Updated Review of Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications
Abstract
1. Introduction
2. Plant-Mediated Synthesis of Metallic Nanoparticles
| Plant Species | Used Part | NPs | NP Size (nm) | UV Absorption (nm) | Activity | References |
|---|---|---|---|---|---|---|
| Abutilon indicum | Leaves | MnO | 80 ± 0.5 | 380 and 460 | Antibacterial and anticancer | [27] |
| Abutilon indicum | Leaves | CrO | 17–42 | 280 and 415 | Antibacterial, anticancer, and antioxidant | [28] |
| Achillea millefolium | Leaves | Ag | 14–18 | 400–700 | Antibacterial and antioxidant | [31] |
| Achillea wilhelmsii | Leaves | Au | 2.7–38.7 | 540 | Antibacterial, antioxidant and electrocatalytic activity | [32] |
| Aerva tomentosa | Root | Ag | - | 443 | Antibacterial and antioxidant | [33] |
| Aesculus hippocastanum | Leaves | Ag | 50 ± 5 | 420–470 | Antibacterial and antioxidant | [34] |
| Ajuga bractosa | - | Ag | 400 | - | Antibacterial | [35] |
| Albizia lebbeck | Stem bark | ZnO | 66.25, 82.52, and 112.87 | 300–800 | Antimicrobial and antioxidant | [36] |
| Alcornea laxiflora | Leaves | Ag | 20–52 | 424–435 | Antibacterial, photocatalytic degradation, and tyrosinase inhibition | [37] |
| Allium ampeloprasum | Aerial parts | Ag | 80–50 | 300–800 | Antioxidant and antibacterial | [38] |
| Atalantia monophyla | Leaves | Ag | 8.3 | 396 | Antimicrobial and antioxidant | [39] |
| Atropa acuminata | Leaves | Ag | 5–20 | 428 | Antioxidant, anti-inflammatory, anticancer, and larvicidal activities | [40] |
| Azadirachta indicia | - | ZnO | 19.57 ± 1.5 | - | Antioxidant, antibacterial, and enzyme inhibitor | [41] |
| Bauhinia purpurea | Leaves | Ag and Au | - | 430 and 560 | Anticancer, antioxidant, antimicrobial, and catalytic agents | [42] |
| Bidens Pilosa | Leaf, stem, and root | Ag | 17 | - | Antimicrobial and anticancer | [43] |
| Brassica oleracae | Leaves | SnO2 NPs | 3.62–6.34 | - | Dye degradation activity | [44] |
| Brassica pekinensis | Leaves | Au | - | Antioxidant and antimicrobial | [45] | |
| Callistemon viminalis | Bark | Ag | 55 | 400–435 | Antioxidant, antibacterial, and catalytic | [46] |
| Cannabis sativa | Leaves | Ag | 69 | 435 | Antibacterial | [47] |
| Cayratia pedate | Leaves | ZnO | 52.24 | 320 | Enzyme immobilization | [17] |
| Chlorophytum borivilianum | Leaves | Ag | 52 | 450 | Antimicrobial | [48] |
| Chromolaena odorata | Leaves | Ag | 27.82–32.89 | 435 | Antibacterial | [49] |
| Cinnamomum tamala | Leaves | FeO | 26–35 | 300–800 | Wastewater treatment | [50] |
| Cinnamomum zelanicum | Leaves | Cu | 19.55–69.70 | - | Antioxidant and anticancer | [51] |
| Citrullus colocynthis | Leaves | ZnO | 50–60 | 374 | Anticancer | [52] |
| Clinacanthus nutans | Leaves and stem | Ag | - | 600 | Antioxidant and antimicrobial | [53] |
| Coptis chinensis | Leaves | Ag | 6–45 | 450 | Antibacterial and anticancer | [54] |
| Coriandrum sativum | Leaves | Au | 32.96 ± 5.2 | 540–550 | Antioxidant and analgesic activity | [55] |
| Costus igneus | Leaves | ZnO | 26.55 | 365 | Antidiabetic, antioxidant, antibacterial, and antibiofilm | [56] |
| Curcuma wenyujin | Herbal | Au | 100 nm | 530 | Anticancer | [57] |
| Derris trifoliata | Seeds | Ag | 16.05 ± 5.0 | 360 | Antioxidant, antibacterial, and antiproliferative activity | [58] |
| Dodonaea viscosa | Leaves | Ag | 20–100 | 441–564 | Antibacterial and anticancer | [59] |
| Emblica Phyllanthus | Leaves | Ag | 30–65 | 425 | Antidiabetic and hypolipidemic | [60] |
| Eryngium planum | Leaves | Ag/FeO | 60 | 450 | Noncorrosive heterogeneous catalysts | [61] |
| Euphorbia tirucalli | Arial parts | Mg andCoO | 100 nm–1 µm | 305 and 508 | Antiproliferative agents for cancer | [62] |
| Fagonia cretica | - | Ag | 11–15 | 440 | Antimicrobial | [63] |
