A Versatile Microfluidic Device System that Lacks a Synthetic Extracellular Matrix Recapitulates the Blood–Brain Barrier and Dynamic Tumor Cell Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design, Fabrication, and Permeability Testing of the Microfluidic System
2.2. Cell Immunophenotype, Proliferation Kinetics, and Vascularization Assay
2.3. Endothelial Vascularization Test
2.4. Cell Line Cultures in the Microfluidic System
2.5. Microenvironment of Blood–Brain Barrier
2.6. Confocal Microscopy
3. Results and Discussion
3.1. Microfluidic System Design and Characterization
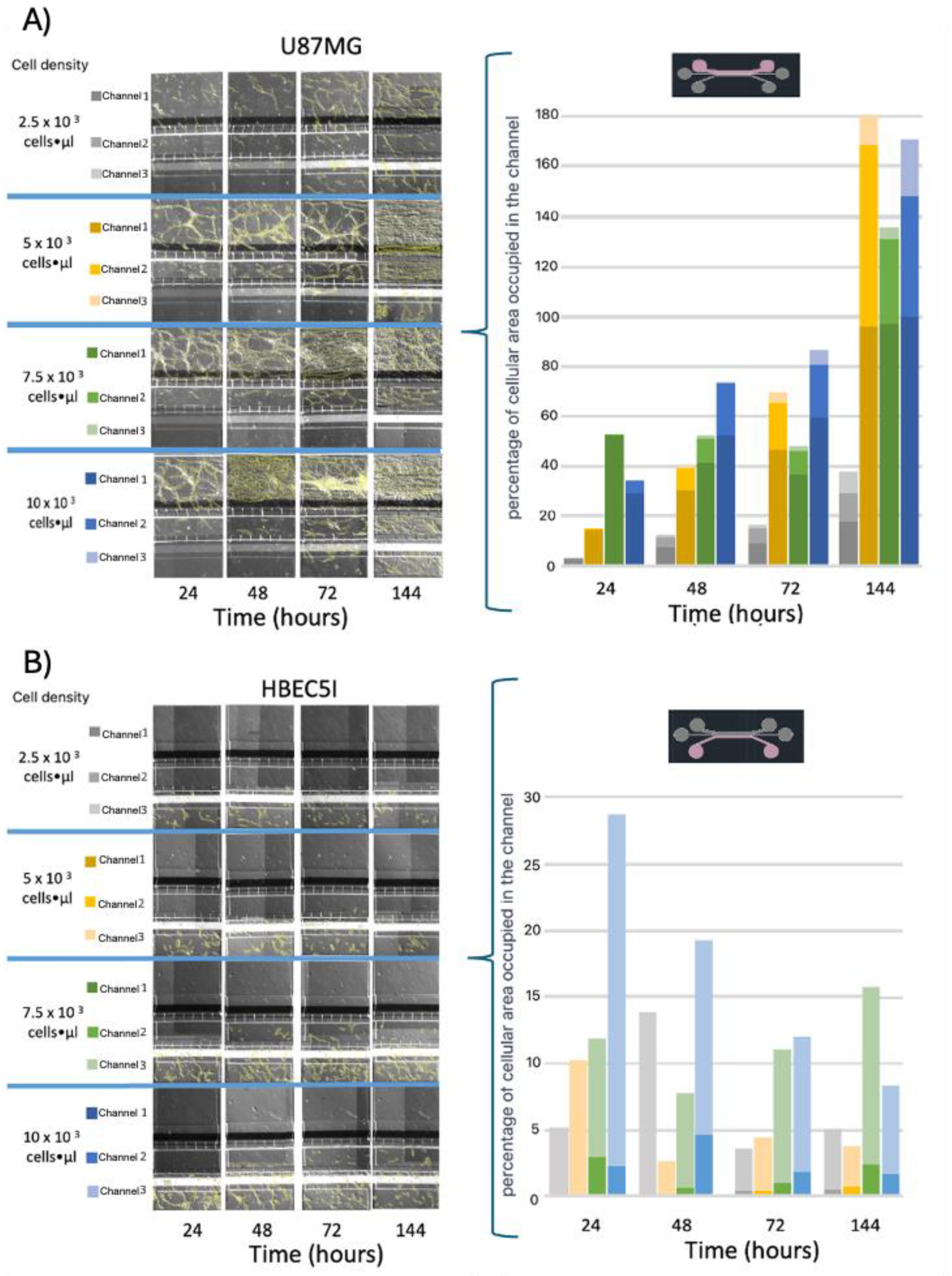
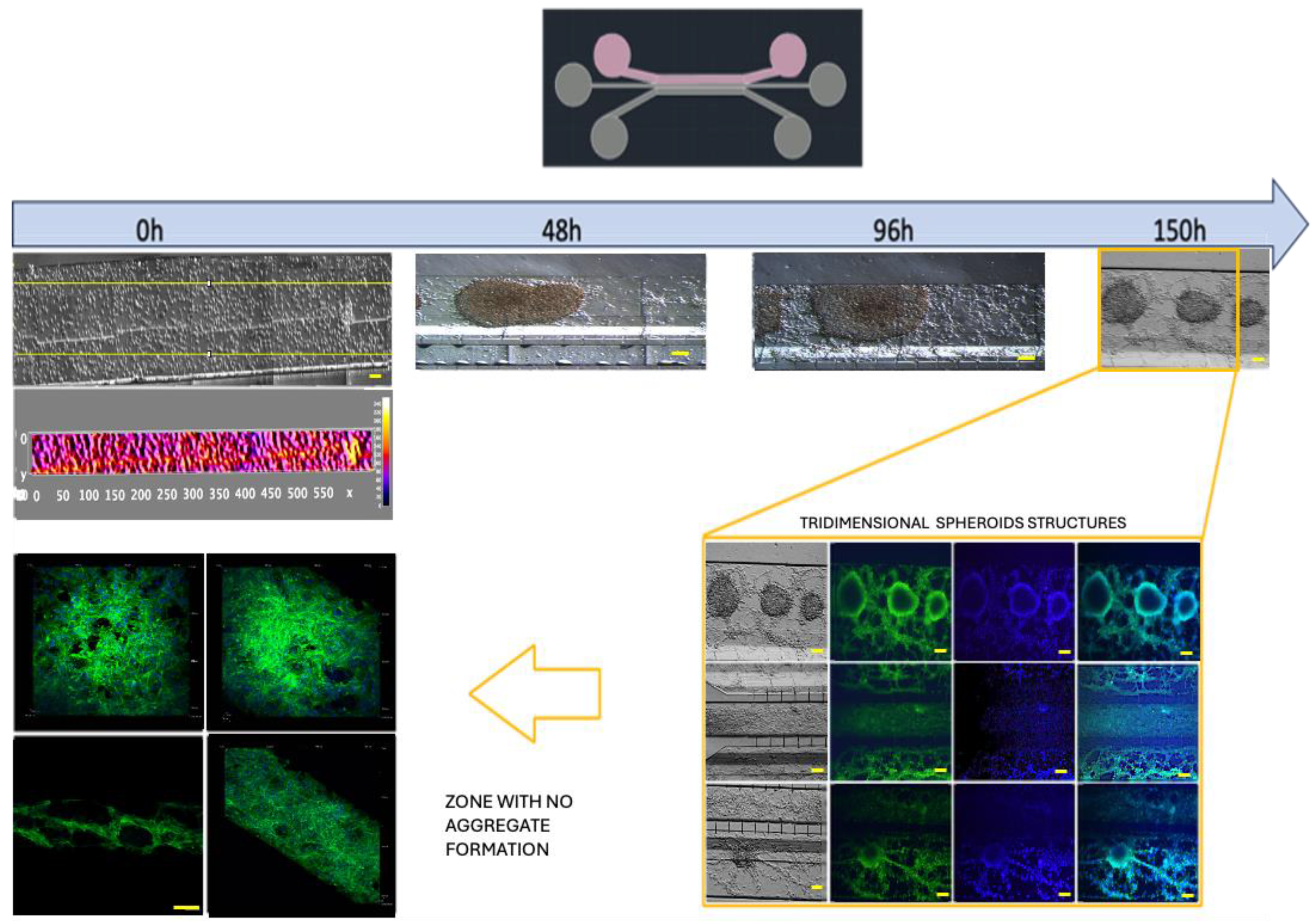
3.2. Cellular Population and Inoculation Cell Density in the Microfluidic System
3.3. Optimization of Inoculation Cell Density in the Microfluidic System
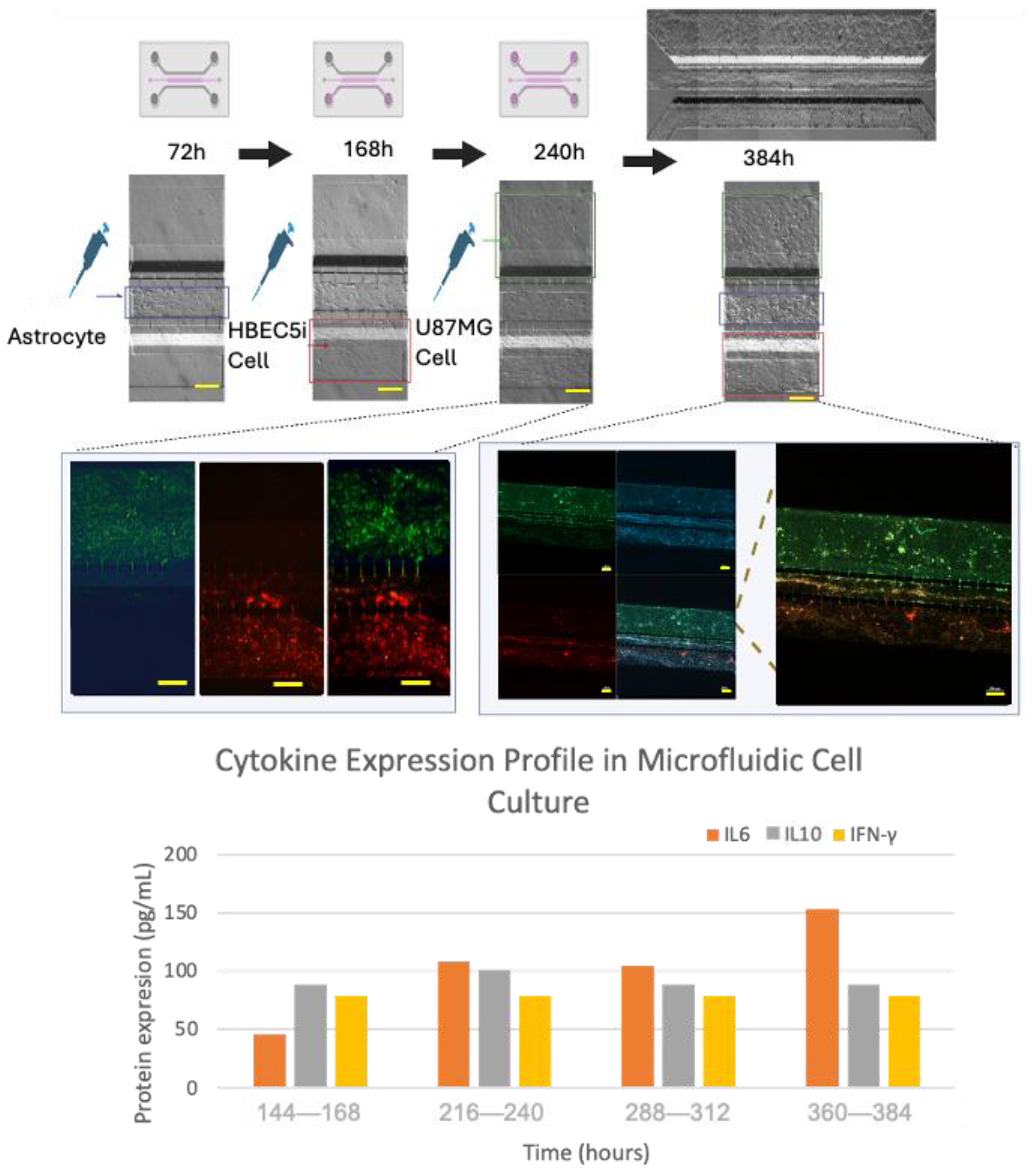
3.4. Cell–Cell Interactions within the Microfluidic System and Pro-Inflammatory Mediators
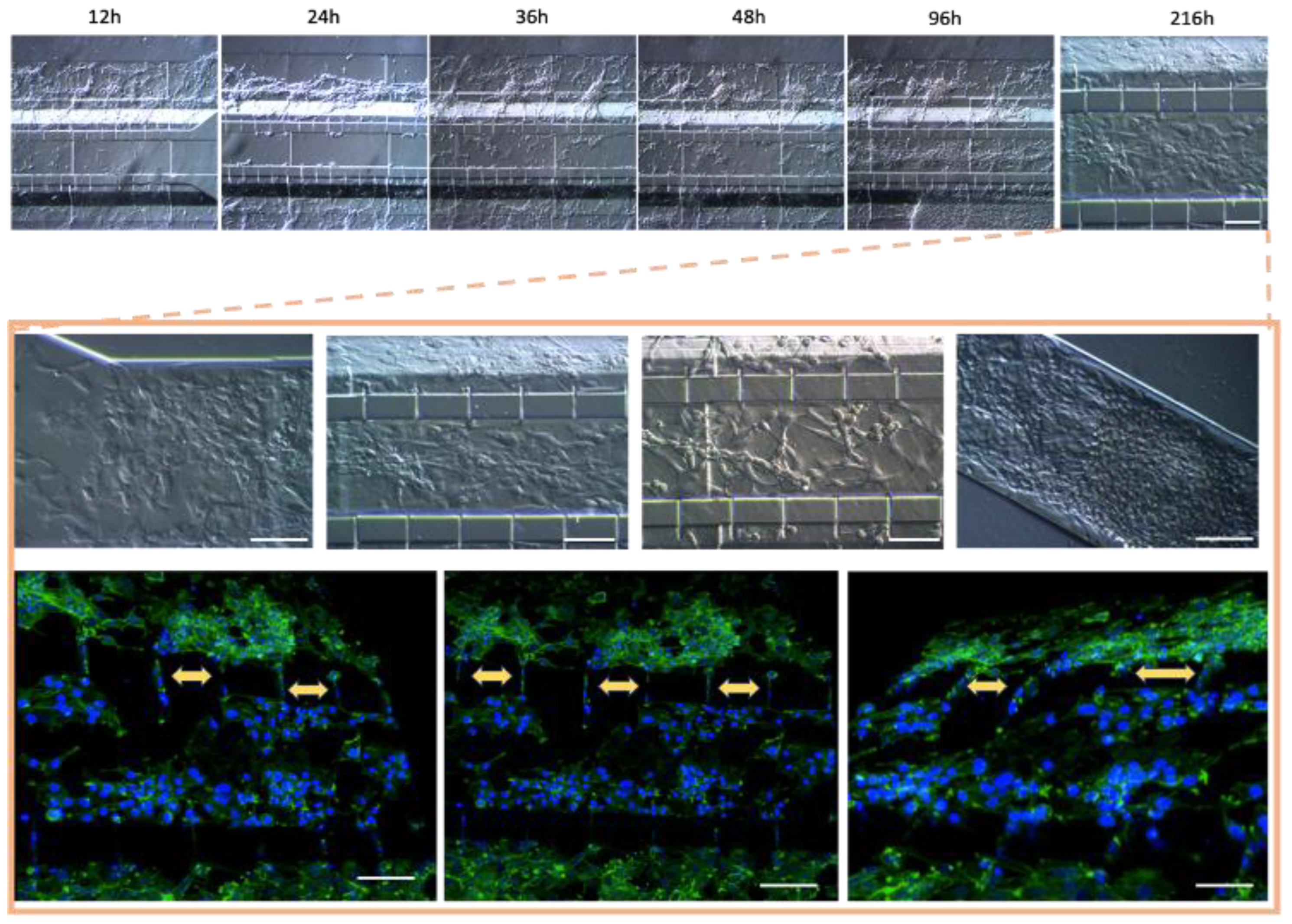
3.5. Cell Detachment in the Microfluidic System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucullo, L.; Oby, E.; Hallene, K.; Aumayr, B.; Rapp, E.; Janigro, D. Artificial blood-Brain Barriers. In Blood-Brain Barriers: FROM Ontogeny to Artificial Interfaces; Dermietzel, R., Spray, D.C., Nedergaard, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 375–402. [Google Scholar]
- Marchi, N.; Banjara, M.; Janigro, D. Blood-brain barrier, bulk flow, and interstitial clearance in epilepsy. J. Neurosci. Methods 2016, 260, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Hacking, R.; Hunt, K. Cerebral abscess: A review of its pathophysiology, diagnosis and management. Br. J. Neurosci. Nurs. 2007, 3, 400–403. [Google Scholar] [CrossRef]
