Full-Thickness Perfused Skin-on-a-Chip with In Vivo-Like Drug Response for Drug and Cosmetics Testing
Abstract
:1. Introduction
2. Materials and Methods
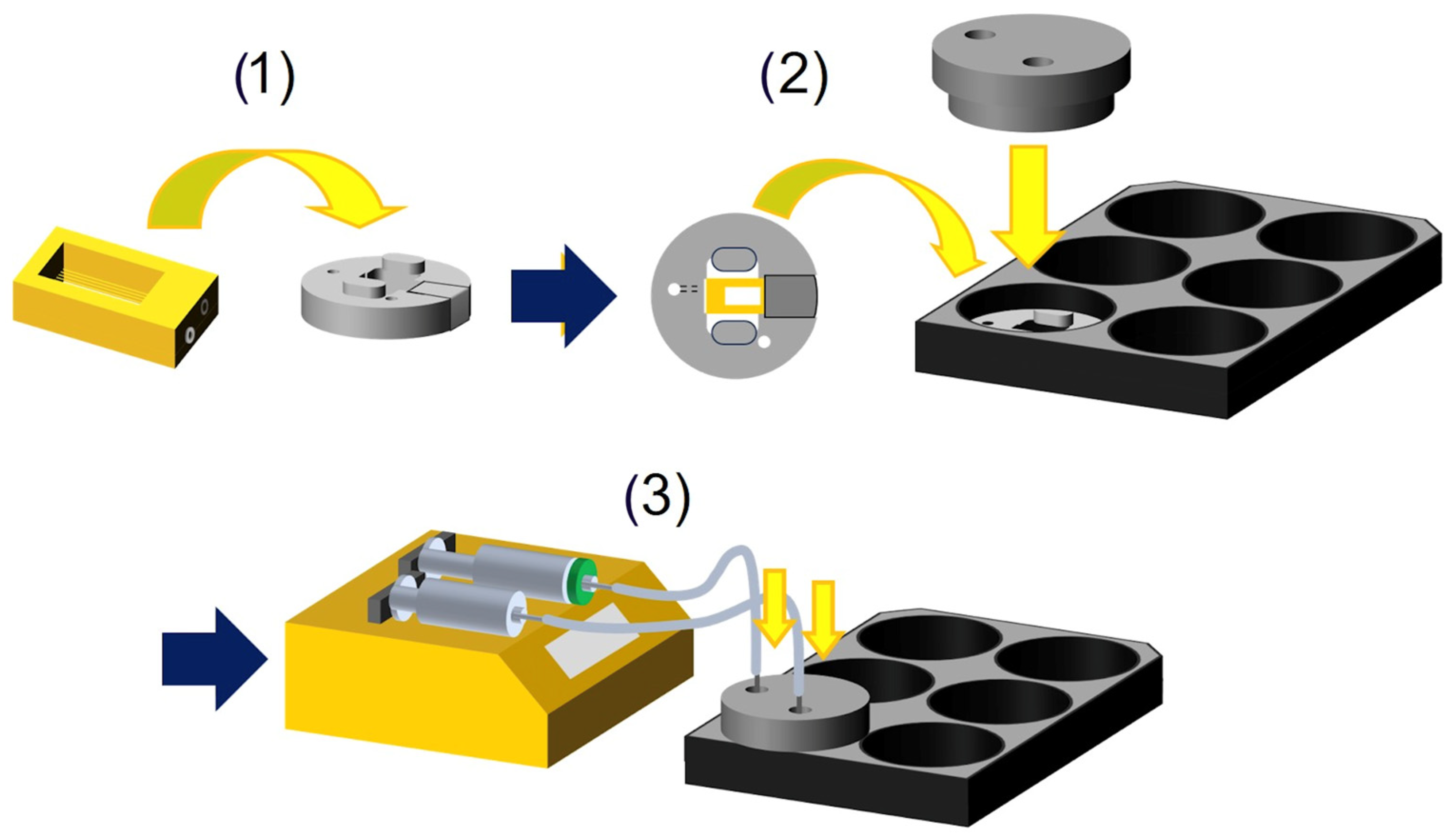
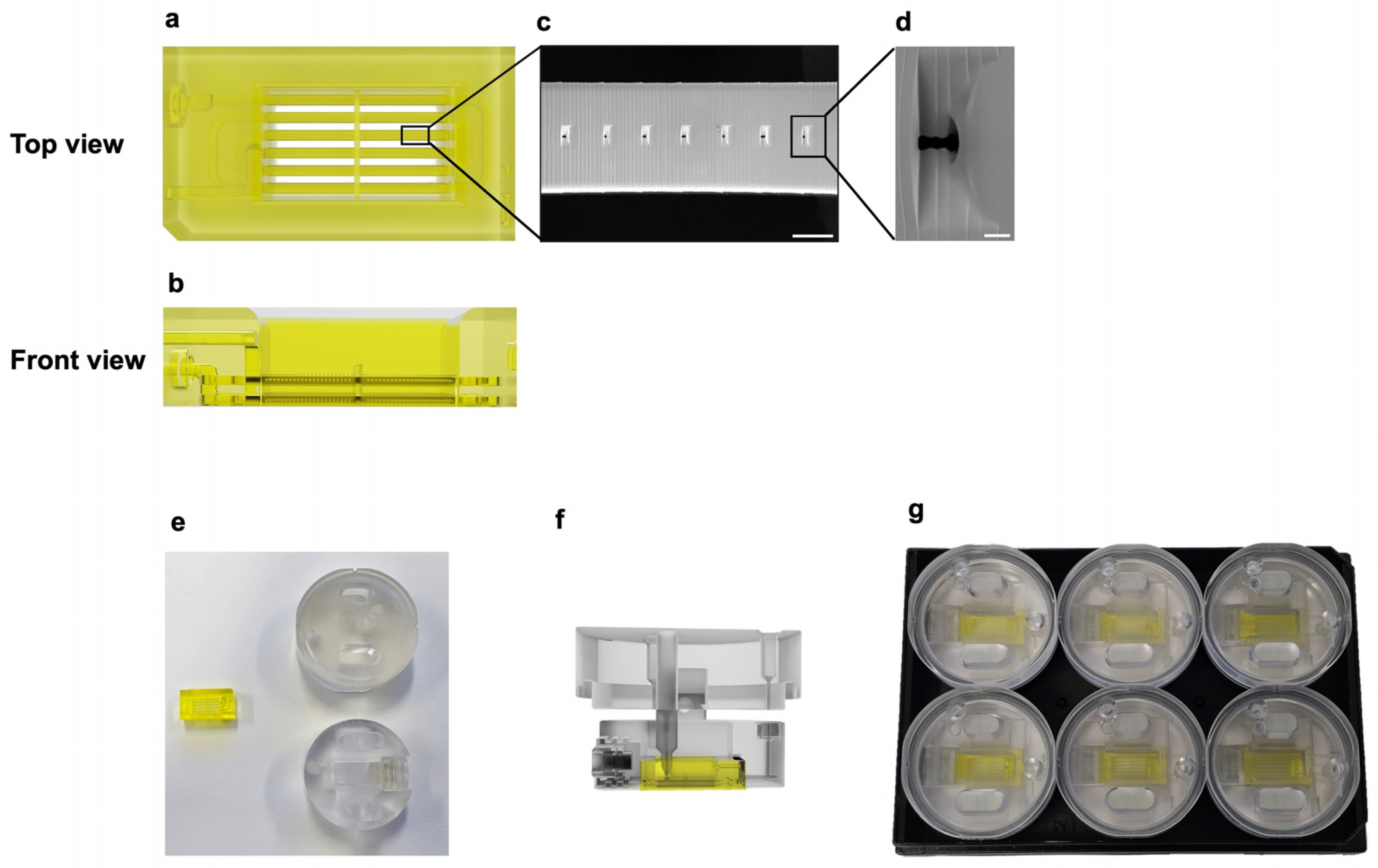
2.1. Chip Design and Fabrication
2.2. Preparation of Chip
2.3. Perfusion Setup
2.4. Flow Testing
2.5. Cell Preparation
2.6. Skin Equivalent Generation
2.7. Two-Dimensional Monoculture
2.8. Skin Biopsy Culture
2.9. Inflammation Induction and Drug Testing
2.10. Immunofluorescence Analysis of Skin Tissue
2.11. Histology
2.12. Cytotoxicity Testing
2.13. Drug Response Analysis
2.14. Statistical Analysis
3. Results
3.1. Design and Fabrication of an Air–Liquid Interface Skin-on-a-Chip System
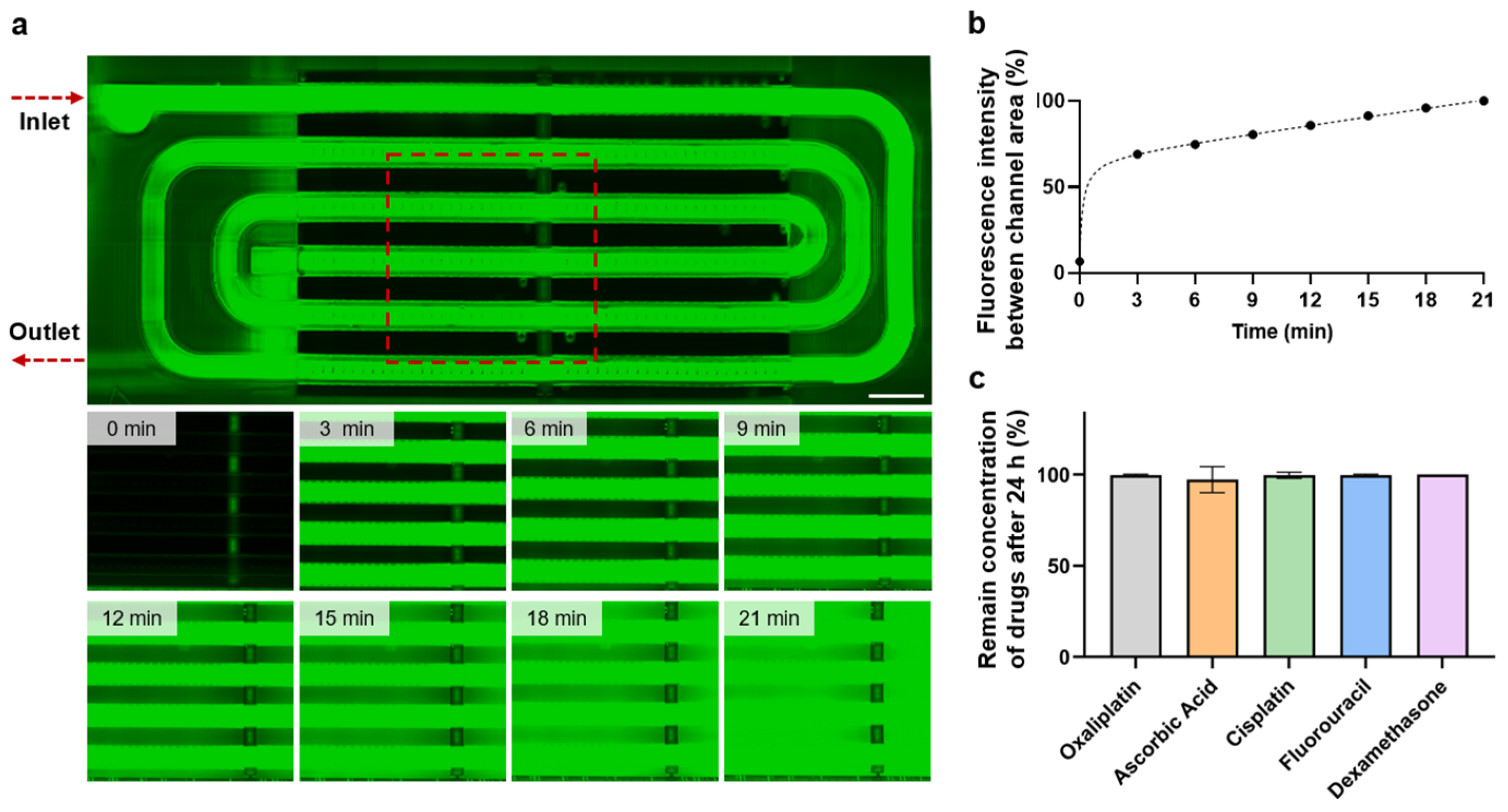
3.2. Perfusion Testing and Small Molecule Absorption
3.3. Formation of Skin Tissue with Perfusion System
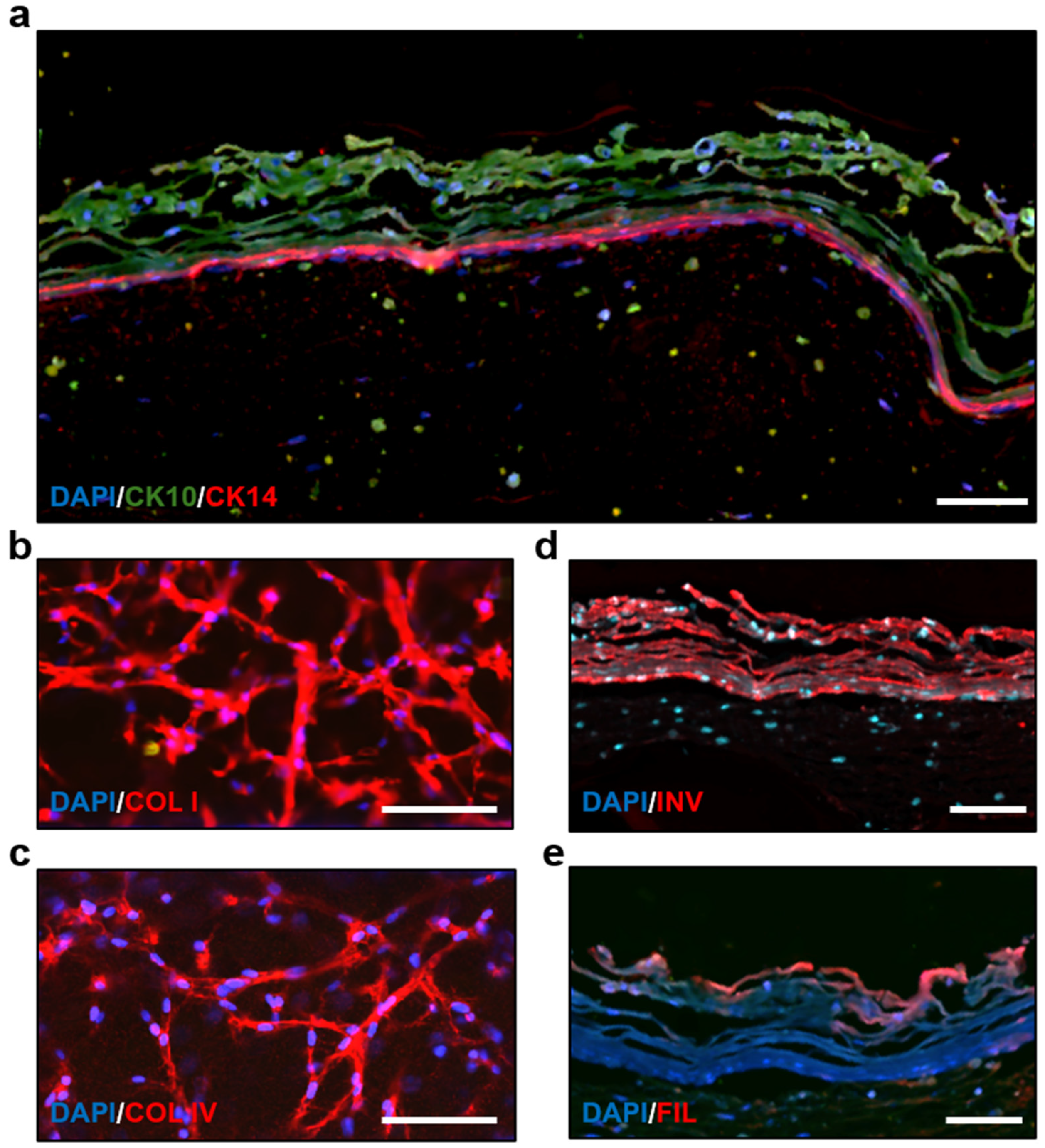
3.4. Immunostaining and Morphology Analysis of the Skin-on-a-Chip
3.5. Drug Treatment on the Skin-on-a-Chip
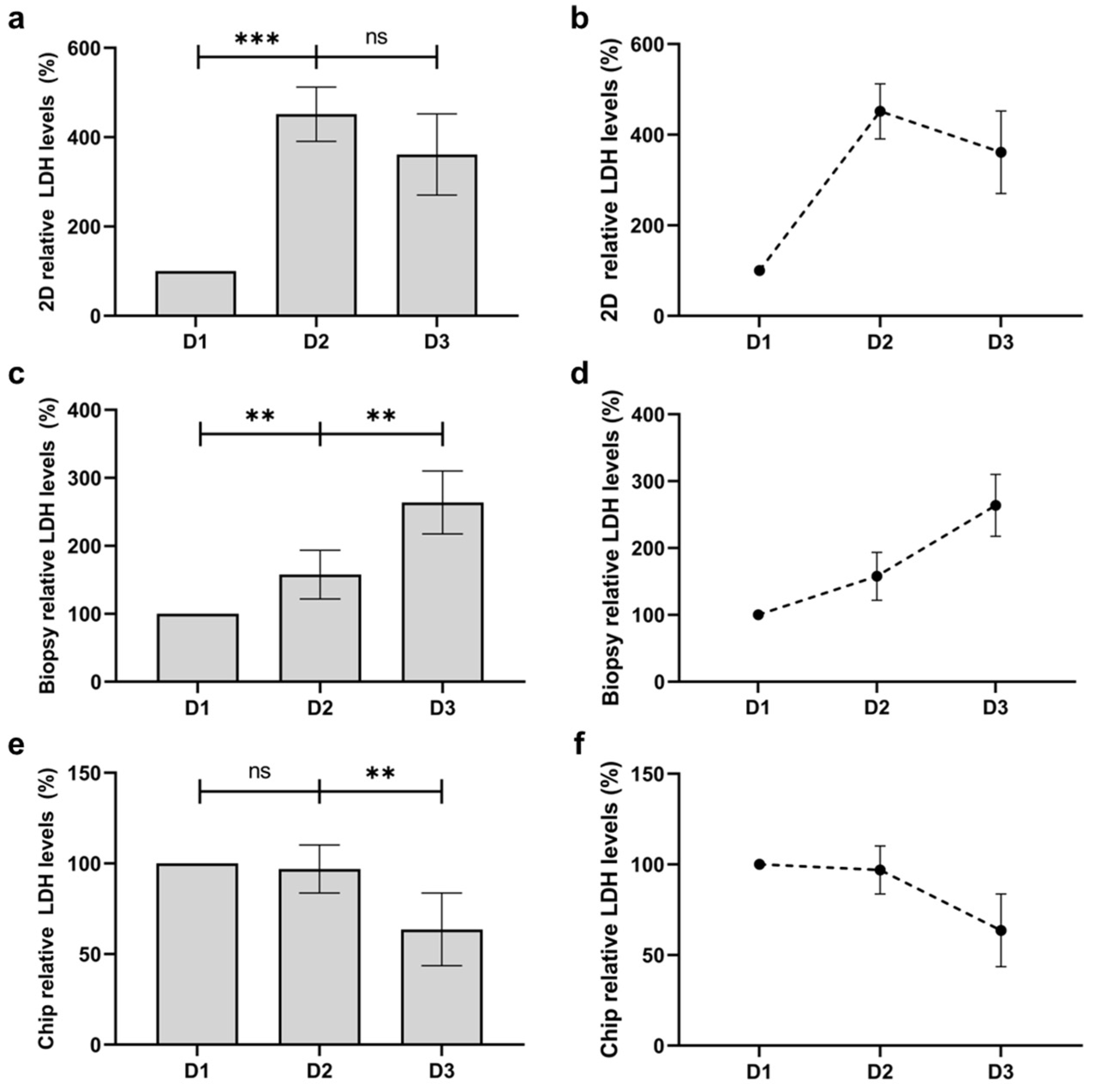
