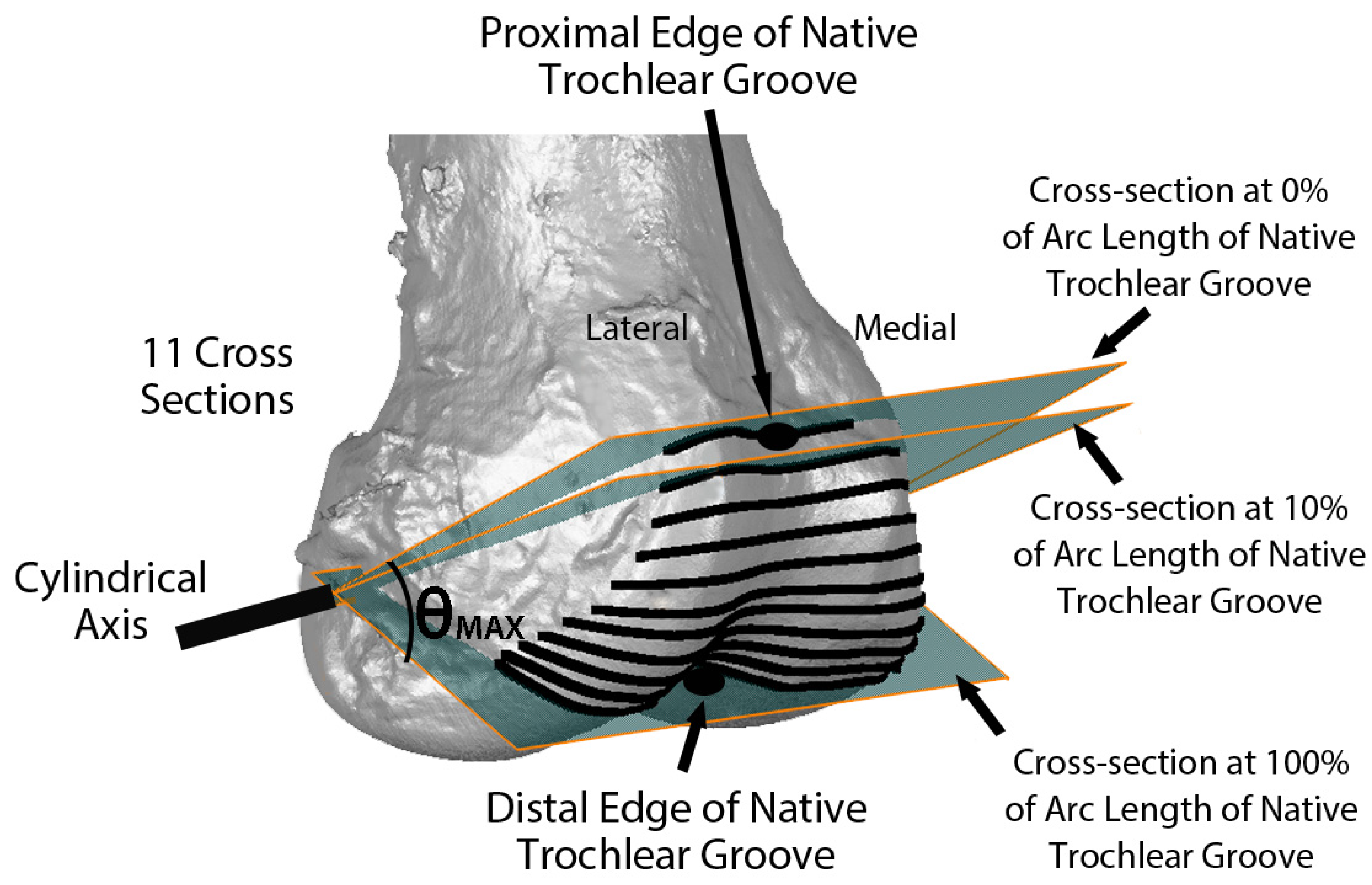
2. Materials and Methods
Statistical Analysis
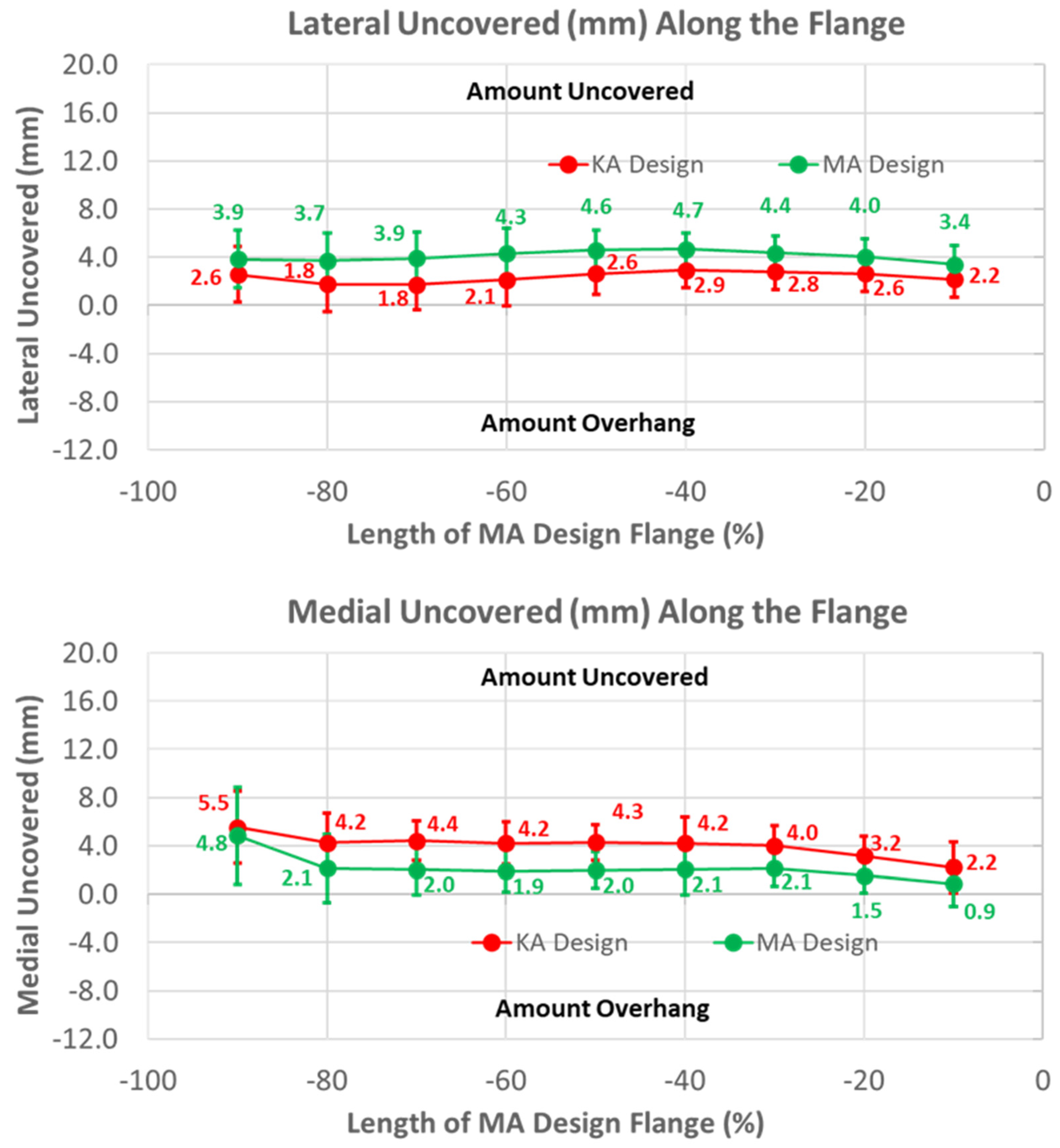
3. Results
3.1. Anterior Flange
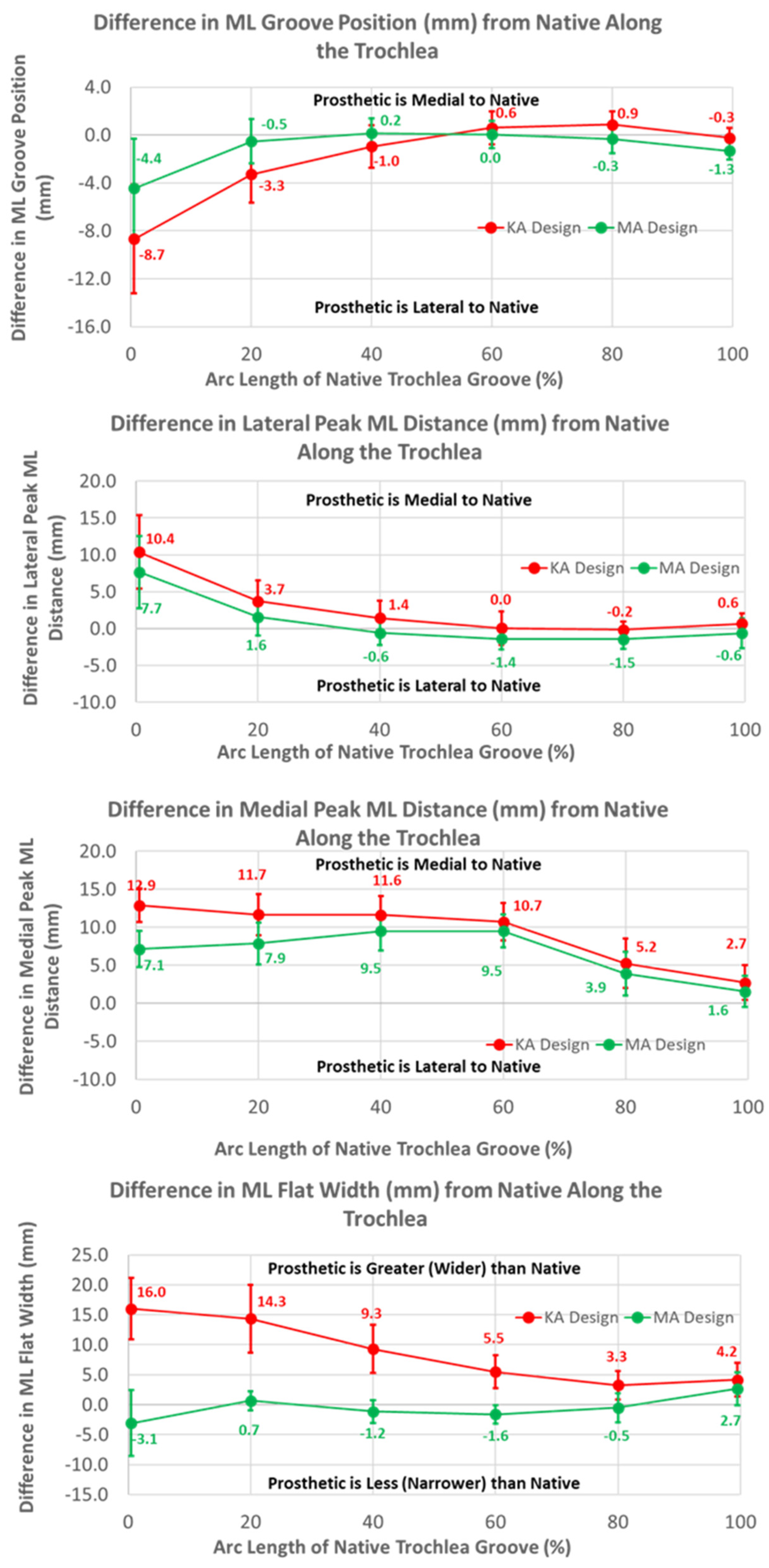
3.2. Arc Length of Native Groove: KA versus MA
3.3. Arc Length of Native Groove: KA versus Native
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assiotis, A.; To, K.; Morgan-Jones, R.; Pengas, I.P.; Khan, W. Patellar complications following total knee arthroplasty: A review of the current literature. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Barrack, R.L.; Schrader, T.; Bertot, A.J.; Wolfe, M.W.; Myers, L. Component rotation and anterior knee pain after total knee arthroplasty. Clin. Orthop. Relat. Res. 2001, 392, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Conditt, M.A.; Noble, P.C.; Allen, B.; Shen, M.; Parsley, B.S.; Mathis, K.B. Surface damage of patellar components used in total knee arthroplasty. J. Bone Joint Surg. 2005, 87, 1265–1271. [Google Scholar] [PubMed]
- Berger, R.A.; Crossett, L.S.; Jacobs, J.J.; Rubash, H.E. Malrotation causing patellofemoral vomplications after total knee arthroplasty. Clin. Orthop. Relat. Res. 1998, 356, 144–153. [Google Scholar] [CrossRef]
- Gonzalez, M.H.; Mekhail, A.O. The failed total knee arthroplasty: Evaluation and etiology. J. Am. Acad. Orthop. Surg. 2004, 12, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Leopold, S.S.; Silverton, C.D.; Barden, R.M.; Rosenberg, A.G. Isolated revision of the patellar component in total knee arthroplasty. J. Bone Jt. Surg. 2003, 85, 41–47. [Google Scholar] [CrossRef]
- Saffarini, M.; Demey, G.; Nover, L.; Dejour, D. Evolution of trochlear compartment geometry in total knee arthroplasty. Ann. Transl. Med. 2015, 4, 7. [Google Scholar] [CrossRef]