- Lecuyer, M.A.; Kebir, H.; Prat, A. Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 472–482. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Ai, X.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Zhang, S. Potential applications of microfluidics-based blood-brain barrier (BBB)-on-chips for in vitro drug development. Biomed. Pharmacother. 2020, 132, 110822. [Google Scholar] [CrossRef]
- Furnari, F.B.; Fenton, T.; Bachoo, R.M.; Mukasa, A.; Stommel, J.M.; Stegh, A.; Hahn, W.C.; Ligon, K.L.; Louis, D.N.; Brennan, C.; et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007, 21, 2683–2710. [Google Scholar] [CrossRef]
- Junttila, M.R.; De Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Becker, J.C.; Andersen, M.H.; Schrama, D.; thor Straten, P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol. Immunother. 2013, 62, 1137–1148. [Google Scholar] [CrossRef]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The brain tumor microenvironment. Glia 2012, 60, 502–514. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Gronseth, E.; Wang, L.; Harder, D.R.; Ramchandran, R. The Role of Astrocytes in Tumor Growth and Progression. In Astrocyte-Physiology and Pathology; Intech: Vienna, Austria, 2018. [Google Scholar]
- Lee, G.; Hall, R.R., III; Ahmed, A.U. Cancer stem cells: Cellular plasticity, niche, and its clinical relevance. J. Stem Cell Res. Ther. 2016, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Abou-Antoun, T.J.; Hale, J.S.; Lathia, J.D.; Dombrowski, S.M. Brain cancer stem cells in adults and children: Cell biology and therapeutic implications. Neurotherapeutics 2017, 14, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Ma YH, V.; Middleton, K.; You, L.; Sun, Y. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsyst. Nanoeng. 2018, 4, 17104. [Google Scholar] [CrossRef]
