Adaptive vs. Conventional Deep Brain Stimulation: One-Year Subthalamic Recordings and Clinical Monitoring in a Patient with Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient History
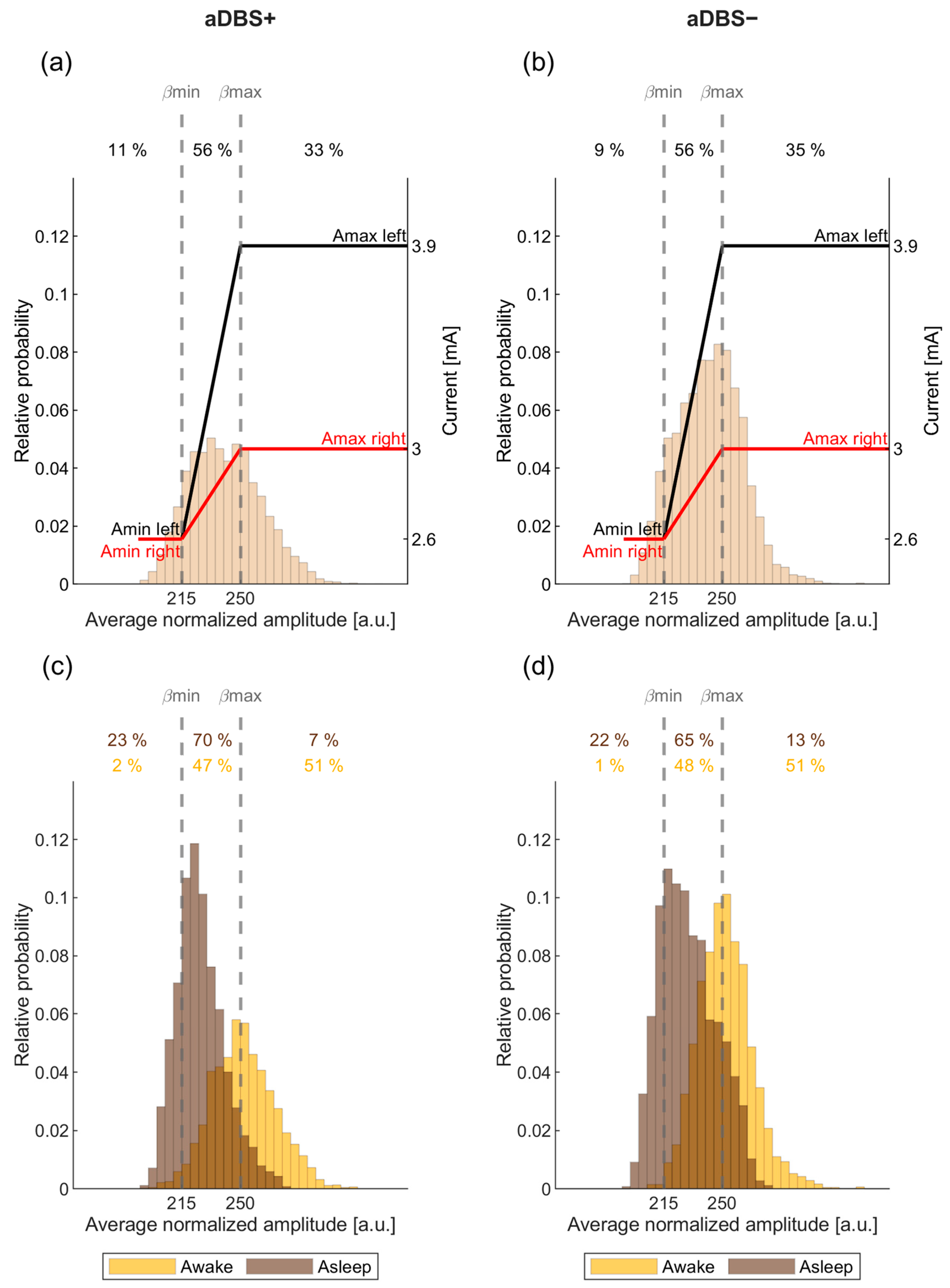
2.2. Adaptive Paradigm and Programming of the AlphaDBS Device
2.3. Spectral Analysis
2.4. Quantification and Statistical Analysis
3. Results
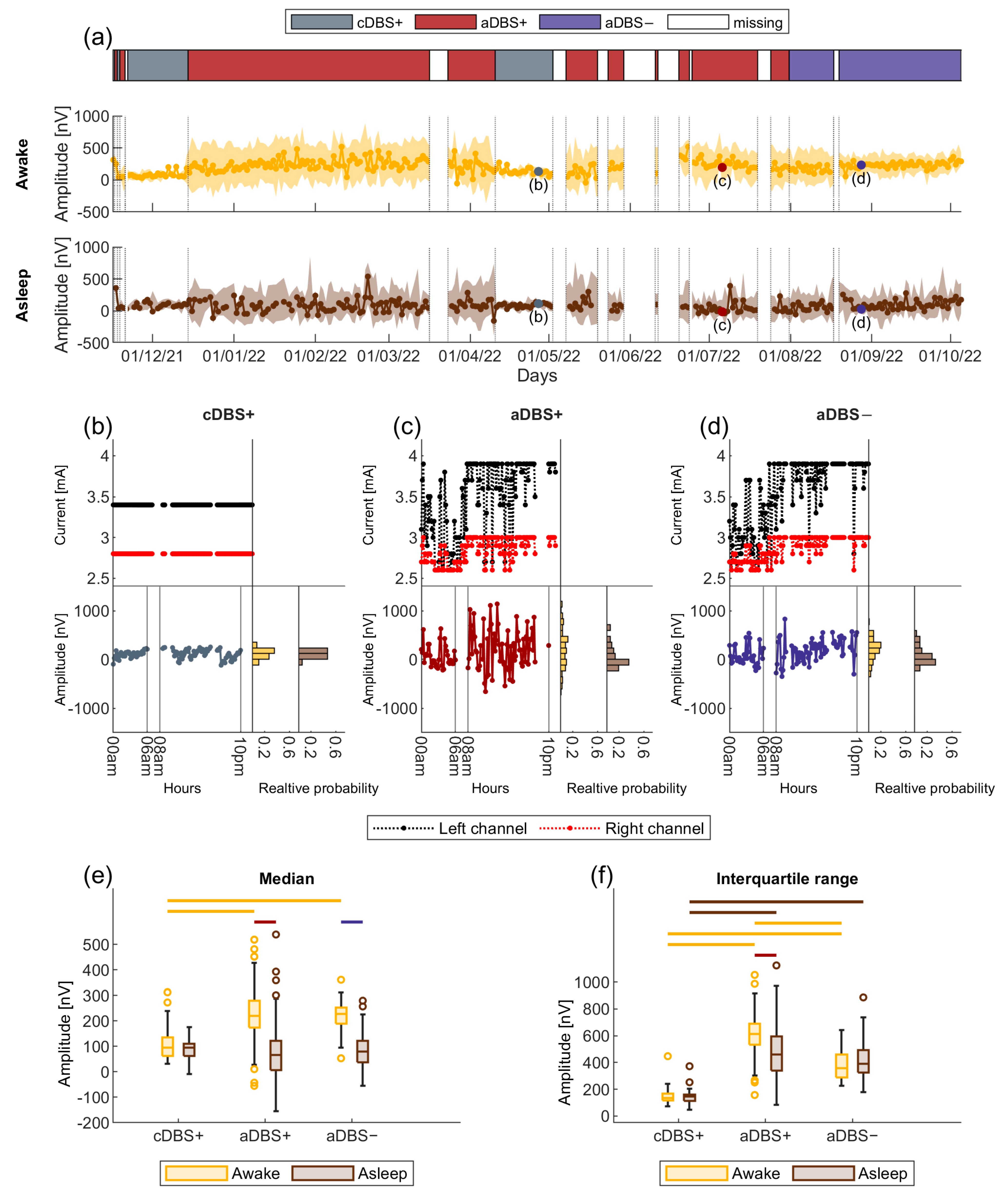
3.1. Consistent and Sustained Long-Term Clinical Improvement with aDBS
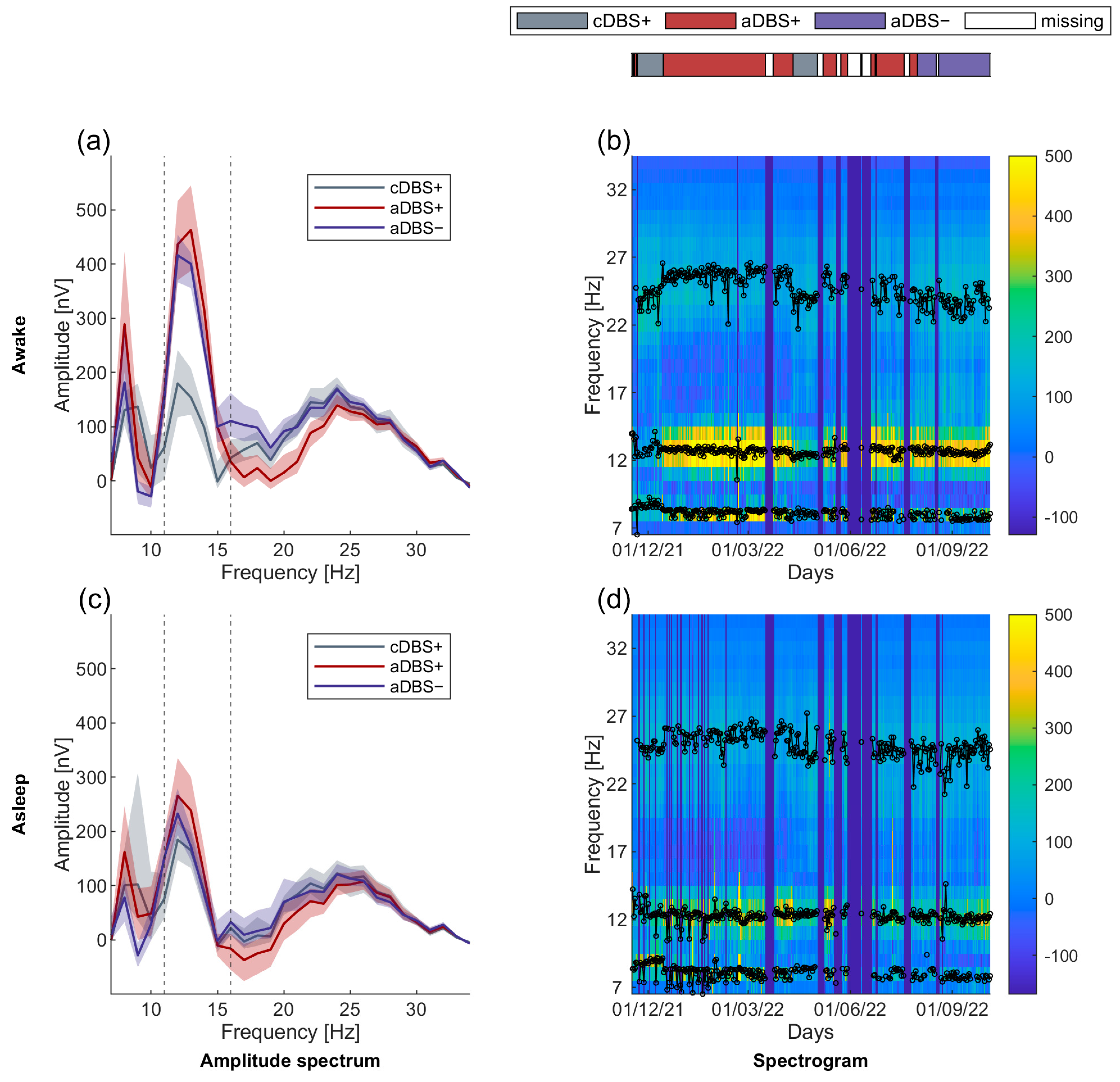
3.2. Long-Term Stable Beta-Band Peak Frequency
3.3. Larger Sleep-Wake Variation in Beta Amplitude in aDBS than in cDBS
3.4. Larger Variability in Beta Amplitude in aDBS than in cDBS
3.5. STN-LFP Beta Amplitude in aDBS Was Informative about Awake and Asleep State
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W.; et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, N.G.; Isaias, I.U. Chapter 19—Adaptive Deep Brain Stimulation: Retuning Parkinson’s Disease. In Handbook of Clinical Neurology; Quartarone, A., Ghilardi, M.F., Boller, F., Eds.; Neuroplasticity; Elsevier: Amsterdam, The Netherlands, 2022; Volume 184, pp. 273–284. [Google Scholar]
- Chen, C.C.; Brücke, C.; Kempf, F.; Kupsch, A.; Lu, C.S.; Lee, S.T.; Tisch, S.; Limousin, P.; Hariz, M.; Brown, P. Deep Brain Stimulation of the Subthalamic Nucleus: A Two-Edged Sword. Curr. Biol. 2006, 16, R952–R953. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Moro, E.; Krack, P. Long-Term Outcomes of Surgical Therapies for Parkinson’s Disease. Mov. Disord. 2012, 27, 1718–1728. [Google Scholar] [CrossRef]
- St George, R.J.; Nutt, J.G.; Burchiel, K.J.; Horak, F.B. A Meta-Regression of the Long-Term Effects of Deep Brain Stimulation on Balance and Gait in PD. Neurology 2010, 75, 1292–1299. [Google Scholar] [CrossRef]
