Targeted Drug Delivery in Periorbital Non-Melanocytic Skin Malignancies
Abstract
:1. Background
2. Methodology
3. Classification
4. Mechanisms of Targeted Drug Delivery
5. Monoclonal Antibodies
5.1. Mechanisms of Action
5.2. Applications in Periorbital Skin Malignancies
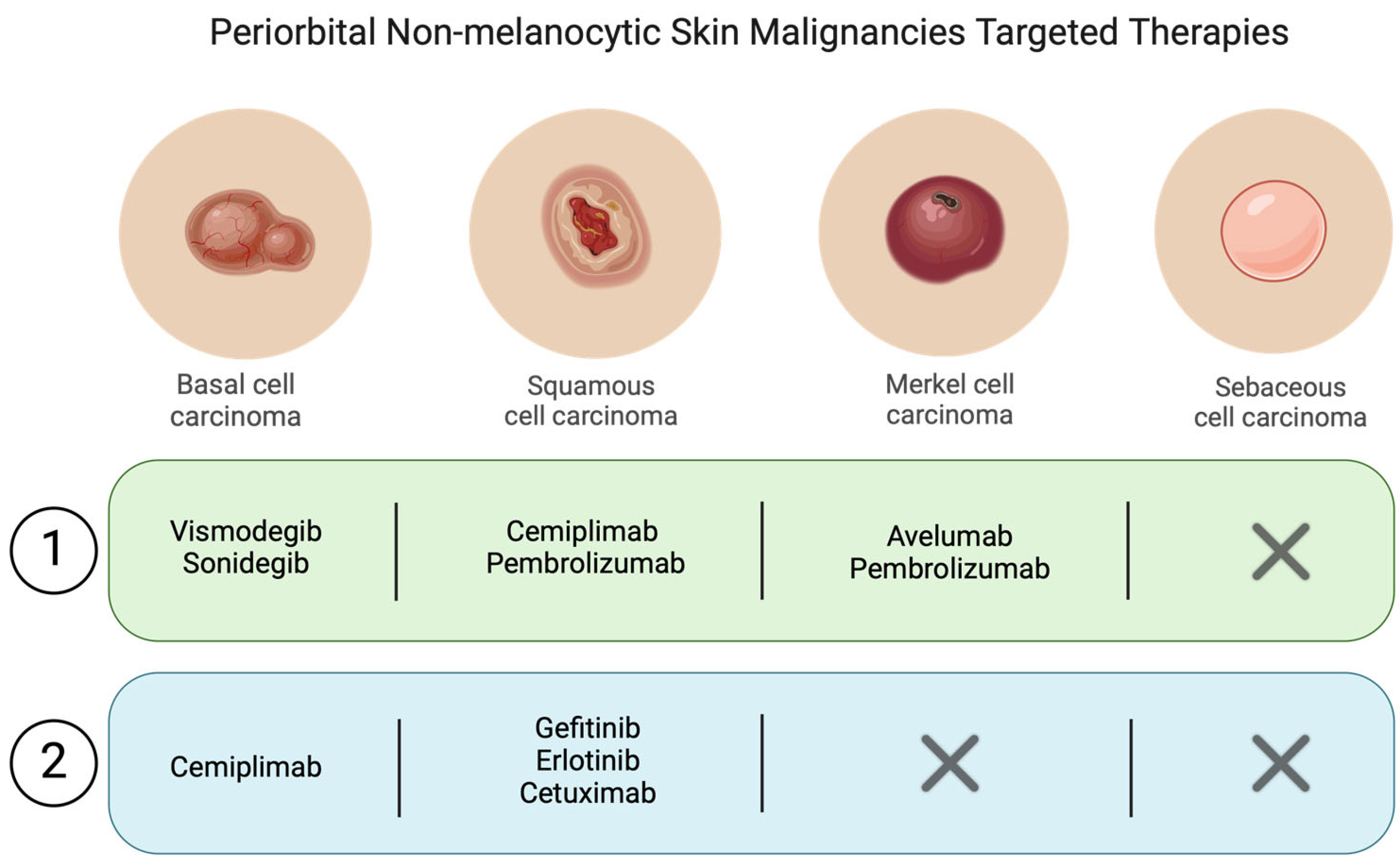
Squamous Cell Carcinoma
6. Immunotherapy with Checkpoint Inhibitors
6.1. Mechanisms of Action
6.2. Applications in Skin Malignancies
6.2.1. Squamous Cell Carcinoma
6.2.2. Merkel Cell Carcinoma
6.2.3. Basal Cell Carcinoma
7. Small Molecule Inhibitors
7.1. Mechanisms of Action
7.2. Applications in Periorbital Skin Malignancies
7.2.1. Basal Cell Carcinoma
7.2.2. Squamous Cell Carcinoma
8. Other Targeted Therapies
8.1. Gene Therapy
8.2. Nanoparticle-Based Delivery Systems
9. Side Effects of Targeted Therapies
9.1. Ocular and Periocular Side Effects
9.2. Management of Side Effects
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Wahida, A.; Buschhorn, L.; Fröhling, S.; Jost, P.J.; Schneeweiss, A.; Lichter, P.; Kurzrock, R. The coming decade in precision oncology: Six riddles. Nat. Rev. Cancer 2023, 23, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Ribeiro, A.B.; Scorilas, A.; Gonçalves, A.C.; Efferth, T.; Trougakos, I.P. The emergence of drug resistance to targeted cancer therapies: Clinical evidence. Drug Resist. Updates 2019, 47, 100646. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Sandhu, N.K.; Chawla, V.; Chawla, P.A. Targeted Drug Delivery: Trends and Perspectives. Curr. Drug Deliv. 2021, 18, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Bayer, V. An Overview of Monoclonal Antibodies. Semin. Oncol. Nurs. 2019, 35, 150927. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.-C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody–drug conjugates for cancer therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Thakur, A.; Huang, M.; Lum, L.G. Bispecific antibody based therapeutics: Strengths and challenges. Blood Rev. 2018, 32, 339–347. [Google Scholar] [CrossRef]
