The Use of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles in the Treatment of Osteoarthritis: Insights from Preclinical Studies
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Steultjens, M. Classification of patients with knee osteoarthritis in clinical phenotypes: Data from the osteoarthritis initiative. PLoS ONE 2018, 13, e0191045. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Allan, R.; Smith, S.L.; Marreiros, S.S.; Steultjens, M. Identification of clinical phenotypes in knee osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2016, 17, 425. [Google Scholar] [CrossRef]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.M.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef]
- Gardashli, M.; Baron, M.; Huang, C.; Kaplan, L.D.; Meng, Z.; Kouroupis, D.; Best, T.M. Mechanical loading and orthobiologic therapies in the treatment of post-traumatic osteoarthritis (PTOA): A comprehensive review. Front. Bioeng. Biotechnol. 2024, 12, 1401207. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Best, T.M.; Huard, J.; Philippon, M.; Hornicek, F.; Duan, Z.; Griswold, A.J.; Kaplan, L.D.; Hare, J.M.; Kouroupis, D. Therapeutic Perspectives for Inflammation and Senescence in Osteoarthritis using Mesenchymal Stem Cells, Mesenchymal Stem Cell-Derived Extracellular Vesicles and Senolytic Agents. Cells 2023, 12, 1421. [Google Scholar] [CrossRef]
- Kouroupis, D.; Wang, X.N.; El-Sherbiny, Y.; McGonagle, D.; Jones, E. The Safety of Non-Expanded Multipotential Stromal Cell Therapies. In Safety, Ethics and Regulations; Pham, P.V., Rosemann, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 91–118. [Google Scholar]
- Kouroupis, D.; Sanjurjo-Rodriguez, C.; Jones, E.; Correa, D. Mesenchymal Stem Cell Functionalization for Enhanced Therapeutic Applications. Tissue Eng. Part B Rev. 2019, 25, 55–77. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Diederichs, S.; Melnik, S.; Riegger, J.; Trivanović, D.; Li, S.; Jenei-Lanzl, Z.; Brenner, R.E.; Huber-Lang, M.; Zaucke, F.; et al. Extracellular Vesicles in Musculoskeletal Pathologies and Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 624096. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Jiang, S.; Yuan, C.; Lin, K. The potential therapeutic role of extracellular vesicles in osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 1022368. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Wong, K.L.; Saseendar, S.; Muthu, S.; Concaro, S.; Fernandes, T.L.; Mahmood, A. Exploring the potential of mesenchymal stem/stromal cell-derived extracellular vesicles as cell-free therapy for osteoarthritis: A narrative review. J. Cartil. Jt. Preserv. 2024, 4, 100184. [Google Scholar] [CrossRef]
- Kodama, J.; Wilkinson, K.J.; Otsuru, S. MSC-EV therapy for bone/cartilage diseases. Bone Rep. 2022, 17, 101636. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surg. 2017, 99, 1769–1779. [Google Scholar] [CrossRef]
- Ramaswamy Reddy, S.H.; Reddy, R.; Babu, N.C.; Ashok, G.N. Stem-cell therapy and platelet-rich plasma in regenerative medicines: A review on pros and cons of the technologies. J. Oral Maxillofac. Pathol. 2018, 22, 367–374. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, Y.; Fang, L.; Yuan, C.; Wang, X.; Lin, K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact. Mater. 2023, 22, 423–452. [Google Scholar] [CrossRef]