| Ganoderma lucidum | Fruit bodies | Ag | 10.72 | 409 | Anticancer | [64] |
| Garcinia Kola | Leaves | Ag | 28.8 | 425.18 | Antibacterial | [65] |
| Gelidium pusillum | - | Au | 7–17 | 529 | Anticancer | [66] |
| Gracilaria crassa | Leaves | Au | 32.0 nm ± 4.0 nm (mean ± S EM) | - | Ecotoxicological potential | [67] |
| Hibiscus cannabinus | Leaves | Ag | 9 | 446 | Antimicrobial | [68] |
| Hylotelephium telephium | Flowers | CuO and ZnO | 83 and 36 | - | Antioxidant and antibacterial | [69] |
| Jatropha curcas | Crude latex | FeO | 20–42 | 300–800 | Wastewater treatment | [51] |
| Juniperus procera | Leaves | Ag | 23 | 424 | Antimicrobial | [70] |
| Lonicera japonica | Flowers | Au | 10–40 | 530–580 | Anticancer | [71] |
| Lythrum salicaria | Leaves | Ag | 20–138 | 395–415 | Antimicrobial, anticancer, and catalytic degradation | [72] |
| Melia azedarach | Leaves | Ag | 14–20 | 420 | Antibacterial, wound healing, antidiabetic, and antioxidant | [73] |
| Mentha pulegium | Leaves | ZnO | 40 | 370 | Antibacterial | [74] |
| Mimusops elengi | Fruits | Ag | 43 | 431 | Antibiofilm, antibacterial, and anticancer | [75] |
| Moringa oleifera | Seeds | Ag | 17.6 | 421 | Wound dressing | [30] |
| Moringa oleifera | Peel | GdO | 26 ± 2 | 280–300 | Antifungal, nontoxic, and photocatalyst | [29] |
| Moringa Oleifera | Leaves | FeO | 18–20 | 668 | Drug delivery | [76] |
| Myristica fragrans | Fruits | ZnO | 66 | 200–700 | Antibacterial | [77] |
| Nymphaea alba | Root | Au | 32–280 | 200 and 300 | Antibacterial and anticancer | [78] |
| Ocimum Americanum | - | ZnO | 21 | 316 | Antioxidant and antibacterial | [79] |
| Ocimum basilicum | Leaves | ZnO | 10–25 | 370 | Antibacterial | [80] |
| Ougeinia oojeinensis | Leaves | Ag | 5–100 | 450–500 | Antioxidant and antimicrobial | [81] |
| P. austroarabica | Leaves | Ag | 16.8 ± 5.4 | - | Catalytic efficacy and antioxidant | [82] |
| Panax ginseng | Roots | Zn | 59.76 nm | 340 | - | [83] |
| Phyllanthus acidus | Leaves | ZnO | 27.14–35.7 | 375 | Anticancer and antioxidant | [84] |
| Picrasma quassioides | Leaves | Ag | 5–40 nm | 412 | Radio sensitizing | [85] |
| Pimpinella anisum | Seeds | Ag-Au | 16–48 and 15 | 428 and 544 | Antioxidant and antimicrobial | [86] |
| Pithecellobium dulce | Peel | ZnO | 30 | 250–300 | Antifungal and photocatalytic activity | [87] |
| Polyalthia longifolia | - | Ag | 45 | 443 | Antiamoebic | [88] |
| Prosopis juliflora | Leaves | Ag | 10–20 | 420 | Wound healing and degradation | [89] |
| Psidium guajava | Leaves | Ag | 5.88 | 425 | Antibacterial and antioxidant | [90] |
| Raphanus sativa | Roots | ZnO | 15–25 | 372 | Wound dressing for diabetic foot ulcers | [91] |
| Rhodiola rosea | Rhizome | Ag | 10 | 437 | Antioxidant and catalytic reduction | [92,93] |
| Rhus javanica | Bark | Ag | 67 | 400–435 | Antioxidant, antibacterial, and catalytic | [46] |
| Ricinus communis | Seeds | ZnO | 10–30 | - | Antioxidant, antifungal, and anticancer | [94] |
| Rubia cordifolia | Leaves and roots | ZnO | 257.1 ± 0.76 | 285 | Antimicrobial and antioxidant | [95] |
| Rumex hastatus | Bark | Ag | 61 | 400–435 | Antioxidant, antibacterial, and catalytic | [46] |
| Sauropus androgynus | Leaves | ZnO | 12–23 | 373 | Antineoplastic agent | [96] |
| Scoparia dulcis | Leaves | Ag | 3–18 | 420 | Antimicrobial | [97] |
| Scutellaria barbata | - | Au | 400–1000 | 525 | Anticancer | [98] |
| Senna alata | Leaves | Ag | 25 | 434 | Antibacterial | [99] |
| Senna auriculata | Flowers | FeO | 160–300 | 300 and 310 | Antibacterial | [100] |
| Sida acuta | Leaves | ZnO | 32.82 | 373 | Antioxidant and photocatalytic activity | [101] |
| Silybum marianum | Seeds | Ag | 13.20 | 448 | Antioxidants | [102] |
| Sonchus asper | Leaves | TiO2 | 9–22 | - | Antimicrobial | [103] |