- Ruiz-Garcia, H.; Alvarado-Estrada, K.; Schiapparelli, P.; Quinones-Hinojosa, A.; Trifiletti, D.M. Engineering three- dimensional tumor models to study glioma cancer stem cells and tumor microenvironment. Front. Cell. Neurosci. 2020, 14, 558381. [Google Scholar] [CrossRef] [PubMed]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 2017, 168, 1135–1148. [Google Scholar] [CrossRef]
- Elbakary, B.; Badhan, R.K. A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Sci. Rep. (Nat. Publ. Group) 2020, 10, 3788. [Google Scholar] [CrossRef]
- van Duinen, V.; Trietsch, S.J.; Joore, J.; Vulto, P.; Hankemeier, T. Microfluidic 3D cell culture: From tools to tissue models. Curr. Opin. Biotechnol. 2015, 35, 118–126. [Google Scholar] [CrossRef]
- Plummer, S.; Wallace, S.; Ball, G.; Lloyd, R.; Schiapparelli, P.; Quiñones-Hinojosa, A.; Hartung, T.; Pamies, D. A Human iPSC-derived 3D platform using primary brain cancer cells to study drug development and personalized medicine. Sci. Rep. 2019, 9, 1407. [Google Scholar] [CrossRef]
- Tsai, H.F.; Trubelja, A.; Shen, A.Q.; Bao, G. Tumour-on-a-chip: Microfluidic models of tumour morphology, growth and microenvironment. J. R. Soc. Interface 2017, 14, 20170137. [Google Scholar] [CrossRef]
- Meng, F.; Meyer, C.M.; Joung, D.; Vallera, D.A.; McAlpine, M.C.; Panoskaltsis-Mortari, A. 3D Bioprinted In Vitro Metastatic Models via Reconstruction of Tumor Microenvironments. Adv. Mater. 2019, 31, 1806899. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Shen, Y. Microfluidics for cancer nanomedicine: From fabrication to evaluation. Small 2018, 14, 1800360. [Google Scholar] [CrossRef]