- Delgado, M.; Garcia-Sanz, J.A. Therapeutic Monoclonal Antibodies against Cancer: Present and Future. Cells 2023, 12, 2837. [Google Scholar] [CrossRef]
- Stasiłojć, G.; Österborg, A.; Blom, A.M.; Okrój, M. New perspectives on complement mediated immunotherapy. Cancer Treat. Rev. 2016, 45, 68–75. [Google Scholar] [CrossRef]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 2018, 10, 693–711. [Google Scholar] [CrossRef]
- Chin, D.S.; Lim, C.S.Y.; Nordin, F.; Arifin, N.; Jun, T.G. Antibody-Dependent Cell-Mediated Cytotoxicity Through Natural Killer (NK) Cells: Unlocking NK Cells for Future Immunotherapy. Curr. Pharm. Biotechnol. 2021, 23, 552–578. [Google Scholar] [CrossRef]
- Cao, X.; Chen, J.; Li, B.; Dang, J.; Zhang, W.; Zhong, X.; Wang, C.; Raoof, M.; Sun, Z.; Yu, J.; et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy. Sci. Adv. 2022, 8, eabl9171. [Google Scholar] [CrossRef]
- Pourakbari, R.; Hajizadeh, F.; Parhizkar, F.; Aghebati-Maleki, A.; Mansouri, S.; Aghebati-Maleki, L. Co-Stimulatory Agonists: An Insight into the Immunotherapy of Cancer. EXCLI J. 2021, 20, 1055–1085. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Huang, J.; Xing, Y.; Dai, H.; Zhu, L.; Li, S.; Feng, J.; Zhou, B.; Li, J.; et al. CD16+ fibroblasts foster a trastuzumab-refractory microenvironment that is reversed by VAV2 inhibition. Cancer Cell 2022, 40, 1341–1357.e13. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Yin, V.T. Targeting EGFR and sonic hedgehog pathways for locally advanced eyelid and periocular carcinomas. World J. Clin. Cases 2014, 2, 432–438. [Google Scholar] [CrossRef]
- Lee, N.G.; Kim, L.A.; Freitag, S.K. The Role of Genetics in the Pathogenesis of Periocular Cutaneous Neoplasms: Implications for Targeted Therapy. Semin. Ophthalmol. 2013, 28, 267–274. [Google Scholar] [CrossRef]
- Uribe, P.; Gonzalez, S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: Molecular bases for EGFR-targeted therapy. Pathol. Res. Pract. 2011, 207, 337–342. [Google Scholar] [CrossRef]