| Tabernaemontana heyneana | Leaves, stem, and callus | ZnO | 6.69 | 370–376 | Antioxidant, anti-inflammatory, antidiabetic, anticancer, and photocatalytic activities | [104] |
| Terminalia mantaly | Leaves | Ag | 11–83 | 350–700 | Antibacterial | [105] |
| Triphala churna | - | FeO | 29–74 | - | Anticancer and super paramagnetism | [106] |
| Vaccinium Arctostaphylos | Leaves | ZnO | 12.4 and 21 | 365 | Antidiabetic, antibacterial, and oxidative | [107] |
| Vallarai Chooranam | - | Ag | 43.1 | 432 | Antibacterials, antioxidant, larvicidal, anti-acetylcholinesterase, and anticancer | [108] |
| Withania coagulans | Leaves | Ag | 14 | 200–700 | Antimicrobial | [109] |
| Withania coagulans | Berries | FeO | 16 ± 2 and 18 ± 2 | 249 | Antimicrobial | [110] |
| Zea mays | Corn flour powder | Ag | - | 420 | Antioxidant | [111] |
| Zingiber officinale | Rhizome | Ag | 18.9–23.8 | 438–443 | Antibacterial | [112] |
3. Microbe-Mediated Synthesis of Metallic Nanoparticles
| Organism | Mode of Synthesis | NPs | NPs Size | UV Abs. | Ref. |
|---|---|---|---|---|---|
| Fungi | |||||
| Agaricus bisporus | Extracellular | Cu-NPs | 2–10 nm | 551 nm | [124] |
| Aspergillus terreus | Extracellular | Ag-Cu NPs | 20–30 nm | 517 nm | [125] |
| A. Niger | Extracellular | AgNPs | 10–100 nm | 430 nm | [126] |
| A. austroafricanus | Extracellular | AgNPs | 2–51.34 nm | 400 nm | [127] |
| Penicillium oxalicum | Extracellular | AgNPs | 60–80 nm | -- | [128] |
| P. oxalicum | Extracellular | CdO-NPs | 40–80 nm | 250–650 nm | [129] |
| P. duclauxii | Extracellular | AgNPs | 3–32 nm | 300–900 nm | [130] |
| P. oxalicum | Intracellular | CdO-NPs | 22.94 nm | 297 nm | [131] |
| Metarhizium robertsii | Extracellular | CuNPs | 15.67–62.56 nm | 670 nm | [132] |
| Cordyceps militaris | Extracellular | ZnO-NPs | 1.83 nm | 354 nm | [133] |
| Enoki mushroom | Extracellular | AgNPs | 10 nm | 435 nm | [134] |
| Flammulina velutipes | Extracellular | AgNPs | 21.4 nm | ---- | [135] |
| Ganoderma applanatum | Extracellular | AgNPs | 58.77 nm | 435 nm | [136] |
| Xylaria acuta | Extracellular | ZnO-NPs | 34–55 nm | 280–500 nm | [137] |
|
Pleurotus florida
(oyster mushroom) | Extracellular | Au-Pt NPs |
Au 17.96 nm
Pt 23.45 nm | 521 nm | [138] |
| P. sajor-caju | Extracellular | AuNPs AgNPs | Au 15–20 nm Ag 16–18 nm | Au 426 nm Ag 531 nm | [139] |
| Ganoderma lucidum (reishi mushroom) | Extracellular | AgNPs | 15–22 nm | 400–460 nm | [140] |
| A. sydowii | Extracellular | AgNPs | 1 and 24 nm | 420 nm | [141] |
| Talaromyces purpureogenus | Extracellular | AgNPs | 30–60 nm | 380–470 nm | [142] |
| P. djamor | Extracellular | TiO2-NPs | 31 nm | 345 nm | [143] |
| Streptomyces sp. | Extracellular | ZnO-NPs | 12–35 nm | 350, 400 nm | [144] |
| Cordyceps militaris | Extracellular | ZnO-NPs | 10.15 nm | 350 nm | [145] |
| Alternaria sp. | Extracellular | AgNPs | 3 and 10 nm. | 435 nm | [146] |
| Bacteria | |||||
| Bacillus megaterium | Extracellular | SeNPs | 45.9 nm | 200–900 nm | [147] |
| Proteus vulgaris | Extracellular | Iron oxide-NPs | 19.23–30.51 nm | 310 nm | [148] |
| Vibrio alginolyticus | Extracellular | AuNps | 100–150 nm. | 300–600 nm | [149] |
| Lactobacillus sp. (LCM5) | Extracellular | AgNPs | 3–35 nm | 420 nm | [150] |
| Enterococcus sp. (RMAA) | Intracellular | AuNPs | 5–10 nm | 360–660 nm | [151] |
| B. cereus (SZT1) | Extracellular | AgNPs | 18–39 nm | 418.99 nm | [152] |
| Pseudomonas poae | Extracellular | AgNPs | 19.8–44.9 nm | 422 nm | [153] |
| B. siamensis | Extracellular | AgNPs | 25–50 nm | 200–800 nm | [154] |
| Cuprividus sp. | Extracellular | AgNPs | 10–50 nm | 420 nm | [155] |
| B. cereus RNT6 | Extracellular | ZnONPs | 21–35 nm | 250–800 nm | [156] |
| Pseudomonas aeruginosa | Extracellular | ZnONPs | 6–21 nm | 200–600 nm. | [157] |