- Labusek, N.; Mouloud, Y.; Köster, C.; Diesterbeck, E.; Tertel, T.; Wiek, C.; Hanenberg, H.; Horn, P.A.; Felderhoff-Müser, U.; Bendix, I.; et al. Extracellular vesicles from immortalized mesenchymal stromal cells protect against neonatal hypoxic-ischemic brain injury. Inflamm. Regen. 2023, 43, 24. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhan, J.; Yan, Z.; Chen, D.; Xue, X.; Pan, X. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J. Cell. Mol. Med. 2021, 25, 7734–7745. [Google Scholar] [CrossRef]
- Xu, H.; Xu, B. BMSC-Derived Exosomes Ameliorate Osteoarthritis by Inhibiting Pyroptosis of Cartilage via Delivering miR-326 Targeting HDAC3 and STAT1//NF-κB p65 to Chondrocytes. Mediat. Inflamm. 2021, 2021, 9972805. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhou, J.; Wang, Z.; Tao, H.; Bai, J.; Ge, G.; Li, W.; Zhang, W.; Hao, Y.; Yang, X.; et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg. Chem. 2021, 113, 104978. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Huang, W.; Chen, Q.; Xu, J.; Yao, G.; Li, B.; Wu, T.; Yin, C.; Cheng, X. LncRNA Malat-1 From MSCs-Derived Extracellular Vesicles Suppresses Inflammation and Cartilage Degradation in Osteoarthritis. Front. Bioeng. Biotechnol. 2021, 9, 772002. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Liebmann, K.; Castillo, M.A.; Jergova, S.; Best, T.M.; Sagen, J.; Kouroupis, D. Modification of Mesenchymal Stem/Stromal Cell-Derived Small Extracellular Vesicles by Calcitonin Gene Related Peptide (CGRP) Antagonist: Potential Implications for Inflammation and Pain Reversal. Cells 2024, 13, 484. [Google Scholar] [CrossRef]
- Leñero, C.; Kaplan, L.D.; Best, T.M.; Kouroupis, D. CD146+ Endometrial-Derived Mesenchymal Stem/Stromal Cell Subpopulation Possesses Exosomal Secretomes with Strong Immunomodulatory miRNA Attributes. Cells 2022, 11, 4002. [Google Scholar] [CrossRef]
- Kouroupis, D.; Kaplan, L.D.; Huard, J.; Best, T.M. CD10-Bound Human Mesenchymal Stem/Stromal Cell-Derived Small Extracellular Vesicles Possess Immunomodulatory Cargo and Maintain Cartilage Homeostasis under Inflammatory Conditions. Cells 2023, 12, 1824. [Google Scholar] [CrossRef]
- Kouroupis, D.; Kaplan, L.D.; Best, T.M. Human infrapatellar fat pad mesenchymal stem cells show immunomodulatory exosomal signatures. Sci. Rep. 2022, 12, 3609. [Google Scholar] [CrossRef]
- Teo, K.Y.W.; Tan, R.; Wong, K.L.; Hey, D.H.W.; Hui, J.H.P.; Toh, W.S. Small extracellular vesicles from mesenchymal stromal cells: The next therapeutic paradigm for musculoskeletal disorders. Cytotherapy 2023, 25, 837–846. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Qu, Z.; Liu, J.; Yang, L.; Zhang, W. Efficacy of Extracellular Vesicles From Mesenchymal Stem Cells on Osteoarthritis in Animal Models: A Systematic Review and Meta-Analysis. Nanomedicine 2021, 16, 1297–1310. [Google Scholar] [CrossRef]