- Maubec, E.; Petrow, P.; Scheer-Senyarich, I.; Duvillard, P.; Lacroix, L.; Gelly, J.; Certain, A.; Duval, X.; Crickx, B.; Buffard, V.; et al. Phase II Study of Cetuximab as First-Line Single-Drug Therapy in Patients with Unresectable Squamous Cell Carcinoma of the Skin. J. Clin. Oncol. 2011, 29, 3419–3426. [Google Scholar] [CrossRef]
- El-Sawy, T.; Sabichi, A.L.; Myers, J.N.; Kies, M.S.; William, W.N.; Glisson, B.S.; Lippman, S.; Esmaeli, B. Epidermal Growth Factor Receptor Inhibitors for Treatment of Orbital Squamous Cell Carcinoma. Arch. Ophthalmol. 2012, 130, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Hitt, R.; Irigoyen, A.; Cortes-Funes, H.; Grau, J.J.; García-Sáenz, J.A.; Cruz-Hernandez, J.J. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann. Oncol. 2012, 23, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- de Mello, R.A.; Gerós, S.; Alves, M.P.; Moreira, F.; Avezedo, I.; Dinis, J. Cetuximab Plus Platinum-Based Chemotherapy in Head and Neck Squamous Cell Carcinoma: A Retrospective Study in a Single Comprehensive European Cancer Institution. PLoS ONE 2014, 9, e86697. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, E. Results of radiotherapy plus Cetuximab for squamous cell carcinoma of the head and neck. Jpn. J. Clin. Radiol. 2015, 60, 1681–1687. [Google Scholar]
- Tahara, M.; Kiyota, N.; Yokota, T.; Hasegawa, Y.; Muro, K.; Takahashi, S.; Onoe, T.; Homma, A.; Taguchi, J.; Suzuki, M.; et al. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann. Oncol. 2018, 29, 1004–1009. [Google Scholar] [CrossRef]
- Wollina, U.; Tchernev, G.; Lotti, T. Chimeric Monoclonal Antibody Cetuximab Targeting Epidermal Growth Factor-Receptor in Advanced Non-Melanoma Skin Cancer. Open Access Maced. J. Med. Sci. 2018, 6, 152–155. [Google Scholar] [CrossRef]
- Howell, J.Y.; Ramsey, M.L. Squamous Cell Skin Cancer. In StatPearls [lntermet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/28722968/ (accessed on 1 September 2024).