- Varadarajan, K.M.; Rubash, H.E.; Li, G. Are current total knee arthroplasty implants designed to restore normal trochlear groove anatomy? J. Arthroplast. 2011, 26, 274–281. [Google Scholar] [CrossRef]
- Howell, S.M.; Papadopoulos, S.; Kuznik, K.T.; Hull, M.L. Accurate alignment and high function after kinematically aligned TKA performed with generic instruments. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2271–2280. [Google Scholar] [CrossRef]
- Gu, Y.; SM, H.; ML, H. Simulation of total knee arthroplasty in 5 degrees or 7 degrees valgus: A study of gap imbalances and changes in limb and knee alignments from native. J. Orthop. Res. 2017, 35, 2031–2039. [Google Scholar] [CrossRef]
- Gu, Y.; Roth, J.D.; Howell, S.M.; Hull, M.L. How frequently do four methods for mechanically aligning a total knee arthroplasty cause collateral ligament imbalance and change alignment from normal in white patients? J. Bone Joint Surg. Am. 2014, 96, e101. [Google Scholar] [CrossRef] [PubMed]
- Dossett, H.G.; Estrada, N.A.; Swartz, G.J.; LeFevre, G.W.; Kwasman, B.G. A randomised controlled trial of kinematically and mechanically aligned total knee replacements: Two-year clinical results. Bone Joint J. 2014, 96, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Campanelli, V.; Howell, S.; Hull, M. Kinematic alignment more closely restores the groove location and the sulcus angle of the native trochlea than mechanical alignment: Implications for prosthetic design. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.S.; Howell, S.M.; Hull, M.L.; Mahfouz, M.R. Does kinematic alignment and flexion of a femoral component designed for mechanical alignment reduce the proximal and lateral reach of the trochlea? J. Arthroplast. 2016, 31, 1808–1813. [Google Scholar] [CrossRef]