3.6. Quantification of Viability and Comparison with Human Skin Biopsy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.; Kligman, A. Barrier functions of human skin: A holistic view. Ski. Pharmacol. Physiol. 2009, 22, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Castaño, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.d.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Abaci, H.; Guo, Z.; Doucet, Y.; Jacków, J.; Christiano, A. Next generation human skin constructs as advanced tools for drug development. Exp. Biol. Med. 2017, 242, 1657–1668. [Google Scholar] [CrossRef]
- Randall, M.J.; Jüngel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the biofabrication of 3D skin in vitro: Healthy and pathological models. Front. Bioeng. Biotechnol. 2018, 6, 154. [Google Scholar] [CrossRef]
- Risueño, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Elston, D.M.; Stratman, E.J.; Miller, S.J. Skin biopsy: Biopsy issues in specific diseases. J. Am. Acad. Dermatol. 2016, 74, 1–16. [Google Scholar] [CrossRef]
- Zhang, Q.; Sito, L.; Mao, M.; He, J.; Zhang, Y.S.; Zhao, X. Current advances in skin-on-a-chip models for drug testing. Microphysiological Syst. 2018, 2, 4. [Google Scholar] [CrossRef]
- Cui, M.; Wiraja, C.; Zheng, M.; Singh, G.; Yong, K.T.; Xu, C. Recent Progress in Skin-on-a-Chip Platforms. Adv. Ther. 2022, 5, 2100138. [Google Scholar] [CrossRef]