- Jiang, R.; Fritz, M.; Que, S.K.T. Cutaneous Squamous Cell Carcinoma: An Updated Review. Cancers 2024, 16, 1800. [Google Scholar] [CrossRef]
- Ju, S.; Rokohl, A.C.; Guo, Y.; Yao, K.; Fan, W.; Heindl, L.M. Personalized treatment concepts in extraocular cancer. Adv. Ophthalmol. Pract. Res. 2024, 4, 69–77. [Google Scholar] [CrossRef]
- Trotier, D.C.; Huang, L.; van Landingham, S.W.; Burr, A.R.; Ma, V.T. Review of recent advances in managing periocular skin malignancies. Front. Oncol. 2024, 14, 1275930. [Google Scholar] [CrossRef]
- Patel, B.; Saba, N.F. Current Aspects and Future Considerations of EGFR Inhibition in Locally Advanced and Recurrent Metastatic Squamous Cell Carcinoma of the Head and Neck. Cancers 2021, 13, 3545. [Google Scholar] [CrossRef]
- Allen, R.C. Molecularly targeted agents in oculoplastic surgery. Curr. Opin. Ophthalmol. 2017, 28, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Foote, M.C.; McGrath, M.; Guminski, A.; Hughes, B.G.M.; Meakin, J.; Thomson, D.; Zarate, D.; Simpson, F.; Porceddu, S.V. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann. Oncol. 2014, 25, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Lin, X.; Lu, X.; Luo, G.; Xiang, H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020, 186, 111876. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Wieland, A.; Nasti, T.; Yang, S.; Zhang, R.; Barber, D.L.; Konieczny, B.T.; Daugherty, C.Z.; Koenig, L.; Yu, K.; et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355, 1423–1427. [Google Scholar] [CrossRef]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.; Osewold, M.; Presser, D.; Meiwes, A.; Garbe, C.; Leiter, U. Advanced cutaneous squamous cell carcinoma: Real world data of patient profiles and treatment patterns. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.F.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Hughes, B.; Munoz-Couselo, E.; Mortier, L.; Bratland, Å.; Gutzmer, R.; Roshdy, O.; Mendoza, R.G.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase II trial. Ann. Oncol. 2021, 32, 1276–1285. [Google Scholar] [CrossRef]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 387, 1557–1568. [Google Scholar] [CrossRef]
- Goldfarb, J.A.; Ferrarotto, R.; Gross, N.; Goepfert, R.; Debnam, J.M.; Gunn, B.; Nagarajan, P.; Esmaeli, B. Immune checkpoint inhibitors for treatment of periorbital squamous cell carcinoma. Br. J. Ophthalmol. 2023, 107, 320–323. [Google Scholar] [CrossRef]
- Steren, B.B.; Burtness, B.; Bhatia, A.M.; Demirci, H.; Shinder, R.; Yoo, D.; Tse, B.; Pointdujour-Lim, R. Cemiplimab for Orbital Squamous Cell Carcinoma in 11 Cases. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 496–502. [Google Scholar] [CrossRef]
- Tiosano, A.; Ben-Ishai, M.; Cnaany, Y.; Markel, G.; Kurman, N.; Popovtzer, A.; Bar Sela, G.; Ben Simon, G.; Gershoni, A.; Yassur, I. Primary cemiplimab treatment for orbital squamous cell carcinoma is effective and may alleviate the need for orbital exenteration. Eye 2023, 37, 2482–2487. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef]
- Hernandez, L.E.; Mohsin, N.; Yaghi, M.; Frech, F.S.; Dreyfuss, I.; Nouri, K. Merkel cell carcinoma: An updated review of pathogenesis, diagnosis, and treatment options. Dermatol. Ther. 2022, 35, e15292. [Google Scholar] [CrossRef] [PubMed]