| Alkalibacillus sp. W7 | Extracellular | ZnONPs | 1–30 nm | 310 nm | [158] |
| Pseudomonas putida (MCC 2989) | Extracellular | ZnONPs | 25–45 nm | 200–800 nm | [159] |
| Paraclostridium benzoelyticum | Extracellular | ZnONPs | 50 nm | 300–800 nm | [160] |
| P. haeundaensis | Extracellular | AuNPs | 20.93 ± 3.46 nm | 535 nm | [161] |
| Algae | |||||
| Cladophora glomerata | Extracellular | ZnONPs | 14.39–37.85 nm | 290–360 nm | [162] |
| Kappaphycus alvarezii | Extracellular | ZnONPs | >100 nm | 300–700 nm | [163] |
| Ulva lactuca | Extracellular | ZnONPs | 12–17 nm | 310 nm | [164] |
3.1. Fungi
3.2. Algae
4. Natural Extract-Mediated Biosynthesis of Metallic NPs
4.1. Flavonoid
4.2. Terpenoids
4.3. Proteins
5. Factors Affecting the Biosynthesis of Metallic NPs
5.1. Light
5.2. Temperature and Heating Rate
5.3. pH
5.4. Time
5.5. Reactant Concentration
5.6. Reducing Agent Concentration
5.7. Capping Agents
5.8. Pressure
6. Encapsulation of Metallic Nanoparticles
7. Applications of Green Synthesized MNPs
7.1. Medical Applications of Metallic NPs
7.1.1. Antioxidant Properties
7.1.2. Anticancer Properties
7.1.3. Anti-Diabetic Properties
7.1.4. Regenerative Medicine
7.1.5. Drug Delivery
7.1.6. SARS-CoV-2
7.2. Environmental Applications
7.2.1. Biosensors
7.2.2. Wastewater Treatment and Catalytic Reduction
7.2.3. Soil Remediation
8. Conclusions
9. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rauta, P.R.; Mohanta, Y.K.; Nayak, D. Nanotechnology in Biology and Medicine: Research Advancements & Future Perspectives; CRC Press: Boca Raton, FL, USA, 2019; ISBN 0429535015. [Google Scholar]
- Khan, S.H. Green Nanotechnology for the Environment and Sustainable Development. In Green Materials for Wastewater Treatment; Springer: Cham, Switzerland, 2020; pp. 13–46. [Google Scholar]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanotechnology-Based Biomaterials for Orthopaedic Applications: Recent Advances and Future Prospects. Mater. Sci. Eng. C 2020, 106, 110154. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, C.O.; Olaniyan, O.T.; Anani, O.A.; Inobeme, A.; Ukhurebor, K.E.; Bodunrinde, R.E.; Adetunji, J.B.; Singh, K.R.B.; Nayak, V.; Palnam, W.D. Bionanomaterials for Green Bionanotechnology. In Bionanomaterials: Fundamentals and Biomedical Applications; IOP Publishing: Bristol, UK, 2021; pp. 10–11. [Google Scholar]
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase Engineering of Nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Badri, A.; Slimi, S.; Guergueb, M.; Kahri, H.; Mateos, X. Green Synthesis of Copper Oxide Nanoparticles Using Prickly Pear Peel Fruit Extract: Characterization and Catalytic Activity. Inorg. Chem. Commun. 2021, 134, 109027. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Y.; Yu, X.; Wang, C.; Wu, C.; Zou, Z. Understanding the Enhanced Catalytic Activity of High Entropy Alloys: From Theory to Experiment. J. Mater. Chem. A Mater. 2021, 9, 19410–19438. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus Interaction: Review on Cellular Processes Underlying Heavy Metal Detoxification and Synthesis of Metal Nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef]
- Yadav, V.K.; Khan, S.H.; Malik, P.; Thappa, A.; Suriyaprabha, R.; Ravi, R.K.; Choudhary, N.; Kalasariya, H.; Gnanamoorthy, G. Microbial Synthesis of Nanoparticles and Their Applications for Wastewater Treatment. In Microbial Biotechnology: Basic Research and Applications; Springer: Singapore, 2020; pp. 147–187. [Google Scholar]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Priya; Naveen; Kaur, K.; Sidhu, A.K. Green Synthesis: An Eco-Friendly Route for the Synthesis of Iron Oxide Nanoparticles. Front. Nanotechnol. 2021, 3, 655062. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]