- Ismayilzada, N.; Tarar, C.; Dabbagh, S.R.; Tokyay, B.K.; Dilmani, S.A.; Sokullu, E.; Abaci, H.E.; Tasoglu, S. Skin-on-a-chip technologies towards clinical translation and commercialization. Biofabrication 2024, 16, 042001. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.L.; Sachs, A.B. Skin biopsies for the measurement of clinical pharmacodynamic biomarkers. Curr. Opin. Biotechnol. 2005, 16, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Katz, S.A. Alternative Toxicological Methods; CRC Press: Boca Raton, FL, USA, 2003; pp. 229–247. [Google Scholar]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects—2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef]
- Almeida, A.; Sarmento, B.; Rodrigues, F. Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. Int. J. Pharm. 2017, 519, 178–185. [Google Scholar] [CrossRef]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef]
- Schmook, F.P.; Meingassner, J.G.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar] [CrossRef]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin Bioprinting: Impending Reality or Fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef]
- Derr, K.; Zou, J.; Luo, K.; Song, M.J.; Sittampalam, G.S.; Zhou, C.; Michael, S.; Ferrer, M.; Derr, P. Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function. Tissue Eng. Part C Methods 2019, 25, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.J.; Tan, Y.; Jia, J.; Yao, H.; Mei, Y. 3D Printing for Tissue Engineering. Isr. J. Chem. 2013, 53, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fukunaga-Kalabis, M.; Herlyn, M. The three-dimensional human skin reconstruct model: A tool to study normal skin and melanoma progression. J. Vis. Exp. 2011, 54, e2937. [Google Scholar] [CrossRef]
- Carlson, M.W.; Alt-Holland, A.; Egles, C.; Garlick, J.A. Three-Dimensional Tissue Models of Normal and Diseased Skin. Curr. Protoc. Cell Biol. 2008, 41, 19.9.1–19.9.17. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, W.-W.; Park, W.; Lee, J.-S.; Choi, M.-J.; Gao, Q.; Gao, G.; Cho, D.-W.; Kim, B.S. 3D biofabrication of diseased human skin models in vitro. Biomater. Res. 2023, 27, 80. [Google Scholar] [CrossRef]
- Di Cristo, L.; Sabella, S. Cell Cultures at the Air-Liquid Interface and Their Application in Cancer Research. Methods Mol. Biol. 2023, 2645, 41–64. [Google Scholar] [CrossRef]
- Lee, S.; Lim, J.; Yu, J.; Ahn, J.; Lee, Y.; Jeon, N.L. Engineering tumor vasculature on an injection-molded plastic array 3D culture (IMPACT) platform. Lab Chip 2019, 19, 2071–2080. [Google Scholar] [CrossRef]