- Thakker, S.; Venna, S.; Belzberg, M.; Jang, S.; DeSimone, J.; Al-Mondhiry, J. Merkel cell carcinoma. J. Am. Acad. Dermatol. 2024, 91, 598–605. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Lebbé, C.; Mortier, L.; Brohl, A.S.; Fazio, N.; Grob, J.-J.; Prinzi, N.; Hanna, G.J.; Hassel, J.C.; Kiecker, F.; et al. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): Primary and biomarker analyses of a phase II study. J. Immunother. Cancer 2021, 9, e002646. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbé, C.; Lewis, K.D.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer 2020, 8, e000674. [Google Scholar] [CrossRef]
- D’Angelo, S.; Bhatia, S.; Brohl, A.; Hamid, O.; Mehnert, J.; Terheyden, P.; Shih, K.; Brownell, I.; Lebbé, C.; Lewis, K.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma (JAVELIN Merkel 200): Updated overall survival data after >5 years of follow-up. ESMO Open 2021, 6, 100290. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J. Immunother. Cancer 2021, 9, e002478. [Google Scholar] [CrossRef]
- Vinohrady, K.; Tumori, I.; Etico, C.; Etico, C.; Devices, M.; Review, W.I. Abstracts 545. MCC 2021, 9, 574–575. [Google Scholar]
- Bhatia, S.; Topalian, S.L.; Sharfman, W.H.; Meyer, T.; Lao, C.D.; Fariñas-Madrid, L.; Devriese, L.A.; Aljumaily, R.; Ferris, R.L.; Honma, Y.; et al. Non-comparative, open-label, international, multicenter phase I/II study of nivolumab (NIVO) ± ipilimumab (IPI) in patients (pts) with recurrent/metastatic merkel cell carcinoma (MCC) (CheckMate 358). J. Clin. Oncol. 2023, 41 (Suppl. S47), 9506. [Google Scholar] [CrossRef]
- Topalian, S.L.; Bhatia, S.; Amin, A.; Kudchadkar, R.R.; Sharfman, W.H.; Lebbé, C.; Delord, J.-P.; Dunn, L.A.; Shinohara, M.M.; Kulikauskas, R.; et al. Neoadjuvant Nivolumab for Patients with Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020, 38, 2476–2487. [Google Scholar] [CrossRef]
- Averbuch, I.; Stoff, R.; Miodovnik, M.; Fennig, S.; Bar-Sela, G.; Yakobson, A.; Daliot, J.; Asher, N.; Fenig, E. Avelumab for the treatment of locally advanced or metastatic Merkel cell carcinoma—A multicenter real-world experience in Israel. Cancer Med. 2023, 12, 12065–12070. [Google Scholar] [CrossRef]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology: Basal Cell Skin Cancer; NCCN Evid Blocks: Plymouth Meeting, PA, USA, 2019; Volume 1, pp. 1–10. [Google Scholar]
- Moujaess, E.; Merhy, R.; Kattan, J.; Sarkis, A.-S.; Tomb, R. Immune Checkpoint Inhibitors for Advanced or Metastatic Basal Cell Carcinoma: How much Evidence do we Need? Immunotherapy 2021, 13, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2023 update. Pharmacol. Res. 2023, 187, 106552. [Google Scholar] [CrossRef] [PubMed]
- Fricker, L.D. Proteasome Inhibitor Drugs. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Profiling, B.P. Small—Molecule Inhibitors of PARPs: From Tools for Investigating ADP—Ribosylation to Therapeutics. Act. Based Protein Profiling 2018, 420, 211–231. [Google Scholar]
- Montagna, E.; Lopes, O.S. Molecular basis of basal cell carcinoma. An. Bras. Dermatol. 2017, 92, 517–520. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Basset-Seguin, N.; Garbe, C.; Gesierich, A.; Lao, C.D.; Miller, C.; Mortier, L.; Murrell, D.F.; Hamid, O.; et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017, 17, 332. [Google Scholar] [CrossRef]
- Basset-Séguin, N.; Hauschild, A.; Kunstfeld, R.; Grob, J.; Dréno, B.; Mortier, L.; Ascierto, P.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer 2017, 86, 334–348. [Google Scholar] [CrossRef]
