Investigation of the Effect of High Shear Stress on Mesenchymal Stem Cells Using a Rotational Rheometer in a Small-Angle Cone–Plate Configuration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concept
2.2. Carrier Fluid
2.3. Media Density
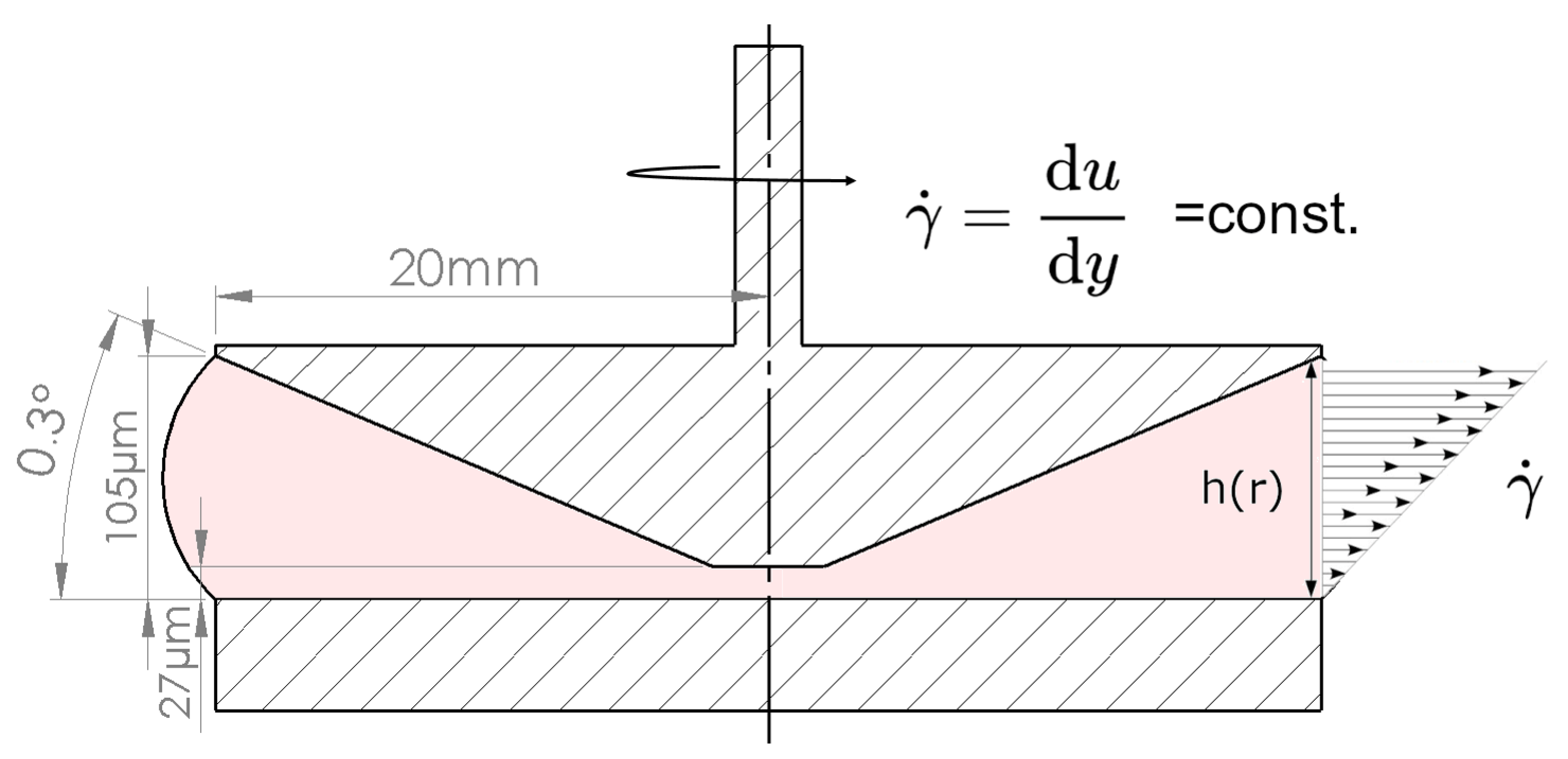
2.4. Rheometer Test Set-Up
2.5. Influence of Exposure Time
2.6. Influence of Shear Rate
2.7. Preparation of Cell Suspension
2.8. Fluid Shear Stress to AD-MSC
2.9. Evaluation of Further Cell Cultivation and Morphological Changes
2.10. Data and Statistics
2.11. Ethics
3. Results
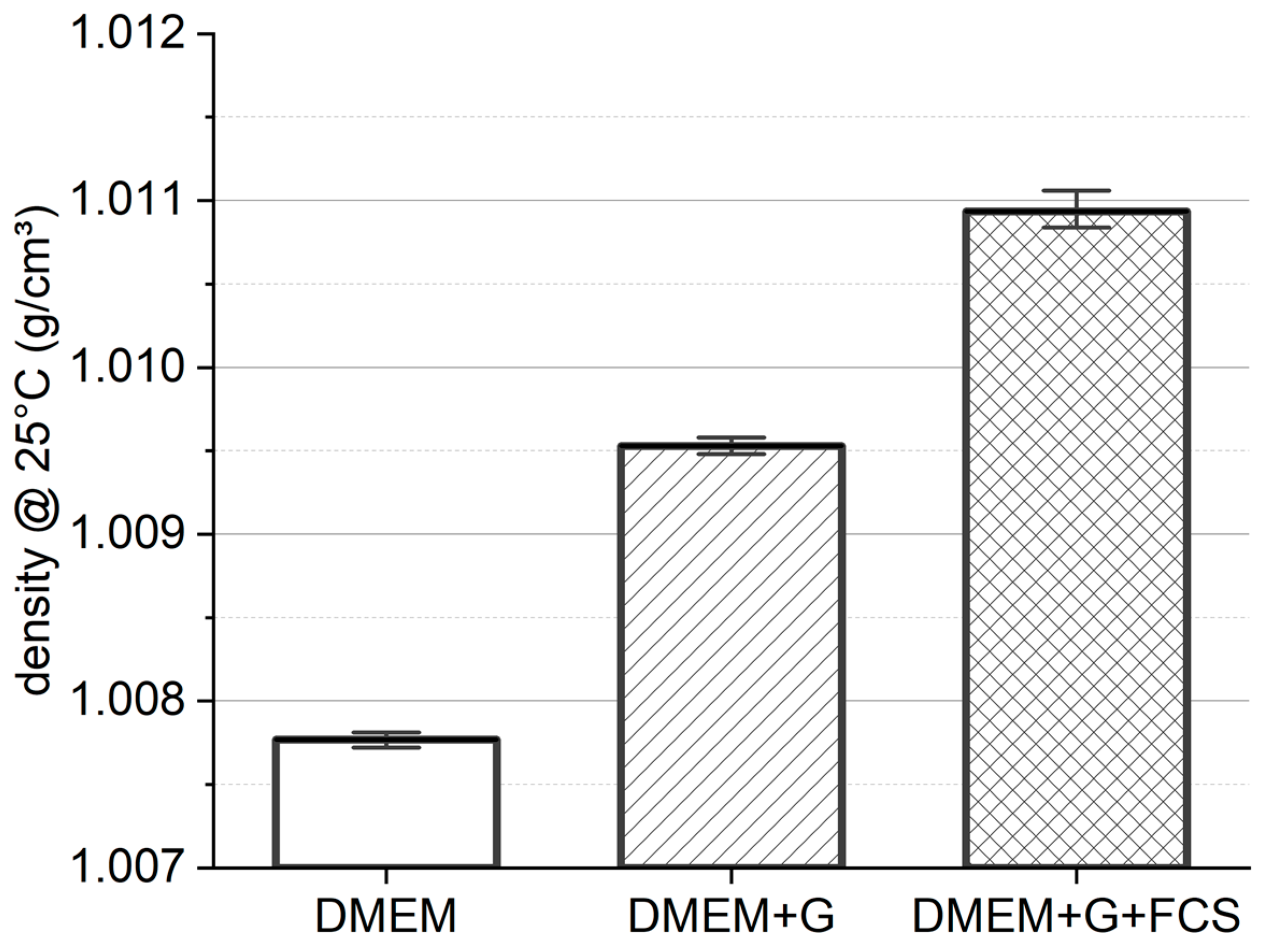
3.1. Media Density
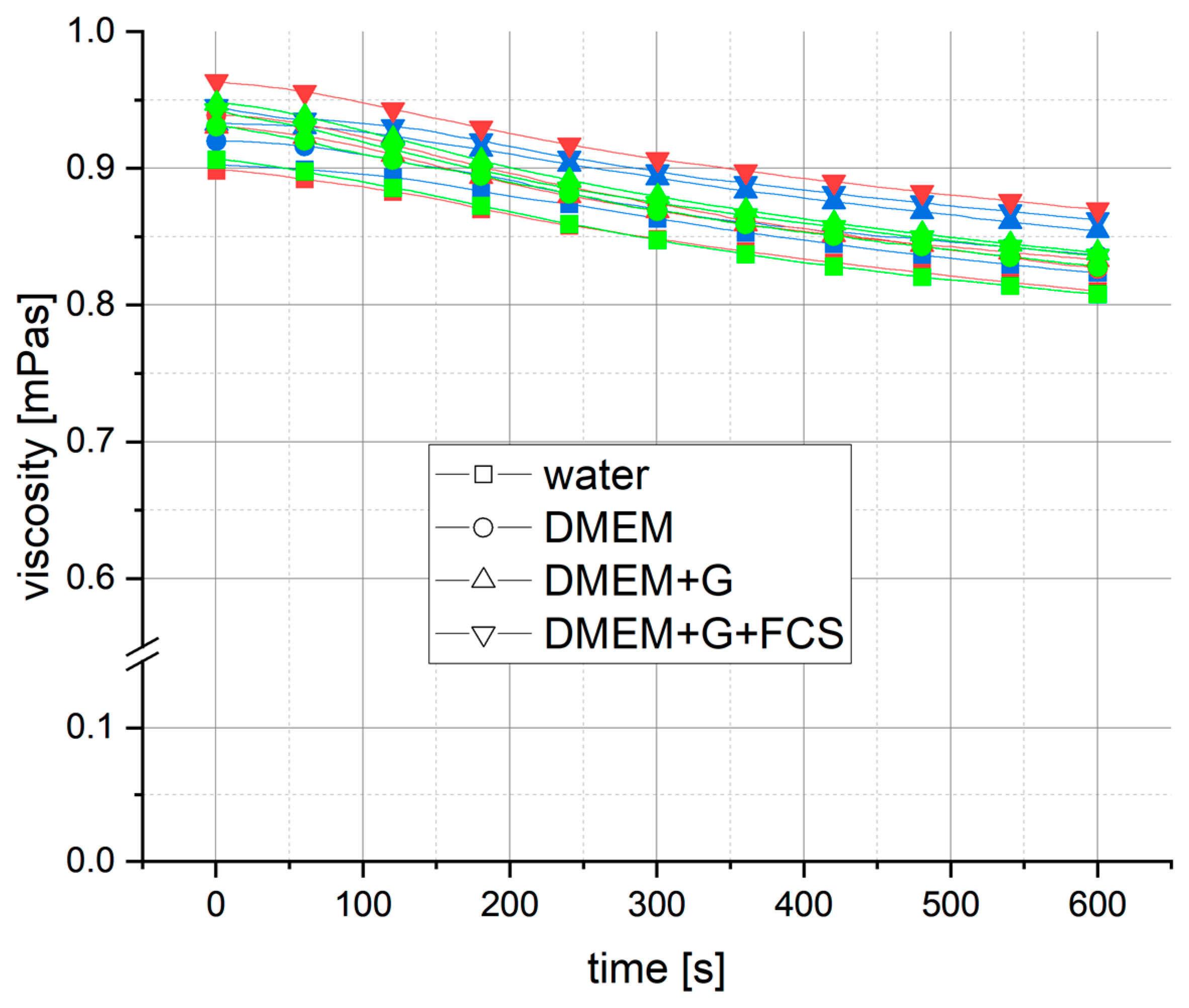
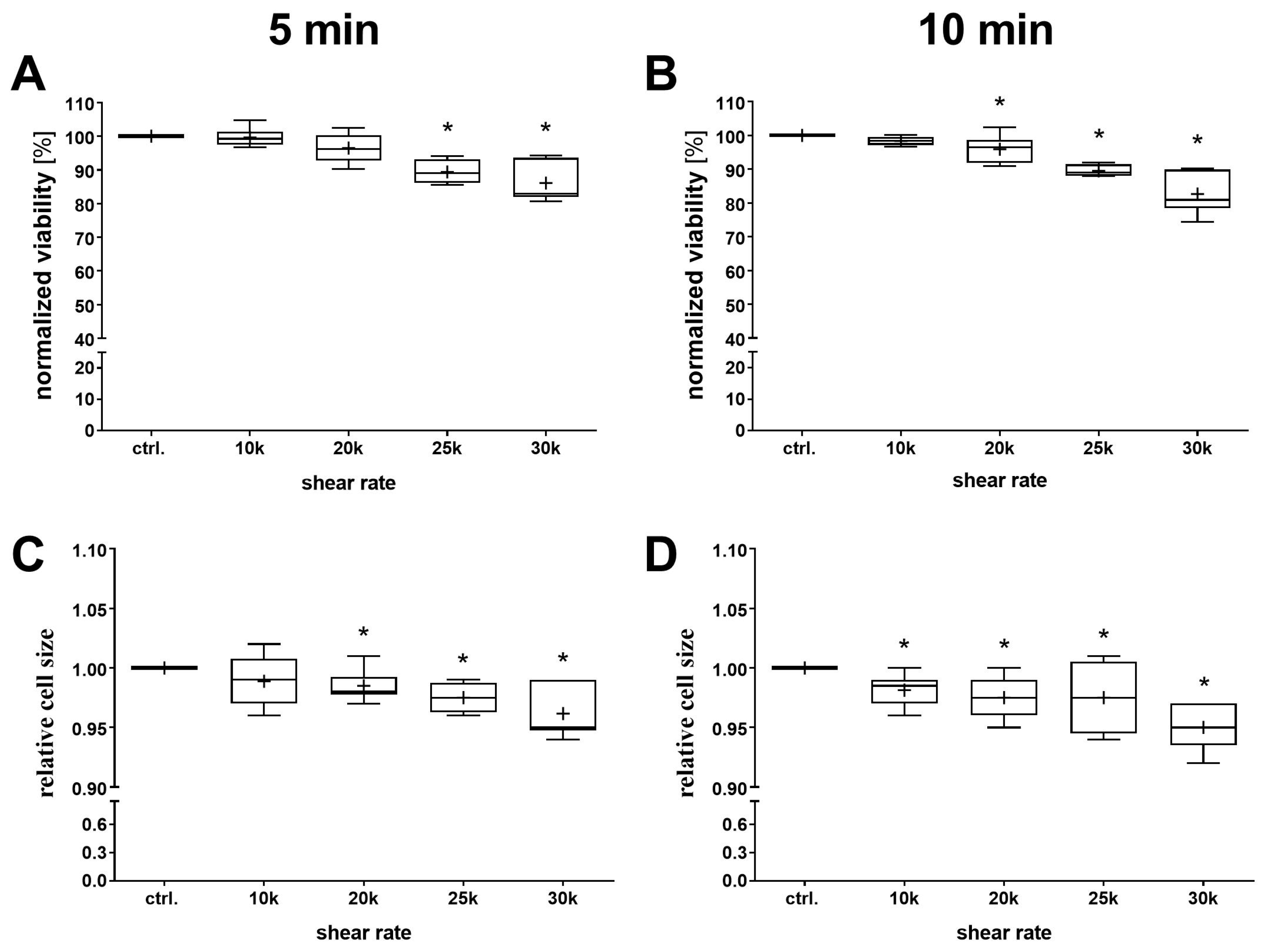
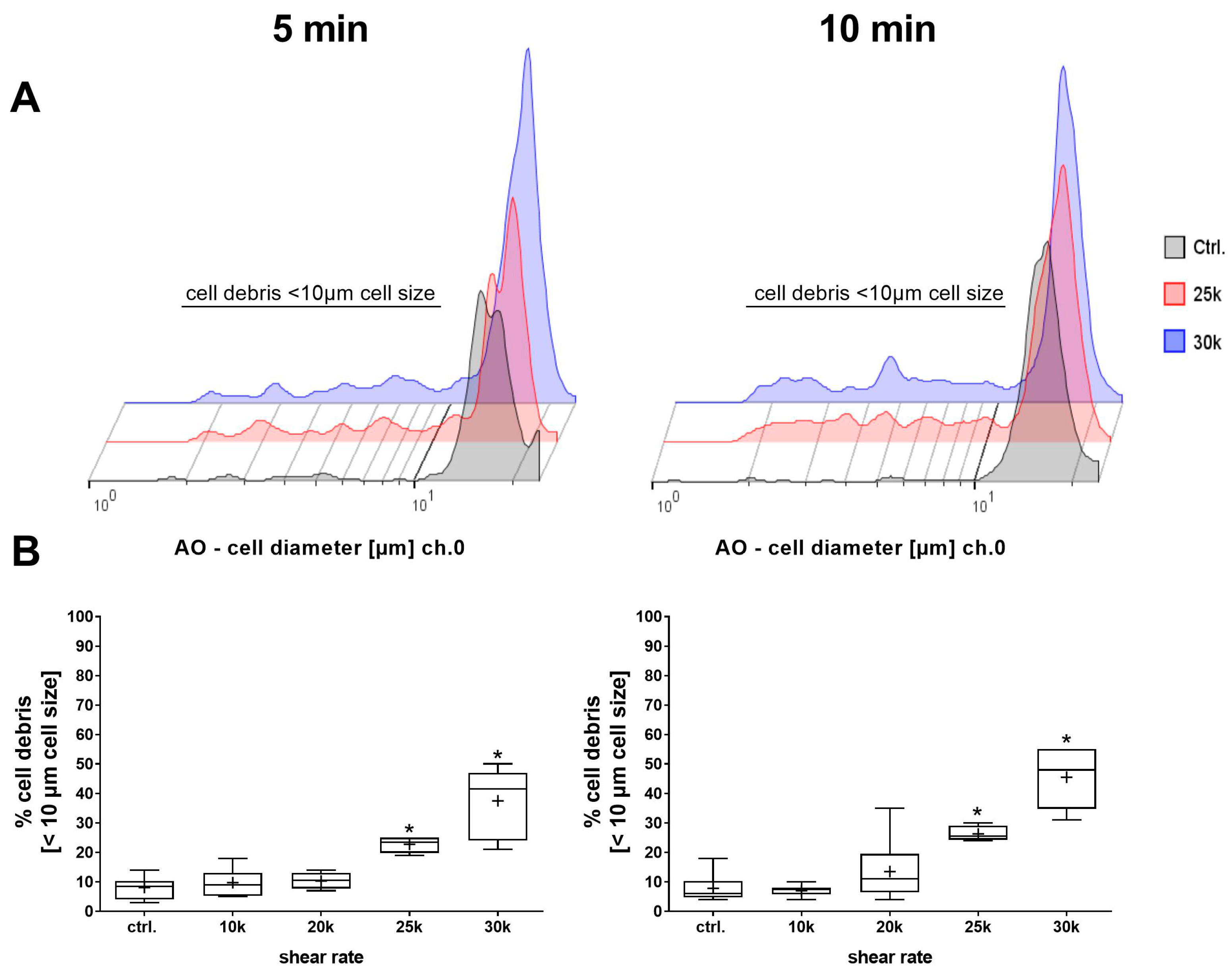
3.2. Influence of Shear Rate
3.3. Influence of Exposure Time