- Ahn, J.; Lim, J.; Jusoh, N.; Lee, J.; Park, T.-E.; Kim, Y.; Kim, J.; Jeon, N.L. 3D Microfluidic Bone Tumor Microenvironment Comprised of Hydroxyapatite/Fibrin Composite. Front. Bioeng. Biotechnol. 2019, 7, 168. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Lee, S.; Jin, S.-P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 2017, 19, 22. [Google Scholar] [CrossRef]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Sung, J.H.; Sung, G.Y. Fabrication of a pumpless, microfluidic skin chip from different collagen sources. J. Ind. Eng. Chem. 2017, 56, 375–381. [Google Scholar] [CrossRef]
- Lim, J.; Rhee, S.; Choi, H.; Lee, J.; Kuttappan, S.; Nguyen, T.T.Y.; Choi, S.; Kim, Y.; Jeon, N.L. Engineering choroid plexus-on-a-chip with oscillatory flow for modeling brain metastasis. Mater. Today Bio 2023, 22, 100773. [Google Scholar] [CrossRef]
- Lee, J.; Jung, S.; Hong, H.K.; Jo, H.; Rhee, S.; Jeong, Y.-L.; Ko, J.; Cho, Y.B.; Jeon, N.L. Vascularized tissue on mesh-assisted platform (VT-MAP): A novel approach for diverse organoid size culture and tailored cancer drug response analysis. Lab Chip 2024, 24, 2208–2223. [Google Scholar] [CrossRef]