- Mohd Noor, N.A.; Abdullah Nurul, A.; Ahmad Mohd Zain, M.R.; Wan Nor Aduni, W.K.; Azlan, M. Extracellular Vesicles from Mesenchymal Stem Cells as Potential Treatments for Osteoarthritis. Cells 2021, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, X.; Li, P.; Fan, Y.; Zhang, L.; Ma, X.; Sun, R.; Liu, Y.; Li, W. microRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021, 272, 119204. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Perucca Orfei, C.; Kouroupis, D.; Ragni, E.; De Luca, P.; ViganÒ, M.; Correa, D.; de Girolamo, L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy 2019, 21, 1179–1197. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Warmink, K.; Rios, J.L.; Varderidou-Minasian, S.; Torres-Torrillas, M.; van Valkengoed, D.R.; Versteeg, S.; Eijkelkamp, N.; Weinans, H.; Korthagen, N.M.; Lorenowicz, M.J. Mesenchymal stem/stromal cells-derived extracellular vesicles as a potentially more beneficial therapeutic strategy than MSC-based treatment in a mild metabolic osteoarthritis model. Stem Cell Res. Ther. 2023, 14, 137. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020, 22, 256. [Google Scholar] [CrossRef]
- Ye, P.; Mi, Z.; Wei, D.; Gao, P.; Ma, M.; Yang, H. miR-3960 from Mesenchymal Stem Cell-Derived Extracellular Vesicles Inactivates SDC1/Wnt/β-Catenin Axis to Relieve Chondrocyte Injury in Osteoarthritis by Targeting PHLDA2. Stem Cells Int. 2022, 2022, 9455152. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, Q.; Lin, F.; Wang, J. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered 2021, 12, 11225–11238. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Z. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing Long Noncoding RNA NEAT1 Relieve Osteoarthritis. Oxidative Med. Cell. Longev. 2022, 2022, 5517648. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cong, M.; Huang, W.; Chen, J.; Zhang, M.; Gu, X.; Sun, C.; Yang, H. The Effect of Human Bone Marrow Mesenchymal Stem Cell-Derived Exosomes on Cartilage Repair in Rabbits. Stem Cells Int. 2022, 2022, 5760107. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell. Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Zavatti, M.; Beretti, F.; Casciaro, F.; Bertucci, E.; Maraldi, T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. Biofactors 2020, 46, 106–117. [Google Scholar] [CrossRef]
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef]
- Li, Y.; Tu, Q.; Xie, D.; Chen, S.; Gao, K.; Xu, X.; Zhang, Z.; Mei, X. Triamcinolone acetonide-loaded nanoparticles encapsulated by CD90(+) MCSs-derived microvesicles drive anti-inflammatory properties and promote cartilage regeneration after osteoarthritis. J. Nanobiotechnol. 2022, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, J.; Fan, A.; Wang, P.; Chen, R.; Lu, L.; Yin, F. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J. Gene Med. 2021, 23, e3379. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, K.; Ge, G.; Zhang, D.; Bai, J.; Guo, X.; Zhou, J.; Xu, T.; Xu, M.; Long, X.; et al. Exosomes derived from miR-155-5p–overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021, 37, 85–96. [Google Scholar] [CrossRef]
- Tao, S.C.; Huang, J.Y.; Gao, Y.; Li, Z.X.; Wei, Z.Y.; Dawes, H.; Guo, S.C. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021, 6, 4455–4469. [Google Scholar] [CrossRef]
- Duan, A.; Shen, K.; Li, B.; Li, C.; Zhou, H.; Kong, R.; Shao, Y.; Qin, J.; Yuan, T.; Ji, J.; et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res. Ther. 2021, 12, 427. [Google Scholar] [CrossRef]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, F.; Yuan, Y.; Shan, L.; Cui, Y.; Qu, J.; Lian, F. Synovial Mesenchymal Stem Cell-Derived EV-Packaged miR-31 Downregulates Histone Demethylase KDM2A to Prevent Knee Osteoarthritis. Mol. Ther. Nucleic Acids 2020, 22, 1078–1091. [Google Scholar] [CrossRef]