- Logun, M.; Zhao, W.; Mao, L.; Karumbaiah, L. Microfluidics in malignant glioma research and precision medicine. Adv. Biosyst. 2018, 2, 170022. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Briggs, R.G.; Homburg, H.B.; Young, I.M.; Davis, E.J.; Lin, Y.-H.; Battiste, J.D.; Sughrue, M.E. Application of microfluidic devices for glioblastoma study: Current status and future directions. Biomed Microdevices 2020, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Prabhakarpandian, B.; Shen, M.C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A Microfluidic Blood Brain Barrier Model. Lab A Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Rapp, E.; Manders, T.; Marchi, N.; Janigro, D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia 2007, 48, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.E.; Sleeboom, J.J.; Ingber, D.E. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef]
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C.; et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflamm. 2016, 13, 306. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef]
- Brown, J.A.; Faley, S.L.; Shi, Y.; Hillgren, K.M.; Sawada, G.A.; Baker, T.K.; Wikswo, J.P.; Lippmann, E.S. Advances in blood-brain barrier modeling in microphysiological systems highlight critical differences in opioid transport due to cortisol exposure. Fluids Barriers CNS 2020, 17, 38. [Google Scholar] [CrossRef]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.-E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef] [PubMed]
- Goral, V.N.; Hsieh, Y.C.; Petzold, O.N.; Clark, J.S.; Yuen, P.K.; Faris, R.A. Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab A Chip 2010, 10, 3380–3386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Liu, J.R.; Patel, B.; Solomon, D.E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Bersini, S.; Gilardi, M.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl. Acad. Sci. USA 2015, 112, 214–219. [Google Scholar] [CrossRef]
- Puech, C.; Hodin, S.; Forest, V.; He, Z.; Mismetti, P.; Delavenne, X.; Perek, N. Assessment of HBEC-5i endothelial cell line cultivated in astrocyte conditioned medium as a human blood-brain barrier model for ABC drug transport studies. Int. J. Pharm. 2018, 551, 281–289. [Google Scholar] [CrossRef]
- Gordillo-Sampedro, S.; Antounians, L.; Wei, W.; Mufteev, M.; Lendemeijer, B.; Kushner, S.A.; de Vrij FM, S.; Zani, A.; Ellis, J. iPSC-derived healthy human astrocytes selectively load miRNAs targeting neuronal genes into extracellular vesicles. Mol. Cell. Neurosci. 2024, 129, 103933. [Google Scholar] [CrossRef]
- Boccellato, C.; Rehm, M. Glioblastoma, from disease understanding towards optimal cell-based in vitro models. Cell. Oncol. 2022, 45, 527–541. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.; Thurgood, P.; Gilliam, C.; Nguyen, N.; Pirogova, E.; Khoshmanesh, K.; Baratchi, S. A microfluidic system for studying the effects of disturbed flow on endothelial cells. Front. Bioeng. Biotechnol. 2019, 7, 81. [Google Scholar] [CrossRef]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab A Chip 2017, 17, 448–459. [Google Scholar] [CrossRef] [PubMed]
- May, S.; Evans, S.; Parry, L. Organoids, organs-on-chips and other systems, and microbiota. Emerg. Top. Life Sci. 2017, 1, 385–400. [Google Scholar] [PubMed]