- Lim, J.; Ching, H.; Yoon, J.-K.; Jeon, N.L.; Kim, Y. Microvascularized tumor organoids-on-chips: Advancing preclinical drug screening with pathophysiological relevance. Nano Converg. 2021, 8, 12. [Google Scholar] [CrossRef]
- Ramadan, Q.; Ting, F.C.W. In vitro micro-physiological immune-competent model of the human skin. Lab Chip 2016, 16, 1899–1908. [Google Scholar] [CrossRef]
- Biglari, S.; Le, T.Y.; Tan, R.P.; Wise, S.G.; Zambon, A.; Codolo, G.; De Bernard, M.; Warkiani, M.; Schindeler, A.; Naficy, S.; et al. Simulating Inflammation in a Wound Microenvironment Using a Dermal Wound-on-a-Chip Model. Adv. Healthc. Mater. 2019, 8, 1801307. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Getschman, A.E.; Hwang, S.; Volkman, B.F.; Klonisch, T.; Levin, D.; Zhao, M.; Santos, S.; Liu, S.; Cheng, J.; et al. Investigations on T cell transmigration in a human skin-on-chip (SoC) model. Lab Chip 2021, 21, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Vahav, I.; Broek, L.J.; Thon, M.; Monsuur, H.N.; Spiekstra, S.W.; Atac, B.; Scheper, R.J.; Lauster, R.; Lindner, G.; Marx, U.; et al. Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J. Tissue Eng. Regen. Med. 2020, 14, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Abaci, H.E.; Guo, Z.; Coffman, A.; Gillette, B.; Lee, W.; Sia, S.K.; Christiano, A.M. Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv. Health Mater. 2016, 5, 1800–1807. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.-W. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef]
- Marino, D.; Luginbühl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med. 2014, 6, 221ra14. [Google Scholar] [CrossRef]
- Salameh, S.; Tissot, N.; Cache, K.; Lima, J.; Suzuki, I.; Marinho, P.A.; Rielland, M.; Soeur, J.; Takeuchi, S.; Germain, S.; et al. A perfusable vascularized full-thickness skin model for potential topical and systemic applications. Biofabrication 2021, 13, 035042. [Google Scholar] [CrossRef]
- O’connor, C.; Brady, E.; Zheng, Y.; Moore, E.; Stevens, K.R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 2022, 7, 702–716. [Google Scholar] [CrossRef]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Yu, J.; Lim, J.; Choi, M.; Chung, M.; Jeon, N.L. From microchannels to microphysiological systems: Development of application specific devices. Microelectron. Eng. 2018, 202, 9–18. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. American National Standards Institute: Washington, DC, USA, 2018.