- Hussain, A.; Oves, M.; Alajmi, M.F.; Hussain, I.; Amir, S.; Ahmed, J.; Rehman, M.T.; El-Seedi, H.R.; Ali, I. Biogenesis of ZnO Nanoparticles Using Pandanus Odorifer Leaf Extract: Anticancer and Antimicrobial Activities. RSC Adv. 2019, 9, 15357–15369. [Google Scholar] [CrossRef]
- Yosri, N.; Khalifa, S.A.M.; Guo, Z.; Xu, B.; Zou, X.; El-Seedi, H.R. Marine Organisms: Pioneer Natural Sources of Polysaccharides/Proteins for Green Synthesis of Nanoparticles and Their Potential Applications. Int. J. Biol. Macromol. 2021, 193, 1767–1798. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.F.; Abd El-Monaem, E.M.; El-Aqapa, H.G.; Elashery, S.E.A.; Eltaweil, A.S.; El Kady, M.; Khalifa, S.A.M.; Hawash, H.B.; El-Seedi, H.R. Iron Oxide Nanoparticles and Their Pharmaceutical Applications. Appl. Surf. Sci. Adv. 2022, 11, 100284. [Google Scholar] [CrossRef]
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Cayratia Pedata Leaf Extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef] [PubMed]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G.; Younesi, H. Facile and Green Synthesis of Cobalt Oxide Nanoparticles Using Ethanolic Extract of Trigonella Foenumgraceum (Fenugreek) Leaves. Adv. Powder Technol. 2020, 31, 3562–3569. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green Approaches for the Synthesis of Metal and Metal Oxide Nanoparticles Using Microbial and Plant Extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-Extract-Mediated Synthesis of Metal Nanoparticles. J. Chem. 2021, 2021, 6562687. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Shafey, A.M. El Green Synthesis of Metal and Metal Oxide Nanoparticles from Plant Leaf Extracts and Their Applications: A Review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; Rangabhashiyam, S.; Saini, A.; Saini, R.V.; Van Le, Q.; Nadda, A.K.; Le, T.-T. Sustainable and Green Trends in Using Plant Extracts for the Synthesis of Biogenic Metal Nanoparticles toward Environmental and Pharmaceutical Advances: A Review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef]
- Aslam, M.; Abdullah, A.Z.; Rafatullah, M. Recent Development in the Green Synthesis of Titanium Dioxide Nanoparticles Using Plant-Based Biomolecules for Environmental and Antimicrobial Applications. J. Ind. Eng. Chem. 2021, 98, 1–16. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic Nanoparticles: A Comprehensive Perspective in Synthesis, Characterization, Application and Its Challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Shahid, B.; Fatima, U.; Abbasi, S.A. Green Synthesis of MnO Nanoparticles Using Abutilon Indicum Leaf Extract for Biological, Photocatalytic, and Adsorption Activities. Biomolecules 2020, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Hanif, S.; Almoallim, H.S.; Alharbi, S.A.; Sellami, H. Green Synthesis of Chromium Oxide Nanoparticles for Antibacterial, Antioxidant Anticancer, and Biocompatibility Activities. Int. J. Mol. Sci. 2021, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.V.; Mohana Roopan, S.; Khan, M.R. Biogenic Approach to Synthesize Rod Shaped Gd2O3 Nanoparticles and Its Optimization Using Response Surface Methodology-Box–Behnken Design Model. Biotechnol. Prog. 2019, 35, e2823. [Google Scholar] [CrossRef]
- Mehwish, H.M.; Liu, G.; Rajoka, M.S.R.; Cai, H.; Zhong, J.; Song, X.; Xia, L.; Wang, M.; Aadil, R.M.; Inam-Ur-Raheem, M. Therapeutic Potential of Moringa Oleifera Seed Polysaccharide Embedded Silver Nanoparticles in Wound Healing. Int. J. Biol. Macromol. 2021, 184, 144–158. [Google Scholar] [CrossRef]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green Synthesis of Silver Nanoparticles and Their Applications as an Alternative Antibacterial and Antioxidant Agents. Mater. Sci. Eng. C 2020, 112, 110901. [Google Scholar] [CrossRef]