- Yan, L.; Liu, G.; Wu, X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J. Orthop. Transl. 2021, 26, 111–120. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, X.; Yan, C.; Xiong, W.; Ma, Z.; Tan, Z.; Wang, J.; Li, Y.; Liu, J.; Duan, A.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res. Ther. 2022, 13, 322. [Google Scholar] [CrossRef]
- Fazaeli, H.; Kalhor, N.; Naserpour, L.; Davoodi, F.; Sheykhhasan, M.; Hosseini, S.K.E.; Rabiei, M.; Sheikholeslami, A. A Comparative Study on the Effect of Exosomes Secreted by Mesenchymal Stem Cells Derived from Adipose and Bone Marrow Tissues in the Treatment of Osteoarthritis-Induced Mouse Model. Biomed. Res. Int. 2021, 2021, 9688138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J Nanobiotechnol. 2021, 19, 194. [Google Scholar] [CrossRef]
- Zhang, S.; Wong, K.L.; Ren, X.; Teo, K.Y.W.; Afizah, H.; Choo, A.B.H.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes Promote Functional Osteochondral Repair in a Clinically Relevant Porcine Model. Am. J. Sports Med. 2022, 50, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, J.; Alahdal, M. Exosomes loaded with chondrogenic stimuli agents combined with 3D bioprinting hydrogel in the treatment of osteoarthritis and cartilage degeneration. Biomed. Pharmacother. 2023, 168, 115715. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef] [PubMed]
- Scalzone, A.; Sanjurjo-Rodríguez, C.; Berlinguer-Palmini, R.; Dickinson, A.M.; Jones, E.; Wang, X.-N.; Crossland, R.E. Functional and Molecular Analysis of Human Osteoarthritic Chondrocytes Treated with Bone Marrow-Derived MSC-EVs. Bioengineering 2024, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Huang, X.; Ma, J.; Zhao, G.; Ma, T.; Chen, K.; Huang, G.; Chen, J.; Shi, J.; Wang, S. Exosomes derived from MSC as drug system in osteoarthritis therapy. Front. Bioeng. Biotechnol. 2024, 12, 1331218. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Zeng, Y.; Pu, W.; Mu, X.; Sun, K.; Peng, Y.; Shen, B. Exosomes rewire the cartilage microenvironment in osteoarthritis: From intercellular communication to therapeutic strategies. Int. J. Oral Sci. 2022, 14, 40. [Google Scholar] [CrossRef]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar] [CrossRef]
- Walsh, D.A.; Mapp, P.I.; Kelly, S. Calcitonin gene-related peptide in the joint: Contributions to pain and inflammation. Br. J. Clin. Pharmacol. 2015, 80, 965–978. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. CMLS 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, T.T.; Sim, W.K.; Zhang, B.; Lim, S.K. A roadmap from research to clinical testing of mesenchymal stromal cell exosomes in the treatment of psoriasis. Cytotherapy 2023, 25, 815–820. [Google Scholar] [CrossRef]
- Toh, W.S.; Yarani, R.; El Andaloussi, S.; Cho, B.S.; Choi, C.; Corteling, R.; De Fougerolles, A.; Gimona, M.; Herz, J.; Khoury, M.; et al. A report on the International Society for Cell & Gene Therapy 2022 Scientific Signature Series, “Therapeutic advances with native and engineered human extracellular vesicles”. Cytotherapy 2023, 25, 810–814. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.J.; Hollinshead, F.; Goodrich, L.R. Extracellular vesicles in the treatment and prevention of osteoarthritis: Can horses help us translate this therapy to humans? Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 151–169. [Google Scholar] [CrossRef] [PubMed]