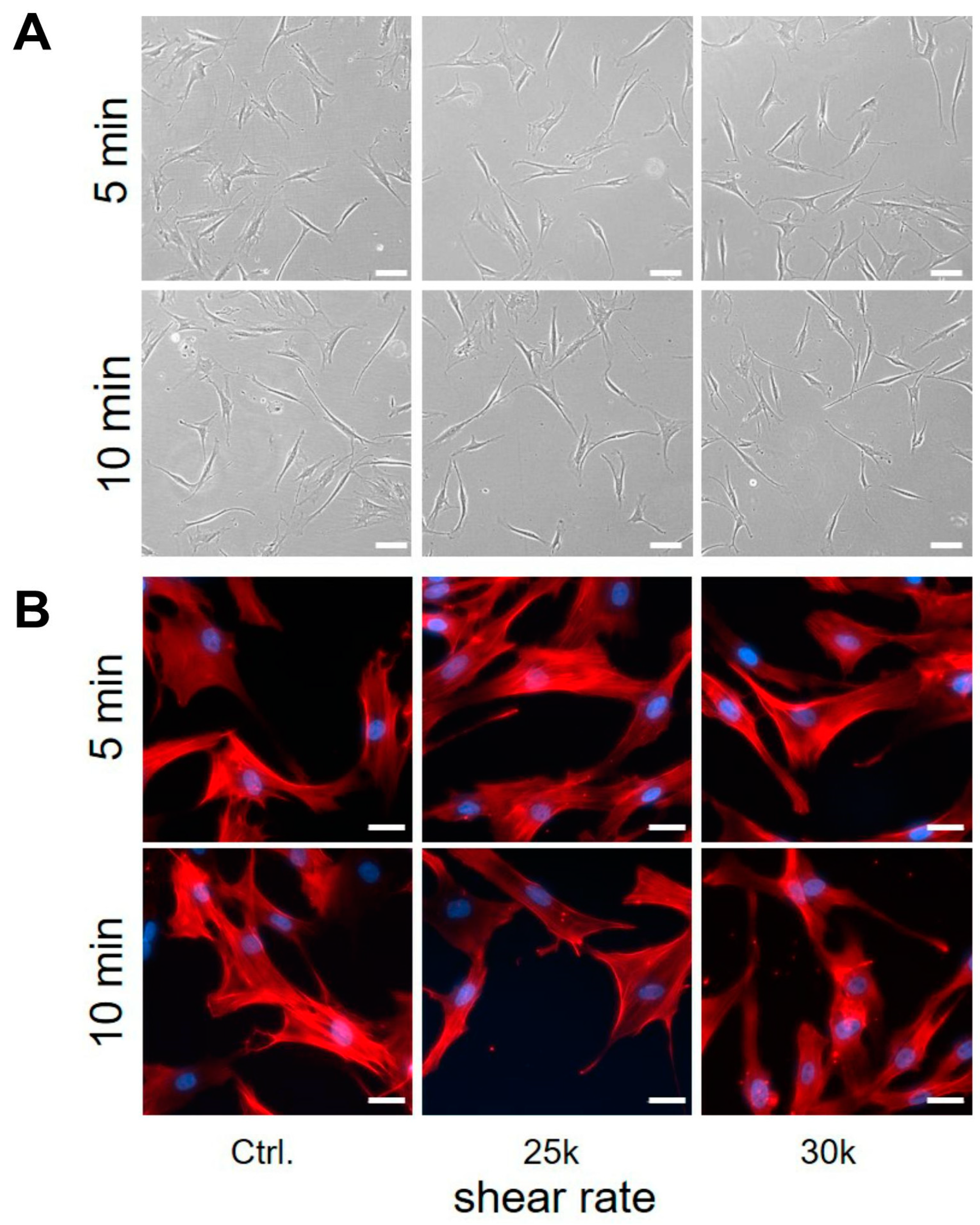
3.4. Shear Stress to Human AD-MSC
4. Discussion
4.1. Media Density
4.2. Influence of Shear Rate
4.3. Influence of Exposure Time
4.4. Shear Stress Application Method
4.5. Shear Response of AD-MSCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuk, P.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, W.; Katz, A.; Benhaim, P.; Lorenz, P.; Hedrick, M. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Ueberreiter, K.; Finckenstein, J.G.v.; Cromme, F.; Herold, C.; Tanzella, U.; Vogt, P.M. BEAULI™—eine neue Methode zur einfachen und zuverlässigen Fettzell-Transplantation. Handchir. Mikrochir. Plast. Chir. 2010, 42, 379–385. [Google Scholar] [CrossRef]