- Asariha, M.; Chahardoli, A.; Karimi, N.; Gholamhosseinpour, M.; Khoshroo, A.; Nemati, H.; Shokoohinia, Y.; Fattahi, A. Green Synthesis and Structural Characterization of Gold Nanoparticles from Achillea Wilhelmsii Leaf Infusion and in Vitro Evaluation. Bull. Mater. Sci. 2020, 43, 57. [Google Scholar] [CrossRef]
- Singh, S.; Kotiya, A.; Lodha, P.; Hassan, M. Green synthesis of silver nanoparticles using root extract of aerva tomentosa forsk. and analyse antibacterial and antioxidant property. Int. J. Pharm. Sci. Res. 2020, 11, 6456. [Google Scholar] [CrossRef]
- Küp, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of Silver Nanoparticles Using Leaf Extract of Aesculus Hippocastanum (Horse Chestnut): Evaluation of Their Antibacterial, Antioxidant and Drug Release System Activities. Mater. Sci. Eng. C 2020, 107, 110207. [Google Scholar] [CrossRef]
- Nazer, S.; Andleeb, S.; Ali, S.; Gulzar, N.; Iqbal, T.; Khan, M.A.R.; Raza, A. Synergistic Antibacterial Efficacy of Biogenic Synthesized Silver Nanoparticles Using Ajuga Bractosa with Standard Antibiotics: A Study against Bacterial Pathogens. Curr. Pharm. Biotechnol. 2020, 21, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of Zinc Oxide Nanoparticles Using Albizia Lebbeck Stem Bark, and Evaluation of Its Antimicrobial, Antioxidant, and Cytotoxic Activities on Human Breast Cancer Cell Lines. Int. J. Nanomed. 2019, 14, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Ekennia, A.C.; Uduagwu, D.N.; Nwaji, N.N.; Nwosa, C.C.; Olowu, O.J.; Nwanji, O.L.; Udu, D.A.; Christopher, S.U.; Andrew, T.A.; Nkwor, A.N. Facile Green Synthesis and Biological Evaluation of Biogenic Silver Nanoparticles Using Aqueous Extract of Alchornea laxiflora Leaf. Inorg. Nano-Metal. Chem. 2022, 52, 979–990. [Google Scholar] [CrossRef]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green Synthesized Silver Nanoparticle from Allium Ampeloprasum Aqueous Extract: Characterization, Antioxidant Activities, Antibacterial and Cytotoxicity Effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Verma, R.; Khan, A.B.; Amar, A.K.; Khan, M.I.K.; Sah, S. Opto-Structural Characteristics and Biomedical Applications of Microwave Irradiated Green Synthesised AM-AgNP from Atalantia monophylla (L.) Leaf Extract. ES Energy Environ. 2022, 17, 44–55. [Google Scholar] [CrossRef]
- Rajput, S.; Kumar, D.; Agrawal, V. Green Synthesis of Silver Nanoparticles Using Indian Belladonna Extract and Their Potential Antioxidant, Anti-Inflammatory, Anticancer and Larvicidal Activities. Plant Cell Rep. 2020, 39, 921–939. [Google Scholar] [CrossRef]
- Sohail, M.F.; Rehman, M.; Hussain, S.Z.; Huma, Z.-E.; Shahnaz, G.; Qureshi, O.S.; Khalid, Q.; Mirza, S.; Hussain, I.; Webster, T.J. Green Synthesis of Zinc Oxide Nanoparticles by Neem Extract as Multi-Facet Therapeutic Agents. J. Drug Deliv. Sci. Technol. 2020, 59, 101911. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Anticancer, Antimicrobial, Antioxidant, and Catalytic Activities of Green-Synthesized Silver and Gold Nanoparticles Using Bauhinia purpurea Leaf Extract. Bioprocess. Biosyst. Eng. 2019, 42, 305–319. [Google Scholar] [CrossRef]
- Mtambo, S.E.; Krishna, S.B.N.; Govender, P. Physico-Chemical, Antimicrobial and Anticancer Properties of Silver Nanoparticles Synthesised from Organ-Specific Extracts of Bidens pilosa L. South Afr. J. Bot. 2019, 126, 196–206. [Google Scholar] [CrossRef]
- Ahmad, W.; Pandey, A.; Rajput, V.; Kumar, V.; Verma, M.; Kim, H. Plant Extract Mediated Cost-Effective Tin Oxide Nanoparticles: A Review on Synthesis, Properties, and Potential Applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100211. [Google Scholar] [CrossRef]