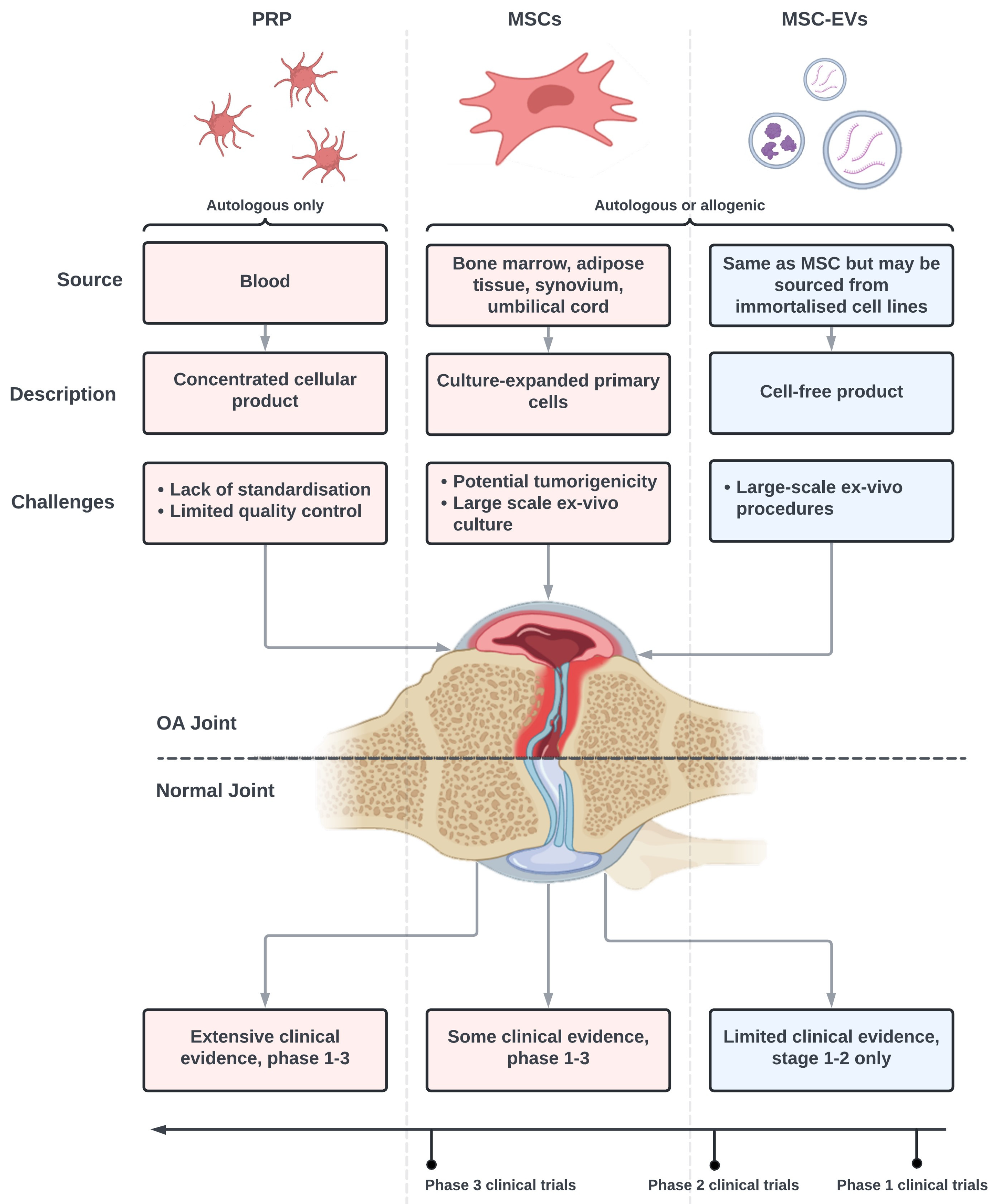
| Source | Cargo | OA Model | EVs Dose | Control Group | Follow Up Period | Outcome Measures | Key Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| hAD-MSC | Various miRNAs | - MIA induced in rats - BMM in mice | - In rat subacute OA group: 30 μL injection once per week for 21 days of hASC-EVs (1 × 108 particles) or PBS and hyaluronic acid - In rat chronic OA group: 30 μL injection twice per week for 40 days of hASC-EVs (1 × 108 particles) or PBS and hyaluronic acid - 6 μL injection once per week of EVs (1 × 108 particles) or PBS | PBS | 4 and 8 weeks | Histology (H), immunohistochemistry (IH), OARSI scores | EVs significantly attenuated OA progression and protected cartilage from degeneration in both the monosodium iodoacetate (MIA) rat and the surgical destabilization of the medial meniscus (DMM) mouse models | [46] |
| hAFSC | Not defined | MIA induced in rats | 100 μg, repeated after 10 days | OA, AFSC (5 × 105 cells) | 3 weeks | H/IH, OARSI scores, pain assessment | Enhanced pain tolerance, lower OARSI scores comparable to AFSC-treated defects | [45] |
| hBM-MSC | miR-92a-3p | Collagenase induced in mice | 15 μL (500 μg/mL), once a week | Healthy, OA, MSC-miR-92a-3p-transfected | 3 weeks | H/IH, WB | Reduced cartilage matrix loss, improved col2a1 and aggrecan expression (further improved in MSC-miR-92a-3p group | [33] |
| hBM-MSC | Not defined | Groove surgery in rats on high-fat diet | 7.77 × 107 particles (from 2 × 106 MSCs), five doses with 5-day intervals | OA, PBS, 2 × 106 MSCs | 11weeks | µCT scans at week 0, 12 and 24, pain behavior, H/IH, OARSI scores | Unchanged OARSI scores, pain behavior and MMP13 staining in cartilage (compared to MSC group where these were unexpectedly aggravated) | [38] |
| hBM-MSC | miR-136-5p | Post-traumatic oleanolic acid (OA) in mice | 100 μL single injection of 1011 particles/mL of EVs or miR-136-5p EVs | Healthy, OA | 1 h | Histology (H), immunohistochemistry (IH) | miR-136-5p was found to reduce the degeneration of cartilage extracellular matrix | [39] |
| hBM-MSC | miR-3960 | DMM in mice | 10 μL MSC-EVs or sterile normal saline were injected into the articular capsule for 3 weeks (once a week) | Sham, OA, MSCs-EVs-agomir-NC | 3 weeks | Histology (H), pain assessment | miR-3960 shuttled by MSC- EVs protected against apoptosis and ECM degradation in chondrocytes | [40] |
| hBM-MSC | miR-125a-5p | ACL rupture in mice | 100 μL single injection of 1011 particles/mL | Healthy, OA | 1 h | WB, qPCR | Alleviation in chondrocyte extracellular matrix degradation | [41] |
| hBM-MSC | miR-361-5p | ACL rupture in rats | 250 ng/5 µL EVs-miR-NC or EVs-miR-361-5p postoperatively for 7 days | Sham, OA | 8 weeks | Histology (H), WB, qPCR | miR-361-5p alleviates cartilage damage | [23] |
| hBM-MSC | LncRNA NEAT1 | DMM in mice | 10 μg EVs or an equivalent amount of PBS, twice a week for 1 month | Sham, OA, DMM + Lv − NC − BMSCs − EVs group, DMM + Lv − NEAT1 – BMSCs − EVs group, DMM + Lv − NEAT1 − BMSCs − EVs + sh − Sesn2 group | 7 weeks | Histology (H), immunohistochemistry (IH), OARSI scores | LncRNA NEAT1 induced the proliferation and autophagy of chondrocytes but inhibited their apoptosis | [42] |
