Preparation and Characterization of Hydroxylated Recombinant Collagen by Incorporating Proline and Hydroxyproline in Proline-Deficient Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
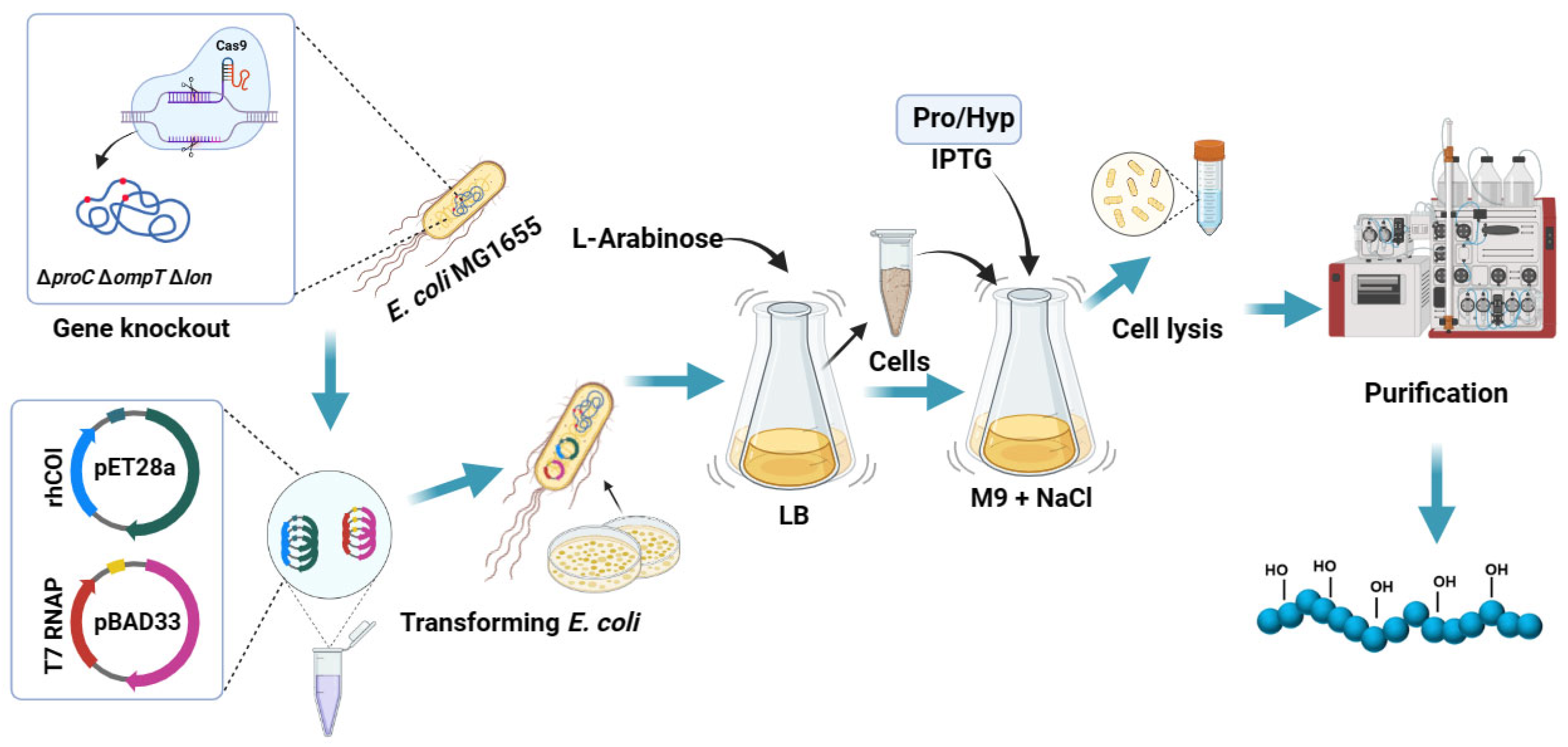
2.1. Construction of Engineered Strains
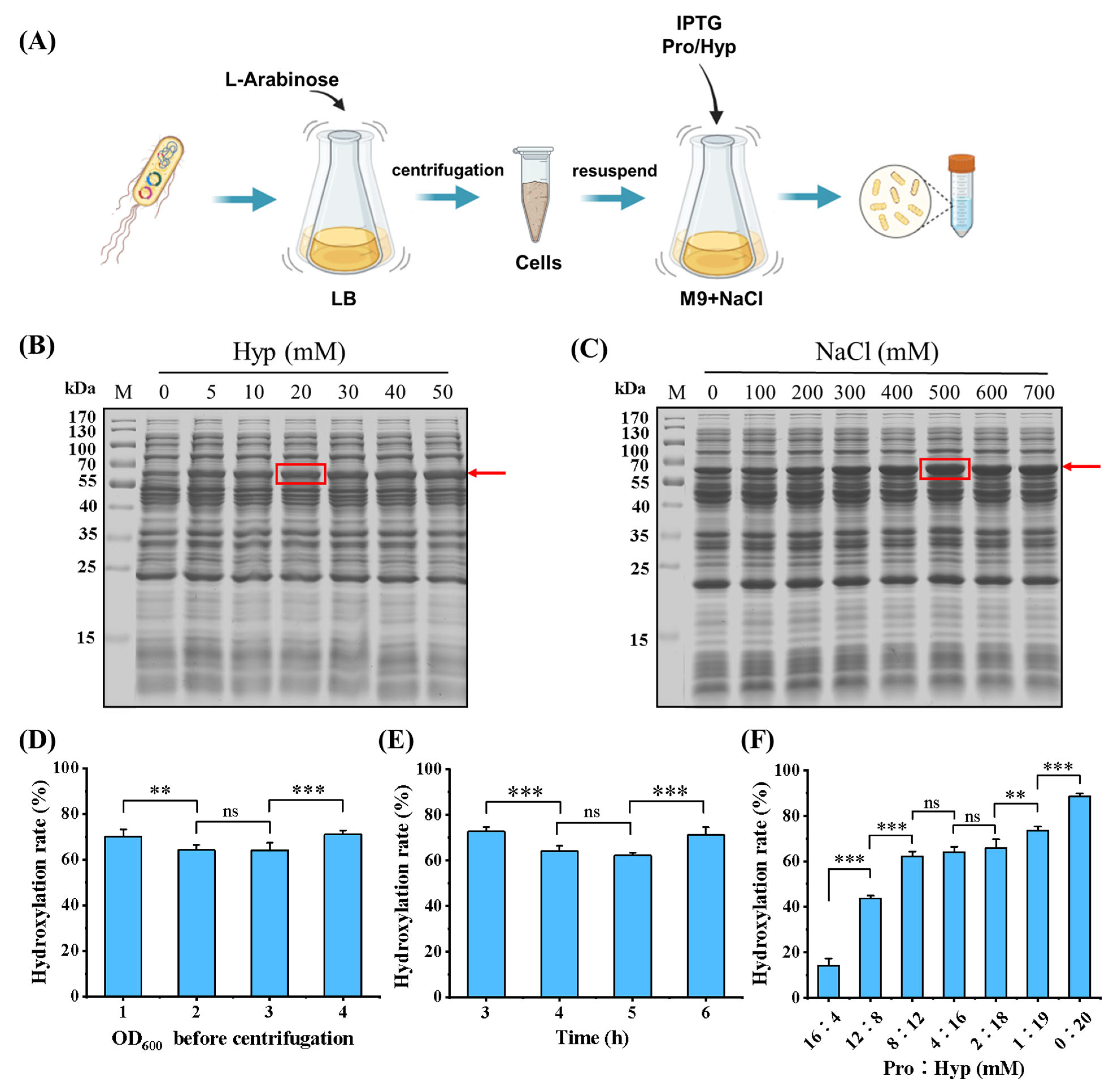
2.2. Expression of Collagen by Incorporating Hyp/Pro
2.3. Expression of Hydroxylated Collagen by Co-Expression of Proline Hydroxylase
2.4. Purification of Recombinant Collagen
2.5. Determination of Collagen Hydroxylation Rate
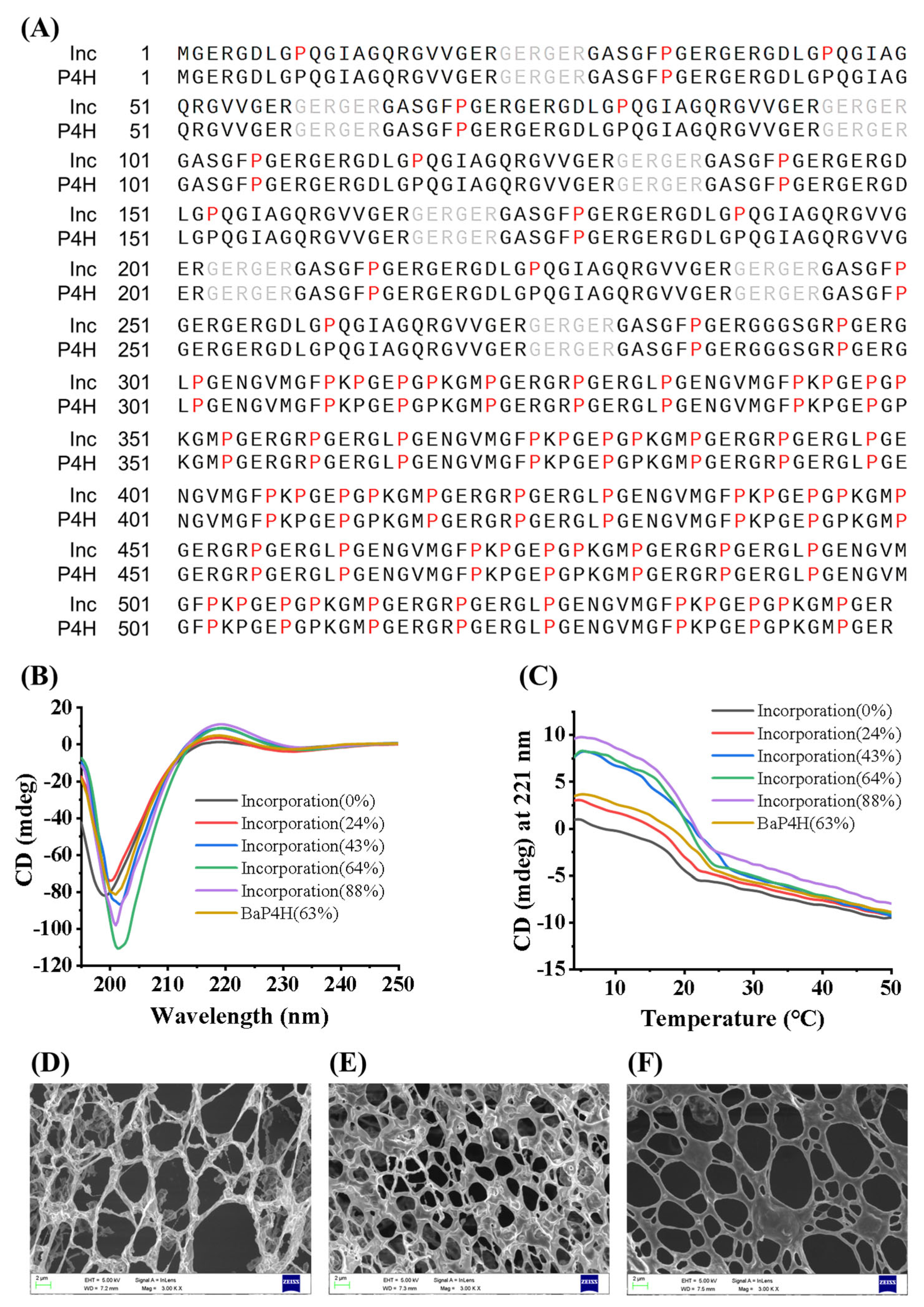
2.6. LC-MS/MS Analysis for Recombinant Collagen
2.7. CD Spectrometry Analysis
2.8. Scanning Electron Microscopy (SEM) Analysis
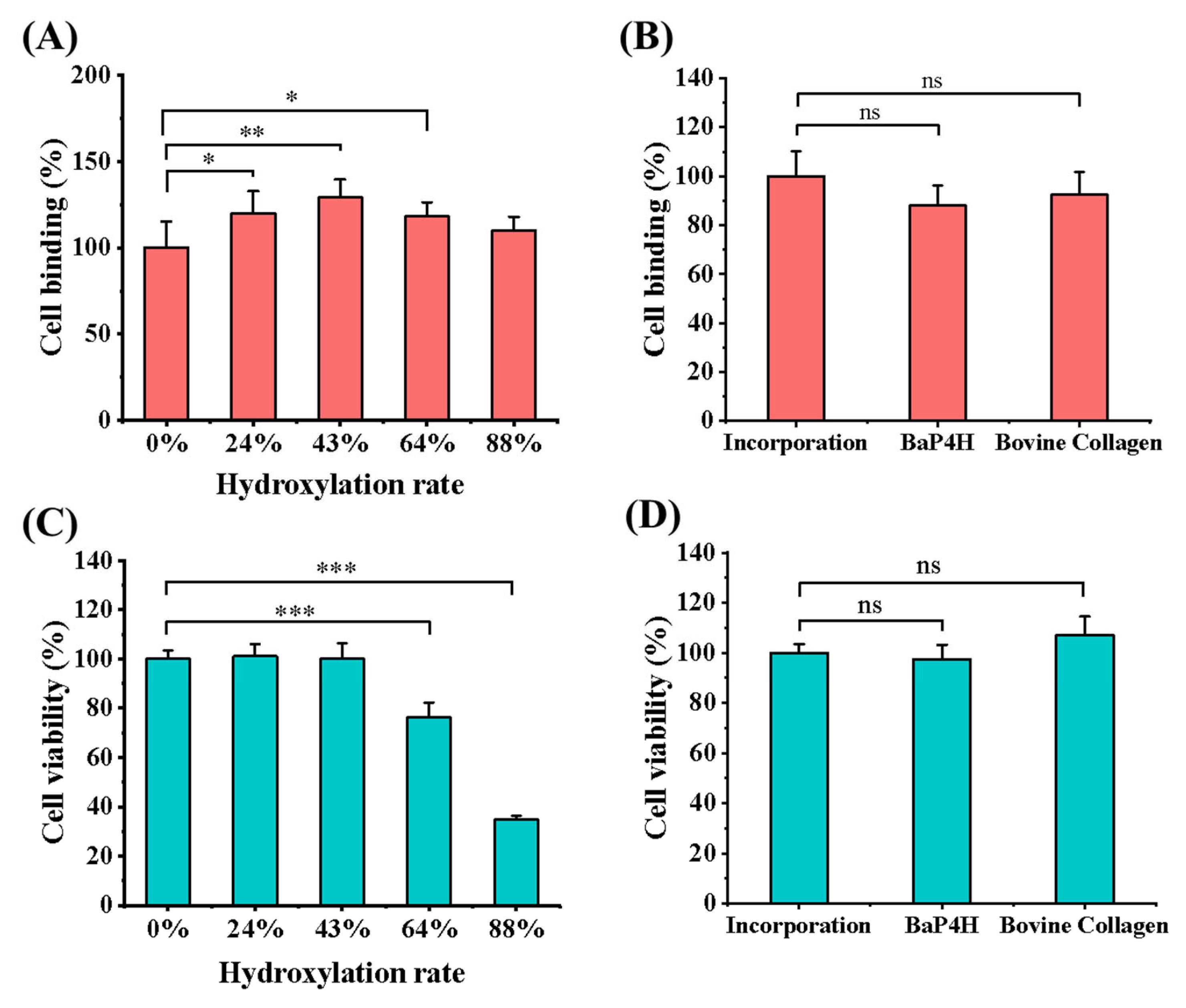
2.9. Cell Binding Assay
2.10. Cell Viability Assay
2.11. Fermentation of Collagen by Incorporation in a 7 L Fermenter
3. Results and Discussion
3.1. Construction of Engineering Bacteria
3.2. Incorporations of Pro and Hyp
3.3. The Structural Characteristics of rhCol
3.4. Cell Binding and Viability of rhCol
3.5. Enhanced Production of rhCol in the 7 L Fermenter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heino, J. The collagen family members as cell adhesion proteins. BioEssays 2007, 29, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.F.; Abdulhussein, R.; Ford, C.E. Sensing extracellular matrix: An update on discoidin domain receptor function. Cell. Signal. 2006, 18, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Parenteau, N. Skin: The first tissue-engineered products. Sci. Am. 1999, 280, 83–84. [Google Scholar] [PubMed]