- Deosarkar, S.P.; Prabhakarpandian, B.; Wang, B.; Sheffield, J.B.; Krynska, B.; Kiani, M.F. A novel dynamic neonatal blood-brain barrier on a chip. PLoS ONE 2015, 10, e0142725. [Google Scholar] [CrossRef] [PubMed]




| Antibody | Primary Antibody | Secondary Antibody | Target Description |
|---|---|---|---|
| Anti Ve-cadherin | 1:200 (Ab33168) | 1:1000 (Ab6718) | Ve-cadherin is an adhesion protein that is present in endothelial cells that form the inner lining of blood vessels. |
| Anti von Willebrand | 1:200 (Ab6994) | 1:1000 (Ab6718) | The Von Willebrand protein is mainly found in endothelial cells that line blood vessels. |
| Anti VEGFR | 1:200 (ab9530) | 1:1000 (ab6786) | The VEGFR (vascular endothelial growth factor receptor) is a protein that plays a crucial role in angiogenesis and blood vessel growth. |
| Anti Oct3/4 | 1:200 (C10-Z210Ms) | 1:1000 (ab6786) | The expression of Oct3/4 in glioblastoma cells, such as U87MG, is an indication of these tumor cells’ ability to acquire more undifferentiated and aggressive characteristics. |
| Anti Sox2 | 1:200 (ZM57-Z236MS) | 1:1000 (ab6786) | Sox2 expression in glioblastoma cells, including U87MG, signals the existence of stem cells that are capable of self-renewal and the generation of differentiated tumor cell subpopulations. |
| Anti GFAP | 1:200 (G3893) | 1:1000 (ab6785) | GFAP (glial fibrillary acidic protein) can be used for identifying and locating astrocytes in cell cultures, brain tissues, and histological sections. |
| Anti Phalloidin-Alexa- Fluor 488 | 1:100 (A12379) | ------------- | The anti-phalloidin antibody can be used to visualize the distribution, organization, and dynamics of actin filaments in living cells or fixed samples. |