- Spiekman, M.; van Dongen, J.A.; Willemsen, J.C.; Hoppe, D.L.; van der Lei, B.; Harmsen, M.C. The power of fat and its adipose-derived stromal cells: Emerging concepts for fibrotic scar treatment. J. Tissue Eng. Regen. Med. 2017, 11, 3220–3235. [Google Scholar] [CrossRef] [PubMed]
- Stasch, T.; Hoehne, J.; Huynh, T.; Baerdemaeker, R.d.; Grandel, S.; Herold, C. Débridement and Autologous Lipotransfer for Chronic Ulceration of the Diabetic Foot and Lower Limb Improves Wound Healing. Plast. Reconstr. Surg. 2015, 136, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, J.A.; Facile, T.; Patrick, R.J.; Francis, K.R.; Milanovich, S.; Weimer, J.M.; Kota, D.J. Concise Review: Fat and Furious: Harnessing the Full Potential of Adipose-Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2017, 6, 1096–1108. [Google Scholar] [CrossRef]
- Paar, A. Influence of Rotational Mode on Rheometry of Low-Viscosity Fluids. Chem. Listy 2016, 110, 306–309. [Google Scholar]
- Madrigal, M.; Rao, K.; Riordan, N. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.-P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Craniomaxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef]
- Fang, B.; Song, Y.; Liao, L.; Zhang, Y.; Zhao, R.C. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant. Proc. 2007, 39, 3358–3362. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A. The Adipose-derived Stem Cell: Looking Back and Looking Ahead. Mol. Biol. Cell 2010, 21, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Kappos, E.A.; Engels, P.; Tremp, M.; Meyer zu Schwabedissen, M.; Summa, P.; Fischmann, A.; Felten, S.v.; Scherberich, A.; Schaefer, D.; Kalbermatten, D. Peripheral Nerve Repair: Multimodal Comparison of the Long-Term Regenerative Potential of Adipose Tissue-Derived Cells in a Biodegradable Conduit. Stem Cells Dev. 2014, 24, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Trottier, V.; Marceau-Fortier, G.; Germain, L.; Vincent, C.; Fradette, J. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008, 26, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Salamon, A.; Herzmann, N.; Adam, S.; Kleine, H.-D.; Matthiesen, I.; Ueberreiter, K.; Peters, K. Isolation and differentiation potential of human mesenchymal stem cells from adipose tissue harvested by water jet-assisted liposuction. Aesthet. Surg. J. 2015, 35, 1030–1039. [Google Scholar] [CrossRef]
- Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC; Publications Office of the European Union: Luxembourg, 2007.