- Reich, M.M.; Brumberg, J.; Pozzi, N.G.; Marotta, G.; Roothans, J.; Åström, M.; Musacchio, T.; Lopiano, L.; Lanotte, M.; Lehrke, R.; et al. Progressive Gait Ataxia Following Deep Brain Stimulation for Essential Tremor: Adverse Effect or Lack of Efficacy? Brain 2016, 139, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Marceglia, S.; Loh, A.; Harmsen, I.E.; Meoni, S.; Foffani, G.; Lozano, A.M.; Moro, E.; Volkmann, J.; Priori, A. Clinical Perspectives of Adaptive Deep Brain Stimulation. Brain Stimul. 2021, 14, 1238–1247. [Google Scholar] [CrossRef]
- Neumann, W.-J.; Degen, K.; Schneider, G.-H.; Brücke, C.; Huebl, J.; Brown, P.; Kühn, A.A. Subthalamic Synchronized Oscillatory Activity Correlates With Motor Impairment in Patients With Parkinson’s Disease. Mov. Disord. 2016, 31, 1748–1751. [Google Scholar] [CrossRef]
- Kühn, A.A.; Kupsch, A.; Schneider, G.-H.; Brown, P. Reduction in Subthalamic 8-35 Hz Oscillatory Activity Correlates with Clinical Improvement in Parkinson’s Disease. Eur. J. Neurosci. 2006, 23, 1956–1960. [Google Scholar] [CrossRef]
- Kühn, A.A.; Kempf, F.; Brücke, C.; Doyle, L.G.; Martinez-Torres, I.; Pogosyan, A.; Trottenberg, T.; Kupsch, A.; Schneider, G.-H.; Hariz, M.I.; et al. High-Frequency Stimulation of the Subthalamic Nucleus Suppresses Oscillatory β Activity in Patients with Parkinson’s Disease in Parallel with Improvement in Motor Performance. J. Neurosci. 2008, 28, 6165–6173. [Google Scholar] [CrossRef]
- Bocci, T.; Prenassi, M.; Arlotti, M.; Cogiamanian, F.M.; Borellini, L.; Moro, E.; Lozano, A.M.; Volkmann, J.; Barbieri, S.; Priori, A.; et al. Eight-Hours Conventional versus Adaptive Deep Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease. npj Parkinson’s Dis. 2021, 7, 88. [Google Scholar] [CrossRef]