- Rivière, C.; Iranpour, F.; Harris, S.; Auvinet, E.; Aframian, A.; Parratte, S.; Cobb, J. Differences in trochlear parameters between native and prosthetic kinematically or mechanically aligned knees. Orthop. Traumatol. Surg. Res. 2018, 104, 165–170. [Google Scholar] [CrossRef]
- Ghosh, K.M.; Merican, A.M.; Iranpour, F.; Deehan, D.J.; Amis, A.A. The effect of overstuffing the patellofemoral joint on the extensor retinaculum of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 1211–1216. [Google Scholar] [CrossRef]
- Mihalko, W.; Fishkin, Z.; Krakow, K. Patellofemoral overstuff and its relationship to flexion after total knee arthroplasty. Clin. Orthop. Relat. Res. 2006, 449, 283–287. [Google Scholar] [CrossRef]
- Hull, M.L.; Howell, S.M. Differences in trochlear morphology from native using a femoral component interfaced with an anatomical patellar prosthesis in kinematic alignment and mechanical alignment. J. Knee Surg. 2020, 35, 625–633. [Google Scholar] [CrossRef]
- Bartlett, J.W.; Frost, C. Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous variables. Ultrasound Obstet. Gynecol. 2008, 31, 466–475. [Google Scholar] [CrossRef]
- Indrayan, A. Methods of Clinical Epidemiology; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Hirschmann, M.T.; Moser, L.B.; Amsler, F.; Behrend, H.; Leclerq, V.; Hess, S. Functional knee phenotypes: A novel classification for phenotyping the coronal lower limb alignment based on the native alignment in young non-osteoarthritic patients. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1394–1402. [Google Scholar] [CrossRef]