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; Sena, G.d.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef]
- Borowski, D.W.; Gill, T.S.; Agarwal, A.K.; Tabaqchali, M.A.; Garg, D.K.; Bhaskar, P. Adipose Tissue-Derived Regenerative Cell-Enhanced Lipofilling for Treatment of Cryptoglandular Fistulae-in-Ano: The ALFA Technique. Surg. Innov. 2015, 22, 593–600. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef]
- Hyang, K. Tissue Grinder and Enzyme Reactor for Cell Culture. WO2022KR10371 20220715, 13 April 2023. [Google Scholar]
- Wurzer, C.; Oberbauer, E.; Redi, H. Non-Enzymatic Method and Milling Device. PCT/EP2017/059934, 2 November 2017. [Google Scholar]
- Aliaghaei, M.; Haun, J.B. Optimization of Mechanical Tissue Dissociation Using an Integrated Microfluidic Device for Improved Generation of Single Cells Following Digestion. Front. Bioeng. Biotechnol. 2022, 10, 841046. [Google Scholar] [CrossRef]
- Al-Mofty, S.; Elsayed, M.; Ali, H.; Ahmed, O.; Altayyeb, A.; Wahby, A.; Abdelgawad, M.; Mousa, N. A microfluidic platform for dissociating clinical scale tissue samples into single cells. Biomed. Microdevices 2021, 23, 10. [Google Scholar] [CrossRef]
- Scheuermann, S.; Lehmann, J.M.; Ramani Mohan, R.; Reißfelder, C.; Rückert, F.; Langejürgen, J.; Pallavi, P. TissueGrinder, a novel technology for rapid generation of patient-derived single cell suspensions from solid tumors by mechanical tissue dissociation. Front. Med. 2022, 9, 721639. [Google Scholar] [CrossRef] [PubMed]
- Horobin, J.T.; Sabapathy, S.; Simmonds, M.J. Red blood cell tolerance to shear stress above and below the subhemolytic threshold. Biomech. Model. Mechanobiol. 2020, 19, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Lommel, M.A.; Goubergrits, L.; Affeld, K.; Kertzscher, U. Couette shearing device for the investigation of shear-induced damage of the primary hemostasis by left ventricular assist devices. Int. J. Artif. Organs 2019, 42, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Taskin, M.E.; Fang, H.-B.; Pampori, A.; Jarvik, R.; Griffith, B.P.; Wu, Z.J. Study of Flow-Induced Hemolysis Using Novel Couette-Type Blood-Shearing Devices. Artif. Organs 2011, 35, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Velasco, V.; Soucy, P.; Keynton, R.; Williams, S.J. A microfluidic impedance platform for real-time, in vitro characterization of endothelial cells undergoing fluid shear stress. Lab. Chip 2022, 22, 4705–4716. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Denais, C.; Chan Celine, M.F.; Wang, Z.; Lammerding, J.; King, M.R. Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am. J. Physiol.-Cell Physiol. 2015, 309, C736–C746. [Google Scholar] [CrossRef]
- Del Giudice, F. A Review of Microfluidic Devices for Rheological Characterisation. Micromachines 2022, 13, 167. [Google Scholar] [CrossRef]
- Barnes, J.M.; Nauseef, J.T.; Henry, M.D. Resistance to Fluid Shear Stress Is a Conserved Biophysical Property of Malignant Cell. PLoS ONE 2012, 7, e50973. [Google Scholar] [CrossRef]
- Hope, J.M.; Bersi Matthew, R.; Dombroski, J.A.; Clinch, A.B.; Pereles, R.S.; Merryman, W.D.; King, M.R. Circulating prostate cancer cells have differential resistance to fluid shear stress-induced cell death. J. Cell Sci. 2021, 134, jcs251470. [Google Scholar] [CrossRef]
- Mitchell, M.J.; King, M.R. Fluid Shear Stress Sensitizes Cancer Cells to Receptor-Mediated Apoptosis via Trimeric Death Receptors. New J. Phys. 2013, 15, 015008. [Google Scholar] [CrossRef]
- Egan, K.; Cooke, N.; Kenny, D. Living in shear: Platelets protect cancer cells from shear induced damage. Clin. Exp. Metastasis 2014, 31, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Moose, D.L.; Krog, B.L.; Kim, T.-H.; Zhao, L.; Williams-Perez, S.; Burke, G.; Rhodes, L.; Vanneste, M.; Breheny, P.; Milhem, M.; et al. Cancer Cells Resist Mechanical Destruction in Circulation via RhoA/Actomyosin-Dependent Mechano-Adaptation. Cell Rep. 2020, 30, 3864–3874.e6. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Shanmugavelayudam, S.K.; Rubenstein, D.A. The effect of physiologically relevant dynamic shear stress on platelet and endothelial cell activation. Thromb. Res. 2011, 127, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Quach, M.E.; Syed, A.K.; Li, R. A Uniform Shear Assay for Human Platelet and Cell Surface Receptors via Cone-plate viscometry. J. Vis. Exp. 2019, 148, e59704. [Google Scholar] [CrossRef]
- Dreyer, L.; Krolitzkia, B.; Autschbach, R.; Vogt, P.; Welte, T.; Ngezahayo, A.; Glasmacher, B. An advanced cone-and-plate reactor for the in vitro-application of shear stress on adherent cells. Clin. Hemorheol. Microcirc. 2011, 49, 391–397. [Google Scholar] [CrossRef]