- Helary, C.; Ovtracht, L.; Coulomb, B.; Godeau, G.; Giraudguille, M. Dense Fibrillar Collagen Matrices: A Model to Study Myofibroblast Behaviour during Wound Healing. Biomaterials 2006, 27, 4443–4452. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Howard, D.; Waller, A.K.; Foster, H.R.; Mueller, A.; Moreau, T.; Evans, A.L.; Arumugam, M.; Chalon, G.B.; Vriend, E. Structurally Graduated Collagen Scaffolds Applied to the Ex Vivo Generation of Platelets from Human Pluripotent Stem Cell-Derived Megakaryocytes: Enhancing Production and Purity. Biomaterials 2018, 182, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Avila Rodriguez, M.I.; Rodriguez Barroso, L.G.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen Scaffolds for Tissue Engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, D.; Shang, L. Exploring the potential of the recombinant human collagens for biomedical and clinical applications: A short review. Biomed. Mater. 2021, 16, 012001. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Young, B.B.; Ezura, Y. Development of tendon structure and function: Regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005, 5, 5–21. [Google Scholar] [PubMed]
- Brodsky, B.; Ramshaw, J.A.M. The Collagen Triple-Helix Structure. Matrix Biol. 1997, 15, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Ramshaw, J.A. Bioengineered Collagens. Subcell. Biochem. 2017, 82, 601–629. [Google Scholar]
- Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V. Recent progress with recombinant collagens produced in Escherichia coli. Curr. Opin. Biomed. Eng. 2019, 10, 149–155. [Google Scholar] [CrossRef]
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline Hydroxylation in Collagen Supports Integrin Binding by Two Distinct Mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef]
- Markolovic, S.; Wilkins, S.E.; Schofield, C.J. Protein Hydroxylation Catalyzed by 2-Oxoglutarate-Dependent Oxygenases. J. Biol. Chem. 2015, 290, 20712–20722. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, P.F. Structural insights into the regulation of aromatic amino acid hydroxylation. Curr. Opin. Struct. Biol. 2015, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, G.; Guo, J.; Takada, M.; Wei, W.; Zhang, Q. New Insights into Protein Hydroxylation and Its Important Role in Human Diseases. In Carolina Digital Repository; University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2016. [Google Scholar]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71B, 343–354. [Google Scholar] [CrossRef]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Werkmeister, J.A. 2.23 Recombinant Proteins as Emerging Biomaterials; Ducheyne, P., Ed.; Comprehensive Biomaterials II; Elsevier: Oxford, UK, 2017; pp. 512–531. [Google Scholar]
- Liu, S.; Li, Y.; Wang, M.; Ma, Y.; Wang, J. Efficient coexpression of recombinant human fusion collagen with prolyl 4-hydroxylase from Bacillus anthracis in Escherichia coli. Biotechnol. Appl. Biochem. 2022, 70, 761–772. [Google Scholar] [CrossRef]