| hBM-MSC | Not defined | Osteochondral defect in rabbits | 300 μL injection once a week for 4 weeks of 1 × 1010 particles/mL (low dosage) or 5 × 1010 particles/mL (high dosage) or PBS | Healthy, OA | 5 weeks | Histology (H), ICRS scores | Facilitates cartilage regeneration and enhances viability of chondrocytes | [43] |
| hBM-MSC | lncRNA MEG-3 | DMM in rats | 100 µL injection per week EVs solution (100 µg) or MSCs (106 cell) | Sham, OA | 8 weeks | Histology (H), immunohistochemistry (IH), OARSI scores, micro-CT | - MSC and MSC-EVs alleviated cartilage destruction and subchondral bone remodeling - lncRNA MEG-3 also reduced the senescence and apoptosis of chondrocytes | [44] |
| hBM-MSC and hAD-MSC | Not defined | Ciproflaxin induced in mice | 25 mL at 100 μg/μL, once a week | Healthy, OA, PBS | 3 weeks | H/IH, OARSI scores, real-time PCR | Lower OARSI scores, upregulated COLII protein and Sox9, COL2 and Aggrecan genes in cartilage, particularly in BM-MSC group | [57] |
| hESC-MSC | Not defined | DMM in mice | 5 μL every 3 days for 4 weeks | OA, PBS | 4 weeks | Histology (H), immunohistochemistry (IH), OARSI scores | Lower OARSI scores, stronger Col II and weaker ADAMTS5 staining of cartilage | [58] |
| hESC-MSC | Not defined | Osteochondral defect in rats | 100 μL injection once a week of 100 μg EVs or PBS | OA, PBS | 12 weeks | Histology (H), immunohistochemistry (IH), multiplex cytokine array | EV-treated defects displayed a regenerative immune phenotype characterized by a higher infiltration of CD163+ regenerative M2 macrophages over CD86+ M1 macrophages, with a concomitant reduction in proinflammatory synovial cytokines IL-1β and TNF-α | [59] |
| hESC-MSC | Not defined | Osteochondral defect in rats | 100 μL injection once a week of 100 μg EVs or PBS | OA, PBS | 12 weeks | Histology (H), ICRS scores | - Enhanced gross appearance and improved histological scores - EV-treated defects displayed complete restoration of cartilage and subchondral bone with characteristic features including a hyaline cartilage with good surface regularity, complete bonding to adjacent cartilage, and extracellular matrix deposition | [60] |
| hIFP-MSC | miR-100-5p | DMM in mice | 10 μL (1010 particles/mL) twice a week | OA, PBS | 4 or 6 weeks | H/IH, OARSI scores, gait analysis | Lower OARSI scores, stronger Col II and weaker ADAMTS5 and MMP13 staining of cartilage, partial improvement of the gait patterns | [25] |
| hIFP-MSC | Various miRNAs | MIA induced in rats | 50 μL single injection of EVs derived from 5 × 105 and 5 × 106 IFP-MSC | Healthy, OA | 4 days | Histology (H), immunohistochemistry (IH) | MSC-EV therapeutic treatment resulted in robust macrophage polarization towards an anti-inflammatory therapeutic M2 phenotype within the synovium/IFP tissues | [29] |
| hIFP-MSC | Various miRNAs and CD10 protein | MIA induced in rats | 50 μL single injection of EVs derived from 1 × 106 IFP-MSC | Healthy, OA | 4 days | Histology (H), immunohistochemistry (IH) | CD10High EV treatment resulted in robust chondroprotective effects by retaining articular cartilage structure/composition and PRG4 (lubricin)-expressing cartilage cells | [28] |