- Franzoni, M.; Cattaneo, I.; Ene-Iordache, B.; Oldani, A.; Righettini, P.; Remuzzi, A. Design of a cone-and-plate device for controlled realistic shear stress stimulation on endothelial cell monolayers. Cytotechnology 2016, 68, 1885–1896. [Google Scholar] [CrossRef]
- Ranucci, M.; Ranucci, M.; Baryshnikova, E. An ex-vivo model of shear-rate-based activation of blood coagulation. Blood Coagul. Fibrinolysis 2018, 29, 172–177. [Google Scholar] [CrossRef]
- Spurell, C.; Baker, A.B. Analysis of a high-throughput cone-and-plate apparatus for the application of defined spatiotemporal flow to cultured cells. Biotechnol. Bioeng. 2013, 110, 1782–1793. [Google Scholar] [CrossRef]
- Ye, C.; Ali, S.; Sun, Q.; Guo, M.; Liu, Y.; Gao, Y.; Huo, B. Novel cone-and-plate flow chamber with controlled distribution of wall fluid shear stress. Comput. Biol. Med. 2019, 106, 140–148. [Google Scholar] [CrossRef]
- Beris, A.N.; Horner, J.S.; Jariwala, S.; Armstrong, M.J.; Wagner, N.J. Recent advances in blood rheology: A review. Soft Matter 2021, 17, 10591–10613. [Google Scholar] [CrossRef]
- Dakhil, H.; Wierschem, A. Measuring the adhesion limit of fibronectin for fibroblasts with a narrow-gap rotational rheometer. Bioprocess Biosyst. Eng. 2018, 41, 353–358. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Lee, S.; Jung, D.H.; Basu, S.K.; Cho, M.-G.; Wierschem, A. Narrow-Gap Rheometry: A Novel Method for Measuring Cell Mechanics. Cells 2022, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.M.; Dombroski, J.A.; Pereles, R.S.; Lopez-Cavestany, M.; Greenlee, J.D.; Schwager, S.C.; Reinhart-King, C.A.; King, M.R. Fluid shear stress enhances T cell activation through Piezo1. BMC Biol. 2022, 20, 61. [Google Scholar] [CrossRef]
- Büyükurgancı, B.; Basu, S.K.; Neuner, M.; Guck, J.; Wierschem, A.; Reichel, F. Shear rheology of methyl cellulose based solutions for cell mechanical measurements at high shear rates. Soft Matter 2023, 19, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, J.; Fortuin, J. A criterion for purely tangential laminar flow in the cone and plate rheometer and the parallel plate rheometer. Chem. Eng. Sci. 1985, 40–41, 111–116. [Google Scholar] [CrossRef]
- Sdougos, H.P.; Bussolari, S.R.; Dewey, C.F. Secondary flow and turbulence in a cone-and-plate device. J. Fluid Mech. 1984, 138, 379–404. [Google Scholar] [CrossRef]
- Drobek, C.; Meyer, J.; Mau, R.; Wolff, A.; Peters, K.; Seitz, H. Volumetric mass density measurements of mesenchymal stem cells in suspension using a density meter. iScience 2023, 26, 105796. [Google Scholar] [CrossRef]
- Dombroski, J.A.; Rowland, S.J.; Fabiano, A.R.; Knoblauch, S.V.; Hope, J.M.; King, M.R. Fluid shear stress enhances dendritic cell activation. Immunobiology 2023, 228, 152744. [Google Scholar] [CrossRef]
- Hahn, O.; Ingwersen, L.-C.; Soliman, A.; Hamed, M.; Fuellen, G.; Wolfien, M.; Scheel, J.; Wolkenhauer, O.; Koczan, D.; Kamp, G.; et al. TGF-ß1 Induces Changes in the Energy Metabolism of White Adipose Tissue-Derived Human Adult Mesenchymal Stem/Stromal Cells In Vitro. Metabolites 2020, 10, 59. [Google Scholar] [CrossRef]
- Peters, K.; Salamon, A.; van Vlierberghe, S.; Rychly, J.; Kreutzer, M.; Neumann, H.-G.; Schacht, E.; Dubruel, P. A New Approach for Adipose Tissue Regeneration Based on Human Mesenchymal Stem Cells in Contact to Hydrogels—An In Vitro Study. Adv. Eng. Mater. 2009, 11, B155–B161. [Google Scholar] [CrossRef]
- Klemenz, A.-C.; Meyer, J.; Ekat, K.; Bartels, J.; Traxler, S.; Schubert, J.K.; Kamp, G.; Miekisch, W.; Peters, K. Differences in the Emission of Volatile Organic Compounds (VOCs) between Non-Differentiating and Adipogenically Differentiating Mesenchymal Stromal/Stem Cells from Human Adipose Tissue. Cells 2019, 8, 697. [Google Scholar] [CrossRef]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J.G. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010, 7, 36. [Google Scholar] [CrossRef]
- Poon, C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. J. Mech. Behav. Biomed. Mater. 2022, 126, 105024. [Google Scholar] [CrossRef]
- Moino, C.; Artusio, F.; Pisano, R. Shear stress as a driver of degradation for protein-based therapeutics: More accomplice than culprit. Int. J. Pharm. 2024, 650, 123679. [Google Scholar] [CrossRef] [PubMed]
- RheoSense. Application Note: Viscosity of DMEM Cell Culture Media. Available online: https://www.rheosense.com/dmem-cell-culture-media (accessed on 20 April 2024).