- Arlotti, M.; Marceglia, S.; Foffani, G.; Volkmann, J.; Lozano, A.M.; Moro, E.; Cogiamanian, F.; Prenassi, M.; Bocci, T.; Cortese, F.; et al. Eight-Hours Adaptive Deep Brain Stimulation in Patients with Parkinson Disease. Neurology 2018, 90, e971–e976. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Pogosyan, A.; Neal, S.; Zavala, B.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J.; et al. Adaptive Deep Brain Stimulation in Advanced Parkinson Disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Beudel, M.; Zrinzo, L.; Foltynie, T.; Limousin, P.; Hariz, M.; Neal, S.; Cheeran, B.; Cagnan, H.; Gratwicke, J.; et al. Bilateral Adaptive Deep Brain Stimulation Is Effective in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Arlotti, M.; Ardolino, G.; Cogiamanian, F.; Marceglia, S.; Di Fonzo, A.; Cortese, F.; Rampini, P.M.; Priori, A. Adaptive Deep Brain Stimulation in a Freely Moving Parkinsonian Patient. Mov. Disord. 2015, 30, 1003–1005. [Google Scholar] [CrossRef]
- Rosa, M.; Arlotti, M.; Marceglia, S.; Cogiamanian, F.; Ardolino, G.; Fonzo, A.D.; Lopiano, L.; Scelzo, E.; Merola, A.; Locatelli, M.; et al. Adaptive Deep Brain Stimulation Controls Levodopa-Induced Side Effects in Parkinsonian Patients: DBS Controls Levodopa-Induced Side Effects. Mov. Disord. 2017, 32, 628–629. [Google Scholar] [CrossRef]
- Little, S.; Tripoliti, E.; Beudel, M.; Pogosyan, A.; Cagnan, H.; Herz, D.; Bestmann, S.; Aziz, T.; Cheeran, B.; Zrinzo, L.; et al. Adaptive Deep Brain Stimulation for Parkinson’s Disease Demonstrates Reduced Speech Side Effects Compared to Conventional Stimulation in the Acute Setting. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1388–1389. [Google Scholar] [CrossRef]
- Isaias, I.U.; Benti, R.; Cilia, R.; Canesi, M.; Marotta, G.; Gerundini, P.; Pezzoli, G.; Antonini, A. [123I]FP-CIT Striatal Binding in Early Parkinson’s Disease Patients with Tremor vs. Akinetic-Rigid Onset. Neuroreport 2007, 18, 1499–1502. [Google Scholar] [CrossRef]
- Arlotti, M.; Colombo, M.; Bonfanti, A.; Mandat, T.; Lanotte, M.M.; Pirola, E.; Borellini, L.; Rampini, P.; Eleopra, R.; Rinaldo, S.; et al. A New Implantable Closed-Loop Clinical Neural Interface: First Application in Parkinson’s Disease. Front. Neurosci. 2021, 15, 763235. [Google Scholar] [CrossRef]