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Morizane, S.; Takiguchi, T.; Iwatsuki, K. Dexamethasone but not tacrolimus suppresses TNF-α-induced thymic stromal lymphopoietin expression in lesional keratinocytes of atopic dermatitis model. J. Dermatol. Sci. 2015, 80, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Machuca, C.; Mendoza-Milla, C.; Córdova, E.; Mejía, S.; Covarrubias, L.; Ventura, J.; Zentella, A. Dexamethasone protection from TNF-alpha-induced cell death in MCF-7 cells requires NF-kappaB and is independent from AKT. BMC Cell Biol. 2006, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Thorlacius, H. Dexamethasone inhibits tumor necrosis factor-alpha-induced expression of macrophage inflammatory protein-2 and adhesion of neutrophils to endothelial cells. Biochem. Biophys. Res. Commun. 2000, 271, 364–367. [Google Scholar] [CrossRef]
- Joyce, D.A.; Kloda, A.; Steer, J.H. Dexamethasone suppresses release of soluble TNF receptors by human monocytes concurrently with TNF-α suppression. Immunol. Cell Biol. 1997, 75, 345–350. [Google Scholar] [CrossRef]
- Uings, I.; Puxeddu, I.; Temkin, V.; Smith, S.J.; Fattah, D.; Ray, K.P.; Levi-Schaffer, F. Effects of dexamethasone on TNF-alpha-induced release of cytokines from purified human blood eosinophils. Clin. Mol. Allergy 2005, 3, 5. [Google Scholar] [CrossRef]
- Gray, R.G.F.; Ryan, D.; Green, A. The cryopreservation of skin biopsies—A technique for reducing workload in a cell culture laboratory. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 1995, 32 Pt 2, 190–192. [Google Scholar] [CrossRef]
- Nault, A.; Zhang, C.; Kim, K.; Saha, S.; Bennett, D.D.; Xu, Y.G. Biopsy Use in Skin Cancer Diagnosis: Comparing Dermatology Physicians and Advanced Practice Professionals. JAMA Dermatol. 2015, 151, 899–902. [Google Scholar] [CrossRef]
- Apaydin, R.A.; Gürbüz, Y.M.; Bayramgürler, D.L.; Bi˙len, N. Cytokeratin contents of basal cell carcinoma, epidermis overlying tumour, and associated stromal amyloidosis: An immunohistochemical study. Amyloid 2005, 12, 41–47. [Google Scholar] [CrossRef]
- Feru, J.; Delobbe, E.; Ramont, L.; Brassart, B.; Terryn, C.; Dupont-Deshorgue, A.; Garbar, C.; Monboisse, J.-C.; Maquart, F.-X.; Brassart-Pasco, S. Aging decreases collagen IV expression in vivo in the dermo-epidermal junction and in vitro in dermal fibroblasts: Possible involvement of TGF-β1. Eur. J. Dermatol. 2016, 26, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E., Jr.; Munderloh, N. Human skin collagen. Presence of type I and type III at all levels of the dermis. J. Biol. Chem. 1978, 253, 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M. Involucrin and other markers of keratinocyte terminal differentiation. J. Investig. Dermatol. 1983, 81, S100–S103. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhee, S.; Xia, C.; Chandra, A.; Hamon, M.; Lee, G.; Yang, C.; Guo, Z.; Sun, B. Full-Thickness Perfused Skin-on-a-Chip with In Vivo-Like Drug Response for Drug and Cosmetics Testing. Bioengineering 2024, 11, 1055. https://doi.org/10.3390/bioengineering11111055
Rhee S, Xia C, Chandra A, Hamon M, Lee G, Yang C, Guo Z, Sun B. Full-Thickness Perfused Skin-on-a-Chip with In Vivo-Like Drug Response for Drug and Cosmetics Testing. Bioengineering. 2024; 11(11):1055. https://doi.org/10.3390/bioengineering11111055
Chicago/Turabian StyleRhee, Stephen, Chunguang Xia, Aditya Chandra, Morgan Hamon, Geonhui Lee, Chen Yang, Zaixun Guo, and Bingjie Sun. 2024. "Full-Thickness Perfused Skin-on-a-Chip with In Vivo-Like Drug Response for Drug and Cosmetics Testing" Bioengineering 11, no. 11: 1055. https://doi.org/10.3390/bioengineering11111055
APA StyleRhee, S., Xia, C., Chandra, A., Hamon, M., Lee, G., Yang, C., Guo, Z., & Sun, B. (2024). Full-Thickness Perfused Skin-on-a-Chip with In Vivo-Like Drug Response for Drug and Cosmetics Testing. Bioengineering, 11(11), 1055. https://doi.org/10.3390/bioengineering11111055