- Sappey-Marinier, E.; Howell, S.M.; Nedopil, A.J.; Hull, M.L. The trochlear groove of a femoral component designed for kinematic alignment is lateral to the quadriceps line of force and better laterally covers the anterior femoral resection than a mechanical alignment design. J. Pers. Med. 2022, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Merican, A.M.; Ghosh, K.M.; Baena, F.R.Y.; Deehan, D.J.; Amis, A.A. Patellar thickness and lateral retinacular release affects patellofemoral kinematics in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.; Vince, K.G. The unstable patella after total knee arthroplasty: Etiology, prevention, and management. J. Am. Acad. Orthop. Surg. 2003, 11, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Barink, M.; Van de Groes, S.; Verdonschot, N.; Malefijt, M.D.W. The difference in trochlear orientation between the natural knee and current prosthetic knee designs; towards a truly physiological prosthetic groove orientation. J. Biomech. 2006, 39, 1708–1715. [Google Scholar] [CrossRef]
- Du, Z.; Chen, S.; Yan, M.; Yue, B.; Wang, Y. Differences between native and prosthetic knees in terms of cross-sectional morphology of the femoral trochlea: A study based on three-dimensional models and virtual total knee arthroplasty. BMC Musculoskelet. Disord. 2017, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Lachiewicz, P.F.; Soileau, E.S. Patella maltracking in posterior-stabilized total knee arthroplasty. Clin. Orthop. Relat. Res. 2006, 452, 155–158. [Google Scholar] [CrossRef]
- Lynch, A.F.; Rorabeck, C.H.; Bourne, R.B. Extensor mechanism complications following total knee arthroplasty. J. Arthroplast. 1987, 2, 135–140. [Google Scholar] [CrossRef]
- Freedman, B.R.; Brindle, T.J.; Sheehan, F.T. Re-evaluating the functional implications of the Q-angle and its relationship to in-vivo patellofemoral kinematics. Clin. Biomech. 2014, 29, 1139–1145. [Google Scholar] [CrossRef]