| Anti Claudin 5 | 1:50 (Ab15106) | 1:1000 (ab6785) | Claudin 5 is a protein that is expressed in the tight junctions of endothelial cells. |
| Anti ZO1 | 1:50 (Ab221547) | 1:1000 (Ab6717) | Zonula occludens-1 is a tight junction protein that plays a key role in the formation and maintenance of tight cellular junctions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santillán-Cortez, D.; Castell-Rodríguez, A.E.; González-Arenas, A.; Suárez-Cuenca, J.A.; Pérez-Koldenkova, V.; Añorve-Bailón, D.; Toledo-Lozano, C.G.; García, S.; Escamilla-Tilch, M.; Mondragón-Terán, P. A Versatile Microfluidic Device System that Lacks a Synthetic Extracellular Matrix Recapitulates the Blood–Brain Barrier and Dynamic Tumor Cell Interaction. Bioengineering 2024, 11, 1008. https://doi.org/10.3390/bioengineering11101008
Santillán-Cortez D, Castell-Rodríguez AE, González-Arenas A, Suárez-Cuenca JA, Pérez-Koldenkova V, Añorve-Bailón D, Toledo-Lozano CG, García S, Escamilla-Tilch M, Mondragón-Terán P. A Versatile Microfluidic Device System that Lacks a Synthetic Extracellular Matrix Recapitulates the Blood–Brain Barrier and Dynamic Tumor Cell Interaction. Bioengineering. 2024; 11(10):1008. https://doi.org/10.3390/bioengineering11101008
Chicago/Turabian StyleSantillán-Cortez, Daniel, Andrés Eliú Castell-Rodríguez, Aliesha González-Arenas, Juan Antonio Suárez-Cuenca, Vadim Pérez-Koldenkova, Denisse Añorve-Bailón, Christian Gabriel Toledo-Lozano, Silvia García, Mónica Escamilla-Tilch, and Paul Mondragón-Terán. 2024. "A Versatile Microfluidic Device System that Lacks a Synthetic Extracellular Matrix Recapitulates the Blood–Brain Barrier and Dynamic Tumor Cell Interaction" Bioengineering 11, no. 10: 1008. https://doi.org/10.3390/bioengineering11101008