- Aghamirzaei, M.; Khiabani, M.S.; Hamishehkar, H.; Mokarram, R.R.; Amjadi, M. Antioxidant, Antimicrobial and Cytotoxic Activities of Biosynthesized Gold Nanoparticles (AuNPs) from Chinese Lettuce (CL) Leave Extract (Brassica rapa Var. Pekinensis). Mater. Today Commun. 2021, 29, 102831. [Google Scholar] [CrossRef]
- Khan, W.; Khan, N.; Jamila, N.; Masood, R.; Minhaz, A.; Amin, F.; Atlas, A.; Nishan, U. Antioxidant, Antibacterial, and Catalytic Performance of Biosynthesized Silver Nanoparticles of Rhus javanica, Rumex hastatus, and Callistemon viminalis. Saudi J. Biol. Sci. 2022, 29, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Csakvari, A.C.; Moisa, C.; Radu, D.G.; Olariu, L.M.; Lupitu, A.I.; Panda, A.O.; Pop, G.; Chambre, D.; Socoliuc, V.; Copolovici, L. Green Synthesis, Characterization, and Antibacterial Properties of Silver Nanoparticles Obtained by Using Diverse Varieties of Cannabis sativa Leaf Extracts. Molecules 2021, 26, 4041. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Long, Y.; Liang, Q.; Purushotham, B.; Swamy, M.K.; Duan, Y. Safed Musli (Chlorophytum borivilianum L.) Callus-Mediated Biosynthesis of Silver Nanoparticles and Evaluation of Their Antimicrobial Activity and Cytotoxicity against Human Colon Cancer Cells. J. Nanomater. 2019, 2019, 2418785. [Google Scholar] [CrossRef]
- Mudasir, M.; Roto, R.; Wijaya, D.; Huda, I.; Sarwana, W.; Umam, K.; Yanuar, E. Preparation and Vibrio Sp Antibacterial Activity of Silver Nanoparticles Mediated by Chromolaena odorata Leaf Extract Using Different Temperatures. Asian J. Biol. 2022, 14, 25–37. [Google Scholar]
- Das, C.; Sen, S.; Singh, T.; Ghosh, T.; Paul, S.S.; Kim, T.W.; Jeon, S.; Maiti, D.K.; Im, J.; Biswas, G. Green Synthesis, Characterization and Application of Natural Product Coated Magnetite Nanoparticles for Wastewater Treatment. Nanomaterials 2020, 10, 1615. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Liu, J.; Nan, K.; Zhang, J.; Guo, L.; Liu, Y. Green Synthesis of Copper Nanoparticles Using Cinnamomum Zelanicum Extract and Its Applications as a Highly Efficient Antioxidant and Anti-Human Lung Carcinoma. J. Exp. Nanosci. 2021, 16, 410–423. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Obeid, M.A.; Bakshi, H.A.; Alshaer, W.; Ennab, R.M.; Al-Trad, B.; Al Khateeb, W.; Al-Batayneh, K.M.; Al-Kadash, A.; Alsotari, S. Synthesis, Characterization, and Assessment of Anti-Cancer Potential of ZnO Nanoparticles in an in Vitro Model of Breast Cancer. Molecules 2022, 27, 1827. [Google Scholar] [CrossRef]
- Chiu, H.I.; Che Mood, C.N.A.; Mohamad Zain, N.N.; Ramachandran, M.R.; Yahaya, N.; Nik Mohamed Kamal, N.N.S.; Tung, W.H.; Yong, Y.K.; Lee, C.K.; Lim, V. Biogenic Silver Nanoparticles of Clinacanthus Nutans as Antioxidant with Antimicrobial and Cytotoxic Effects. Bioinorg. Chem. Appl. 2021, 2021, 9920890. [Google Scholar] [CrossRef]
- Pei, J.; Fu, B.; Jiang, L.; Sun, T. Biosynthesis, Characterization, and Anticancer Effect of Plant-Mediated Silver Nanoparticles Using Coptis Chinensis. Int. J. Nanomed. 2019, 14, 1969–1978. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Chen, J. Biofabrication of AuNPs Using Coriandrum sativum Leaf Extract and Their Antioxidant, Analgesic Activity. Sci. Total Environ. 2021, 767, 144914. [Google Scholar] [CrossRef] [PubMed]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO Nanoparticles Using Insulin-Rich Leaf Extract: Anti-Diabetic, Antibiofilm and Anti-Oxidant Properties. J. Photochem. Photobiol. B 2019, 197, 111541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yu, J.; Liu, P.; Chang, J.; Ali, D.; Tian, X. Gold Nanoparticles Synthesized from Curcuma Wenyujin Inhibits HER-2/Neu Transcription in Breast Cancer Cells (MDA-MB-231/HER2). Arab. J. Chem. 2020, 13, 7264–7273. [Google Scholar] [CrossRef]