- Zhao, T.-X.; Li, M.; Zheng, X.; Wang, C.-H.; Zhao, H.-X.; Zhang, C.; Xing, X.-H. Improved Production of Trans-4-Hydroxy-l-Proline by Chromosomal Integration of the Vitreoscilla Hemoglobin Gene into Recombinant Escherichia coli with Expression of Proline-4-Hydroxylase. J. Biosci. Bioeng. 2017, 123, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, X.; Hang, B.; Li, J.; Huang, L.; Huang, F.; Xu, Z. Efficient Production of Hydroxylated Human-like Collagen via the Co-Expression of Three Key Genes in Escherichia coli Origami (DE3). Appl. Biochem. Biotechnol. 2015, 178, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, X.; Gao, Y.; Fan, D.; Zhu, C.; Mi, Y.; Xue, W. Hydroxylation of human type III collagen α chain by recombinant coexpression with a viral prolyl 4-hydroxylase in Escherichia coli. Protein J. 2017, 36, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Zhang, C.; Pei, C.-H.; Han, M.-N.; Xu, Z.-D.; Li, C.-H.; Li, W. Efficient Production of Trans-4-Hydroxy-L-Proline from Glucose by Metabolic Engineering of Recombinant Escherichia coli. Lett. Appl. Microbiol. 2018, 66, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Buechter, D.D.; Paolella, D.N.; Leslie, B.S.; Brown, M.S.; Mehos, K.A.; Gruskin, E.A. Co-Translational Incorporation of Trans-4-Hydroxyproline into Recombinant Proteins in Bacteria. J. Biol. Chem. 2003, 278, 645–650. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Nebl, T.; Glattauer, V.; Ramshaw, J.A. Incorporation of Hydroxyproline in Bacterial Collagen from Streptococcus Pyogenes. Acta Biomater. 