- Deep Brain Stimulation Programming: Mechanisms, Principles and Practice-Montgomery Jr, Erwin B: 9780190259600—AbeBooks. Available online: https://www.abebooks.it/9780190259600/Deep-Brain-Stimulation-Programming-Mechanisms-0190259604/plp (accessed on 2 August 2024).
- Değirmenci, Y. Current DBS Programming. Deep Brain Stimul. 2024, 4, 29–31. [Google Scholar] [CrossRef]
- van Rheede, J.J.; Feldmann, L.K.; Busch, J.L.; Fleming, J.E.; Mathiopoulou, V.; Denison, T.; Sharott, A.; Kühn, A.A. Diurnal Modulation of Subthalamic Beta Oscillatory Power in Parkinson’s Disease Patients during Deep Brain Stimulation. npj Parkinson’s Dis. 2022, 8, 88. [Google Scholar] [CrossRef]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T.; et al. Parameterizing Neural Power Spectra into Periodic and Aperiodic Components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Mixed (Between/Within Subjects) ANOVA. Available online: https://it.mathworks.com/matlabcentral/fileexchange/27080-mixed-between-within-subjects-anova (accessed on 12 June 2023).
- Algina, J.; Keselman, H.J.; Penfield, R.D. An Alternative to Cohen’s Standardized Mean Difference Effect Size: A Robust Parameter and Confidence Interval in the Two Independent Groups Case. Psychol. Methods 2005, 10, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, S.; Treves, A. Analytical Estimates of Limited Sampling Biases in Different Information Measures. Network 1996, 7, 87–107. [Google Scholar] [CrossRef]
- Magri, C.; Whittingstall, K.; Singh, V.; Logothetis, N.K.; Panzeri, S. A Toolbox for the Fast Information Analysis of Multiple-Site LFP, EEG and Spike Train Recordings. BMC Neurosci. 2009, 10, 81. [Google Scholar] [CrossRef]