- Bertrand, N.; Guerreschi, P.; Basset-Seguin, N.; Saiag, P.; Dupuy, A.; Dalac-Rat, S.; Dziwniel, V.; Depoortère, C.; Duhamel, A.; Mortier, L. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: First results of a multicenter, open-label, phase 2 trial (VISMONEO study): Neoadjuvant Vismodegib in Locally Advanced Basal Cell Carcinoma. eClinicalMedicine 2021, 35, 100844. [Google Scholar] [CrossRef]
- Kahana, A.; Unsworth, S.P.; Andrews, C.A.; Chan, M.P.; Bresler, S.C.; Bichakjian, C.K.; Durham, A.B.; Demirci, H.; Elner, V.M.; Nelson, C.C.; et al. Vismodegib for Preservation of Visual Function in Patients with Advanced Periocular Basal Cell Carcinoma: The VISORB Trial. Oncololgy 2021, 26, e1240–e1249. [Google Scholar] [CrossRef]
- Demirci, H.; Worden, F.; Nelson, C.C.; Elner, V.M.M.; Kahana, A.M. Efficacy of Vismodegib (Erivedge) for Basal Cell Carcinoma Involving the Orbit and Periocular Area. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 463–466. [Google Scholar] [CrossRef]
- Gill, H.S.; Moscato, E.E.; Chang, A.L.S.; Soon, S.; Silkiss, R.Z. Vismodegib for Periocular and Orbital Basal Cell Carcinoma. JAMA Ophthalmol. 2013, 131, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, O.K.; Yin, V.; Chou, E.; Ball, S.; Kies, M.; William, W.N.; Migden, M.; Thuro, B.A.; Esmaeli, B. Hedgehog Pathway Inhibition for Locally Advanced Periocular Basal Cell Carcinoma and Basal Cell Nevus Syndrome. Am. J. Ophthalmol. 2015, 160, 220–227.e2. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, S.P.; Tingle, C.F.; Heisel, C.J.; Eton, E.A.; Andrews, C.A.; Chan, M.P.; Bresler, S.C.; Kahana, A. Analysis of residual disease in periocular basal cell carcinoma following hedgehog pathway inhibition: Follow up to the VISORB trial. PLoS ONE 2022, 17, e0265212. [Google Scholar] [CrossRef] [PubMed]
- Gershoni, A.; Tiosano, A.; Ben Ishai, M.; Barayev, E.; Ben Simon, G.J.; Yassur, I. Vismodegib improves quality of life in patients with periocular locally advanced basal cell carcinoma: Subgroup analysis, STEVIE trial. Eye 2022, 36, 407–413. [Google Scholar] [CrossRef]
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.; Lewis, K.; Chang, A.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Dermatol. 2020, 182, 1369–1378. [Google Scholar] [CrossRef]
- Lewis, C.M.; Glisson, B.S.; Feng, L.; Wan, F.; Tang, X.; Wistuba, I.I.; El-Naggar, A.K.; Rosenthal, D.I.; Chambers, M.S.; Lustig, R.A.; et al. A Phase II Study of Gefitinib for Aggressive Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2012, 18, 1435–1446. [Google Scholar] [CrossRef]
- Engelhardt, C.; Curiel-Lewandrowski, C.; Warneke, J.; Cranmer, L. Metastatic cutaneous squamous cell carcinoma responding to erlotinib therapy. J. Am. Acad. Dermatol. 2011, 65, 237–238. [Google Scholar] [CrossRef]
- Bulaklak, K.; Gersbach, C.A. The once and future gene therapy. Nat. Commun. 2020, 11, 5820. [Google Scholar] [CrossRef]
- Cesur-Ergün, B.; Demir-Dora, D. Gene therapy in cancer. J. Gene Med. 2023, 25, e3550. [Google Scholar] [CrossRef]
- LE, B.T.; Raguraman, P.R.; Kosbar, T.R.; Fletcher, S.; Wilton, S.D.; Veedu, R.N. Antisense Oligonucleotides Targeting Angiogenic Factors as Potential Cancer Therapeutics. Mol. Ther. Nucleic Acids 2019, 14, 142–157. [Google Scholar] [CrossRef]
- Patil, S.D.; Rhodes, D.G.; Burgess, D.J. DNA-based therapeutics and DNA delivery systems: A comprehensive review. AAPS J. 2005, 7, E61–E77. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable Manufacturing of CAR T Cells for Cancer Immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef]
- Jommi, C.; Bramanti, S.; Pani, M.; Ghirardini, A.; Santoro, A. CAR T-Cell Therapies in Italy: Patient Access Barriers and Recommendations for Health System Solutions. Front. Pharmacol. 2022, 13, 915342. [Google Scholar] [CrossRef]