2018, 80, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Ilamaran, M.; Janeena, A.; Valappil, S.; Ramudu, K.N.; Shanmugam, G.; Niraikulam, A. A Self-Assembly and Higher Order Structure Forming Triple Helical Protein as a Novel Biomaterial for Cell Proliferation. Biomater. Sci. 2019, 7, 2191–2199. [Google Scholar] [CrossRef]
- Ye, C.; Yang, Y.; Chen, X.; Yang, L.; Hua, X.; Yang, M.; Zeng, X.; Qiao, S. Metabolic Engineering of Escherichia coli BW25113 for the Production of 5-Aminolevulinic Acid Based on CRISPR/Cas9 Mediated Gene Knockout and Metabolic Pathway Modification. J. Biol. Eng. 2022, 16, 26. [Google Scholar] [CrossRef]
- Wren, J.; Wiggall, P. An improved colorimetric method for the determination of proline in the presence of other ninhydrin-positive compounds. Biochem. J. 1965, 94, 216–220. [Google Scholar] [CrossRef]
- Woessner, J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.M.; Weis, M.; Eyre, D.R. Insights on the Evolution of Prolyl 3-Hydroxylation Sites from Comparative Analysis of Chicken and Xenopus Fibrillar Collagens. PLoS ONE 2011, 6, e19336. [Google Scholar] [CrossRef] [PubMed]
- Kotch, F.W.; Raines, R.T. Self-Assembly of Synthetic Collagen Triple Helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.F.; Josephs, R.; Segal, D.M. Physical chemical studies on proteins and polypeptides. Ann. Rev. Biochem. 1966, 35, 599–650. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Polavarapu, P.L.; Láng, E.; Majer, Z. Conformational Analysis of Amyloid Precursor Protein Fragment Containing Amino Acids 667–676, and the Effect of D-Asp and Iso-Asp Substitution at Asp672 Residue. J. Struct. Biol. 2012, 177, 621–629. [Google Scholar] [CrossRef]