- Horn, A.; Kühn, A.A. Lead-DBS: A Toolbox for Deep Brain Stimulation Electrode Localizations and Visualizations. NeuroImage 2015, 107, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Neumann, W.-J.; Degen, K.; Schneider, G.-H.; Kühn, A.A. Toward an Electrophysiological “Sweet Spot” for Deep Brain Stimulation in the Subthalamic Nucleus. Hum. Brain Mapp. 2017, 38, 3377–3390. [Google Scholar] [CrossRef]
- Horn, A.; Kühn, A.A.; Merkl, A.; Shih, L.; Alterman, R.; Fox, M. Probabilistic Conversion of Neurosurgical DBS Electrode Coordinates into MNI Space. NeuroImage 2017, 150, 395–404. [Google Scholar] [CrossRef]
- Wilkins, K.B.; Petrucci, M.N.; Lambert, E.F.; Melbourne, J.A.; Gala, A.S.; Akella, P.; Parisi, L.; Cui, C.; Kehnemouyi, Y.M.; Hoffman, S.L.; et al. Beta Burst-Driven Adaptive Deep Brain Stimulation Improves Gait Impairment and Freezing of Gait in Parkinson’s Disease. arXiv 2024, arXiv:2024.06.26.24309418. [Google Scholar]
- Vissani, M.; Palmisano, C.; Volkmann, J.; Pezzoli, G.; Micera, S.; Isaias, I.U.; Mazzoni, A. Impaired Reach-to-Grasp Kinematics in Parkinsonian Patients Relates to Dopamine-Dependent, Subthalamic Beta Bursts. npj Parkinson’s Dis. 2021, 7, 53. [Google Scholar] [CrossRef]
- Canessa, A.; Palmisano, C.; Isaias, I.U.; Mazzoni, A. Gait-Related Frequency Modulation of Beta Oscillatory Activity in the Subthalamic Nucleus of Parkinsonian Patients. Brain Stimul. 2020, 13, 1743–1752. [Google Scholar] [CrossRef]
- Arlotti, M.; Palmisano, C.; Minafra, B.; Todisco, M.; Pacchetti, C.; Canessa, A.; Pozzi, N.G.; Cilia, R.; Prenassi, M.; Marceglia, S.; et al. Monitoring Subthalamic Oscillations for 24 Hours in a Freely Moving Parkinson’s Disease Patient. Mov. Disord. 2019, 34, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Kilavik, B.E.; Ponce-Alvarez, A.; Trachel, R.; Confais, J.; Takerkart, S.; Riehle, A. Context-Related Frequency Modulations of Macaque Motor Cortical LFP Beta Oscillations. Cerebral. Cortex. 2012, 22, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