- Pipe, C.J.; Majmudar, T.S.; McKinley, G.H. High shear rate viscometry. Rheol. Acta 2008, 47, 621–642. [Google Scholar] [CrossRef]
- Raposio, E.; Caruana, G.; Bonomini, S.; Libondi, G. A novel and effective strategy for the isolation of adipose-derived stem cells: Minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast. Reconstr. Surg. 2014, 133, 1406–1409. [Google Scholar] [CrossRef]
- Dong, J.; Gu, Y.; Li, C.; Wang, C.; Feng, Z.; Qiu, R.; Chen, B.; Li, J.; Zhang, S.; Wang, Z.; et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol. Sin. 2009, 30, 530–536. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Chang, T.-H.; Hsu, W.-T.; Zhou, J.; Lee, H.-H.; Hui-Chun Ho, J.; Chien, S.; Lee, O.K.-S. Oscillatory shear stress mediates directional reorganization of actin cytoskeleton and alters differentiation propensity of mesenchymal stem cells. Stem Cells 2015, 33, 429–442. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Liu, Y.-A.; Huang, C.-J.; Yen, M.-H.; Tseng, C.-T.; Chien, S.; Lee, O.K. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci. Rep. 2015, 5, 16522. [Google Scholar] [CrossRef]
- Sonam, S.; Sathe, S.R.; Yim, E.K.F.; Sheetz, M.P.; Lim, C.T. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci. Rep. 2016, 6, 20415. [Google Scholar] [CrossRef]
- Arora, S.; Srinivasan, A.; Leung, C.M.; Toh, Y.-C. Bio-mimicking Shear Stress Environments for Enhancing Mesenchymal Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2020, 15, 414–427. [Google Scholar] [CrossRef]
- Becquart, P.; Cruel, M.; Hoc, T.; Sudre, L.; Pernelle, K.; Bizios, R.; Logeart-Avramoglou, D.; Petite, H.; Bensidhoum, M. Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur. Cells Mater. 2016, 31, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.J.; Coughlin, T.R.; Varsanik, M.A.; Niebur, G.L. Shear Stress in Bone Marrow has a Dose Dependent Effect on cFos Gene Expression in In Situ Culture. Cell. Mol. Bioeng. 2019, 12, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Jossen, V.; Pörtner, R.; Kaiser, S.C.; Kraume, M.; Eibl, D.; Eibl, R. Mass Production of Mesenchymal Stem Cells—Impact of Bioreactor Design and Flow Conditions on Proliferation and Differentiation. In Cells and Biomaterials in Regenerative Medicine; InTech: London, UK, 2014. [Google Scholar] [CrossRef]

| Fluid | Mean Viscosity [mPas] | Max SD [± %] | Corrected Viscosity [mPas] |
|---|---|---|---|
| Water | 0.8905 | 0.60 | 0.8921 |
| DMEM | 0.9105 | 0.56 | 0.9125 |
| DMEM+G | 0.9111 | 0.57 | 0.9121 |
| DMEM+G+FCS | 0.9572 | 1.85 | 0.9642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mand, M.; Hahn, O.; Meyer, J.; Peters, K.; Seitz, H. Investigation of the Effect of High Shear Stress on Mesenchymal Stem Cells Using a Rotational Rheometer in a Small-Angle Cone–Plate Configuration. Bioengineering 2024, 11, 1011. https://doi.org/10.3390/bioengineering11101011
Mand M, Hahn O, Meyer J, Peters K, Seitz H. Investigation of the Effect of High Shear Stress on Mesenchymal Stem Cells Using a Rotational Rheometer in a Small-Angle Cone–Plate Configuration. Bioengineering. 2024; 11(10):1011. https://doi.org/10.3390/bioengineering11101011
Chicago/Turabian StyleMand, Mario, Olga Hahn, Juliane Meyer, Kirsten Peters, and Hermann Seitz. 2024. "Investigation of the Effect of High Shear Stress on Mesenchymal Stem Cells Using a Rotational Rheometer in a Small-Angle Cone–Plate Configuration" Bioengineering 11, no. 10: 1011. https://doi.org/10.3390/bioengineering11101011