- Drzewiecki, K.E.; Grisham, D.R.; Parmar, A.S.; Nanda, V.; Shreiber, D.I. Circular Dichroism Spectroscopy of Collagen Fibrillogenesis: A New Use for an Old Technique. Biophys. J. 2016, 111, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Makhatadze, G.I.; Privalov, P.L. Energetics of Protein Structure; Anfinsen, C.B., Richards, F.M., Edsall, J.T., Eisenberg, D.S., Eds.; Advances in Protein Chemistry; Academic Press: Cambridge, MA, USA, 1995; pp. 307–425. [Google Scholar]
- Jithendra, P.; Rajam, A.M.; Kalaivani, T.; Mandal, A.B.; Rose, C. Preparation and Characterization of Aloe Vera Blended Collagen-Chitosan Composite Scaffold for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2013, 5, 7291–7298. [Google Scholar] [CrossRef]
- Okada, T.; Isobe, C.; Wada, T.; Ezaki, S.; Minoura, N. Switchable Binding Affinity of Mannose Tethered to Collagen Peptide by Temperature-Dependent Triple-Helix Formation. Bioconjug. Chem. 2013, 24, 841–845. [Google Scholar] [CrossRef]
- Seo, N.; Russell, B.H.; Rivera, J.J.; Liang, X.; Xu, X.; Afshar-Kharghan, V.; Höök, M. An Engineered α1 Integrin-Binding Collagenous Sequence. J. Biol. Chem. 2010, 285, 31046–31054. [Google Scholar] [CrossRef]