- Fallah, A.; Heidari, H.R.; Bradaran, B.; Sisakht, M.M.; Zeinali, S.; Molavi, O. A gene-based anti-angiogenesis therapy as a novel strategy for cancer treatment. Life Sci. 2019, 239, 117018. [Google Scholar] [CrossRef]
- Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 739–748. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.; Khaydukov, E.V.; Yamada, M.; Zvyagin, A.V.; Leelahavanichkul, A.; Leanse, L.G.; Dai, T.; Prow, T. Nanoparticle enhanced blue light therapy. Adv. Drug Deliv. Rev. 2022, 184, 114198. [Google Scholar] [CrossRef]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef]
- Yoon, H.-M.; Kang, M.-S.; Choi, G.-E.; Kim, Y.-J.; Bae, C.-H.; Yu, Y.-B.; Jeong, Y.-I. Stimuli-Responsive Drug Delivery of Doxorubicin Using Magnetic Nanoparticle Conjugated Poly(ethylene glycol)-g-Chitosan Copolymer. Int. J. Mol. Sci. 2021, 22, 13169. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, X.; Liu, R.; Zhao, D.; Guo, C.; Zhu, A.; Wen, M.; Liu, Z.; Qu, G.; Meng, H. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Zaimy, M.A.; Saffarzadeh, N.; Mohammadi, A.; Pourghadamyari, H.; Izadi, P.; Sarli, A.; Moghaddam, L.K.; Paschepari, S.R.; Azizi, H.; Torkamandi, S.; et al. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther. 2017, 24, 233–243. [Google Scholar] [CrossRef]



| Malignancy | Common Subtypes | Characteristics |
|---|---|---|
| Basal Cell Carcinoma | Nodular, Superficial, Morpheaform (sclerosing), Pigmented | Most common; slow-growing, low metastatic risk; significant local tissue destruction |
| Squamous Cell Carcinoma | Keratoacanthoma, Intraepidermal (Bowen’s disease), Invasive SCC | More aggressive; higher risk of local invasion and metastasis; arises from precancerous lesions |
| Sebaceous Gland Carcinoma | Papillary, Nodular, Pagetoid | Rare, highly aggressive; originates in meibomian glands; high risk of recurrence and metastasis |
| Merkel Cell Carcinoma | N/A | Rare, aggressive neuroendocrine tumor; early metastasis; poor prognosis |
| Other Rare Malignancies | Dermatofibrosarcoma Protuberans (DFSP), Microcystic Adnexal Carcinoma (MAC) | Less common; includes various rare non-melanocytic tumors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirone, B.; Scarabosio, A.; Surico, P.L.; Parodi, P.C.; D’Esposito, F.; Avitabile, A.; Foti, C.; Gagliano, C.; Zeppieri, M. Targeted Drug Delivery in Periorbital Non-Melanocytic Skin Malignancies. Bioengineering 2024, 11, 1029. https://doi.org/10.3390/bioengineering11101029
Tirone B, Scarabosio A, Surico PL, Parodi PC, D’Esposito F, Avitabile A, Foti C, Gagliano C, Zeppieri M. Targeted Drug Delivery in Periorbital Non-Melanocytic Skin Malignancies. Bioengineering. 2024; 11(10):1029. https://doi.org/10.3390/bioengineering11101029
Chicago/Turabian StyleTirone, Benedetta, Anna Scarabosio, Pier Luigi Surico, Pier Camillo Parodi, Fabiana D’Esposito, Alessandro Avitabile, Caterina Foti, Caterina Gagliano, and Marco Zeppieri. 2024. "Targeted Drug Delivery in Periorbital Non-Melanocytic Skin Malignancies" Bioengineering 11, no. 10: 1029. https://doi.org/10.3390/bioengineering11101029
APA StyleTirone, B., Scarabosio, A., Surico, P. L., Parodi, P. C., D’Esposito, F., Avitabile, A., Foti, C., Gagliano, C., & Zeppieri, M. (2024). Targeted Drug Delivery in Periorbital Non-Melanocytic Skin Malignancies. Bioengineering, 11(10), 1029. https://doi.org/10.3390/bioengineering11101029