| Dependent Variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Radial Distance to Groove | Lateral Height | Medial Height | Lateral Slope | Medial Slope | ML Groove Location | Lateral Peak ML Distance | Medial Peak ML Distance | ML Flat Portion | |
| Repeatability | 0.32 mm | 0.18 mm | 0.32 mm | 0.03 mm/mm | 0.03 mm/mm | 0.54 mm | 0.73 mm | 0.77 mm | 0.93 mm |
| Intraobserver ICC | 0.99 | 0.74 | 0.86 | 0.58 | 0.33 | 0.83 | 0.61 | 0.76 | 0.78 |
| Interobserver ICC | 0.93 | 0.65 | 0.71 | 0.50 | 0.28 | 0.54 | 0.54 | 0.71 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hull, M.L.; Simileysky, A.; Howell, S.M. Differences in Trochlear Morphology of a New Femoral Component Designed for Kinematic Alignment from a Mechanical Alignment Design. Bioengineering 2024, 11, 62. https://doi.org/10.3390/bioengineering11010062
Hull ML, Simileysky A, Howell SM. Differences in Trochlear Morphology of a New Femoral Component Designed for Kinematic Alignment from a Mechanical Alignment Design. Bioengineering. 2024; 11(1):62. https://doi.org/10.3390/bioengineering11010062
Chicago/Turabian StyleHull, Maury L., Alexander Simileysky, and Stephen M. Howell. 2024. "Differences in Trochlear Morphology of a New Femoral Component Designed for Kinematic Alignment from a Mechanical Alignment Design" Bioengineering 11, no. 1: 62. https://doi.org/10.3390/bioengineering11010062
APA StyleHull, M. L., Simileysky, A., & Howell, S. M. (2024). Differences in Trochlear Morphology of a New Femoral Component Designed for Kinematic Alignment from a Mechanical Alignment Design. Bioengineering, 11(1), 62. https://doi.org/10.3390/bioengineering11010062