- Yoshizumi, A.; Yu, Z.; Silva, T.; Thiagarajan, G.; Ramshaw, J.A.M.; Inouye, M.; Brodsky, B. Self-Association of Streptococcus Pyogenes Collagen-like Constructs into Higher Order Structures. Protein Sci. 2009, 18, 1241–1251. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Chung, H.J.; Steplewski, A.; Chung, K.Y.; Uitto, J.; Fertala, A. Collagen Fibril Formation: A New Target to Limit Fibrosis. Matrix Biol. 2008, 27, 48. [Google Scholar] [CrossRef]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of Prolyl Hydroxylation in the Molecular Interactions of Collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Hong, B.; Li, Y.; Wang, J. Preparation and Characterization of Hydroxylated Recombinant Collagen by Incorporating Proline and Hydroxyproline in Proline-Deficient Escherichia coli. Bioengineering 2024, 11, 975. https://doi.org/10.3390/bioengineering11100975
Cheng Z, Hong B, Li Y, Wang J. Preparation and Characterization of Hydroxylated Recombinant Collagen by Incorporating Proline and Hydroxyproline in Proline-Deficient Escherichia coli. Bioengineering. 2024; 11(10):975. https://doi.org/10.3390/bioengineering11100975
Chicago/Turabian StyleCheng, Zhimin, Bin Hong, Yanmei Li, and Jufang Wang. 2024. "Preparation and Characterization of Hydroxylated Recombinant Collagen by Incorporating Proline and Hydroxyproline in Proline-Deficient Escherichia coli" Bioengineering 11, no. 10: 975. https://doi.org/10.3390/bioengineering11100975
APA StyleCheng, Z., Hong, B., Li, Y., & Wang, J. (2024). Preparation and Characterization of Hydroxylated Recombinant Collagen by Incorporating Proline and Hydroxyproline in Proline-Deficient Escherichia coli. Bioengineering, 11(10), 975. https://doi.org/10.3390/bioengineering11100975