| Factors | Median F(2,296) = 21.23, p < 0.001 | Interquartile Range F(2,296) = 16.73, p < 0.001 | |||||
|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | p-Value | rCoh | p-Value | rCoh | |
| Awake | cDBS+ | aDBS+ | aDBS− | <0.001 | <0.001 | ||
| cDBS+ | aDBS+ | <0.001 | −1.69 | <0.001 | −4.04 | ||
| cDBS+ | aDBS− | <0.001 | −2.32 | <0.001 | −2.39 | ||
| aDBS+ | aDBS− | 1 | <0.001 | 1.95 | |||
| Asleep | cDBS+ | aDBS+ | aDBS− | 0.15 | <0.001 | ||
| cDBS+ | aDBS+ | <0.001 | −1.86 | ||||
| cDBS+ | aDBS− | <0.001 | −2.68 | ||||
| aDBS+ | aDBS− | 0.19 | |||||
| cDBS+ | Awake | Asleep | 0.058 | 0.92 | |||
| aDBS+ | Awake | Asleep | <0.001 | 1.86 | <0.001 | 0.91 | |
| aDBS− | Awake | Asleep | <0.001 | 2.10 | 0.17 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffi, L.; Romito, L.M.; Palmisano, C.; Aloia, V.; Arlotti, M.; Rossi, L.; Marceglia, S.; Priori, A.; Eleopra, R.; Levi, V.; et al. Adaptive vs. Conventional Deep Brain Stimulation: One-Year Subthalamic Recordings and Clinical Monitoring in a Patient with Parkinson’s Disease. Bioengineering 2024, 11, 990. https://doi.org/10.3390/bioengineering11100990
Caffi L, Romito LM, Palmisano C, Aloia V, Arlotti M, Rossi L, Marceglia S, Priori A, Eleopra R, Levi V, et al. Adaptive vs. Conventional Deep Brain Stimulation: One-Year Subthalamic Recordings and Clinical Monitoring in a Patient with Parkinson’s Disease. Bioengineering. 2024; 11(10):990. https://doi.org/10.3390/bioengineering11100990
Chicago/Turabian StyleCaffi, Laura, Luigi M. Romito, Chiara Palmisano, Vanessa Aloia, Mattia Arlotti, Lorenzo Rossi, Sara Marceglia, Alberto Priori, Roberto Eleopra, Vincenzo Levi, and et al. 2024. "Adaptive vs. Conventional Deep Brain Stimulation: One-Year Subthalamic Recordings and Clinical Monitoring in a Patient with Parkinson’s Disease" Bioengineering 11, no. 10: 990. https://doi.org/10.3390/bioengineering11100990