| iMSC and hSynovium-MSC | Not defined | Collagenase induced in mice | 8 μL injection once per week for 21 days of iMSC-EVs (1.0 × 1010/mL) or MSC-EVs (1.0 × 1010/mL) or PBS | Healthy, OA | 4 weeks | Histology (H), immunohistochemistry (IH), OARSI scores | The injection of iMSC-EVs and MSC-EVs both attenuated OA in the mouse OA model, but iMSC EVs had a superior therapeutic effect compared with MSC-EVs | [53] |
| hSynovium-MSC | Not defined | ACL rupture in rabbits and rats | For rabbits: 12 mg once per week of TA, T-NP, T-RNP, CD90@MV, T-CD90@NP For rats: 0.25 mg once per week of TA, T-NP, T-RNP, CD90@MV, T-CD90@NP | Sham, OA | 24 weeks for rabbits and 2 weeks for rats | Histology (H), immunohistochemistry (IH), micro-CT, RNAseq | - CD90 EVs enhanced repair of damaged cartilage and effective anti-inflammatory ability - CD90 EVs promoted the regeneration of chondrocytes, reduced apoptosis via the FOXO pathway, and influenced type 2 macrophage polarization to regulate inflammation through IL-10 | [47] |
| hSynovium-MSC | miR-26a-5p | DMM in rats | 30 μL injection per week of GW inhibitor or EVs or EV-NC or EV-inhibitor (1011 particles/mL) or PBS | Sham, OA | 4 weeks | Histology (H), ELISA, qPCR | miR-26a-5p MSC EVs inhibit apoptosis and inflammation and ameliorate cartilage damage of OA | [48] |
| hSynovium-MSC | miR-155-5p | DMM in mice | 30 μL injection of MSC-EVs (1011 EVs particles/mL) or MSC-155-5p-EVs (1011 EVs particles/mL) | Healthy, OA | 2 weeks | Histology (H), immunohistochemistry (IH), OARSI scores | miR-155-5p EVs prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes | [49] |
| hSynovium-MSC | circRNA3503 | DMM in rats | 100 μL injection of PLEL@SMSC-EVs or PLEL@Wnt5a/b-dKO-EVs or PLEL@circRNA3503-OE-EVs or PLEL@dKO-OE-EVs or PLEL@Saline | Healthy, OA | 24 weeks | Histology (H) | - circRNA3503-OE-EVs alleviate inflammation-induced apoptosis and the imbalance between ECM synthesis and ECM degradation - circRNA3503-OE-EVs promote chondrocyte renewal to alleviate the progressive loss of chondrocytes | [50] |
| hSynovium-MSC | Not defined | DMM in mice | 10 μL injection twice weekly of PBS-EVs (1011 particles/mL) or PBS-LPS-pre EVs (1011 particles/mL) or PBS | Sham, PBS | 6 weeks | Histology (H), immunohistochemistry (IH), OARSI scores | EVs derived from LPS-preconditioned MSC inhibit extracellular matrix degradation and prevent osteoarthritis | [51] |
| hSynovium-MSC | miR-140-5p | DMM in rats | 100 μL injection of Synovium-MSC-EVs (1011 EVs particles/mL) or SMSC-140 EVs (1011 EVs particles/mL) | Healthy, OA | 12 weeks | Histology (H), immunohistochemistry (IH), OARSI scores | miR-140-5p EVs enhance cartilage tissue regeneration and prevent osteoarthritis | [52] |
| hSynovium-MSC | miR-31 | DMM in mice | 5 μL injection every 3 days for 4 weeks of Synovium-MSC-EVs or EVs (miR-31 mimic) or PBS | Sham, OA | 12 weeks | Histology (H), immunohistochemistry (IH), OARSI scores, ELISA | MSC EVs and EVs from miR-31-overexpressed MSC alleviated cartilage damage and inflammation in knee joints in vivo | [54] |
| hUC-MSC | LncRNA H19 | Osteochondral defect in rats | 100 μL injection once per week of EVs from UC-MSC transfected with siRNA H19 (si-EVs, 1 mg/mL) or EVs from UC-MSC with mechanical stimulation (S-EVs, 1 mg/mL) or PBS | PBS | 4 and 8 weeks | Behavioral analysis, histology (H), MRI, ICRS scores | LncRNA H19 relieve pain levels during the early stages of cartilage repair via enhanced chondrocyte proliferation and matrix synthesis | [55] |
| hUC-MSC | miR-1208 | DMM in mice | 10 μL injection twice per week of MSC-EVs (1011 particles/mL) or antagomiR-NC (200 nmol/mL) or antagomiR-1208 (200 nmol/mL) or PBS | Sham, PBS | 6 weeks | Histology (H), immunohistochemistry (IH), micro-CT, ELISA | MSC-EVs inhibited the secretion of proinflammatory factors and the degradation of cartilage ECM | [56] |
| hUC-MSC | circHIPK3 | Collagenase induced in mice | Single injection of MSC-EVs or MSC-circHIPK3-EVs or circHIPK3 or PBS | Healthy, OA | Not reported | Histology (H), immunohistochemistry (IH), WB, qPCR | MSC-circHIPK3 EVs inhibited cartilage degradation | [61] |
| EV Source | Patients | Dose | Type of Trial | Delivery Method | Follow Up Period | Primary Outcome | Trial ID |
|---|---|---|---|---|---|---|---|
| Allogeneic UC MSCs | KL 2–3 knee OA, n = 10 | 3–5 × 1011 particles | Phase 1, safety and efficacy trial | Single IA injection | 12 months | Adverse events, pain and disability reduction | NCT05060107 |
| Allogeneic UC MSCs | KL 2–3 knee OA, n = 12, n = 4 per group | 2 × 109 particles/dose; 6 × 109 particles/dose; 2 × 1010 particles/dose | Phase 1, open-label dose-escalation trial | Single IA injection | 12 months | Adverse events, pain and disability reduction, percentage of responders at 52 weeks | NCT06431152 |
| Autologous SF-MSCs | Bilateral degenerative meniscus, early OA, 3 groups of n = 10 | EVs from 106 SF MSCs; 106 SF MSCs; control | Phase 2, randomized safety and efficacy trial | Single IA injection | 12 months | Adverse events, pain reduction, cytokine measurements, knee motion and physical activity | NCT05261360 |
| Allogeneic MSCs | KL 1–3 knee OA in both knees, n = 20 | Not reported | Phase 1, safety and efficacy trial | IA injection, day 1 and day 90 | 1, 3, and 6 months | Adverse events, evaluation of pain, measurements of knee function | NCT06466850 |
| Platelets: PEP (Purified EVs Product); 2 doses; with or without 1% sodium hyaluronate | KL 2–3 knee OA, n = 24 | 1 or 2 vials | Phase 1, randomized safety and exploratory efficacy dose-escalation trial | Single IA injection | 90 days (primary safety); 12 months (long-term safety) | Primary and long-term safety, clinical improvements after 12 months | NCT06463132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, M.; Jones, E.; Kouroupis, D. The Use of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles in the Treatment of Osteoarthritis: Insights from Preclinical Studies. Bioengineering 2024, 11, 961. https://doi.org/10.3390/bioengineering11100961
Jones M, Jones E, Kouroupis D. The Use of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles in the Treatment of Osteoarthritis: Insights from Preclinical Studies. Bioengineering. 2024; 11(10):961. https://doi.org/10.3390/bioengineering11100961
Chicago/Turabian StyleJones, Mitch, Elena Jones, and Dimitrios Kouroupis. 2024. "The Use of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles in the Treatment of Osteoarthritis: Insights from Preclinical Studies" Bioengineering 11, no. 10: 961. https://doi.org/10.3390/bioengineering11100961