- Cyril, N.; George, J.B.; Joseph, L.; Raghavamenon, A.C.; Sylas, V.P. Assessment of Antioxidant, Antibacterial and Anti-Proliferative (Lung Cancer Cell Line A549) Activities of Green Synthesized Silver Nanoparticles from Derris trifoliata. Toxicol. Res. 2019, 8, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Gurumallesh Prabu, H. Green Synthesis of Anisotropic Silver Nanoparticles from the Aqueous Leaf Extract of Dodonaea viscosa with Their Antibacterial and Anticancer Activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.W.A.; Qureshi, M.T.; Hussain, Z.; Ullah, I.; Kalsoom, U.E.; Rahim, F.; Rahman, S.S.U.; Sultana, N.; Khan, M.K. Antidiabetic and Hypolipidemic Potential of Green AgNPs against Diabetic Mice. ACS Appl. Bio Mater. 2021, 4, 3433–3442. [Google Scholar] [CrossRef]
- Dehghan, Z.; Ranjbar, M.; Govahi, M.; Khakdan, F. Green Synthesis of Ag/Fe3O4 Nanocomposite Utilizing Eryngium planum L. Leaf Extract and Its Potential Applications in Medicine. J. Drug Deliv. Sci. Technol. 2022, 67, 102941. [Google Scholar] [CrossRef]
- Kgosiemang, I.K.; Lefojane, R.; Direko, P.; Madlanga, Z.; Mashele, S.; Sekhoacha, M. Green Synthesis of Magnesium and Cobalt Oxide Nanoparticles Using Euphorbia Tirucalli: Characterization and Potential Application for Breast Cancer Inhibition. Inorg. Nano-Metal. Chem. 2020, 50, 1070–1080. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Zafar, A.; Rasheed, M.N.; Ali, Z.; Mehmood, K.; Mazher, A.; Hasan, M.; Mahmood, N. Synthesis of Silver Nanoparticles Using: Fagonia Cretica and Their Antimicrobial Activities. Nanoscale Adv. 2019, 1, 1707–1713. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Synthesis of Biogenic Silver Nanoparticles with Eco-Friendly Processes Using Ganoderma lucidum Extract and Evaluation of Their Theranostic Applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Ademosun, O.T. Instrumental Characterization and Antibacterial Investigation of Silver Nanoparticles Synthesized from Garcinia Kola Leaf. J. Drug Deliv. Ther. 2019, 9, 58–64. [Google Scholar] [CrossRef]
- Jeyarani, S.; Vinita, N.M.; Puja, P.; Senthamilselvi, S.; Devan, U.; Velangani, A.J.; Biruntha, M.; Pugazhendhi, A.; Kumar, P. Biomimetic Gold Nanoparticles for Its Cytotoxicity and Biocompatibility Evidenced by Fluorescence-Based Assays in Cancer (MDA-MB-231) and Non-Cancerous (HEK-293) Cells. J. Photochem. Photobiol. B 2020, 202, 111715. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, C.; Karthi, S.; Reegan, A.D.; Balasubramani, G.; Ramkumar, G.; Kalaivani, K.; Zahir, A.A.; Deepak, P.; Senthil-Nathan, S.; Rahman, M.M. Green Synthesis of Gold Nanoparticles Using Gracilaria crassa Leaf Extract and Their Ecotoxicological Potential: Issues to Be Considered. Environ. Res. 2022, 213, 113711. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, M.R.; Umadevi, M. Synthesis of Monodispersed Silver Nanoparticles Using Hibiscus cannabinus Leaf Extract and Its Antimicrobial Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, G.; Jagathambal, M.; Kumar, G.S.; Kolesnikov, E. Hylotelephium Telephium Flower Extract-Mediated Biosynthesis of CuO and ZnO Nanoparticles with Promising Antioxidant and Antibacterial Properties for Healthcare Applications. Jom 2020, 72, 1264–1272. [Google Scholar] [CrossRef]
- Khan, M.; Karuppiah, P.; Alkhathlan, H.Z.; Kuniyil, M.; Khan, M.; Adil, S.F.; Shaik, M.R. Green Synthesis of Silver Nanoparticles Using Juniperus procera Extract: Their Characterization, and Biological Activity. Crystals 2022, 12, 420. [Google Scholar] [CrossRef]
- Patil, M.P.; Bayaraa, E.; Subedi, P.; Piad, L.L.A.; Tarte, N.H.; Kim, G.-D. Biogenic Synthesis, Characterization of Gold Nanoparticles Using Lonicera japonica and Their Anticancer Activity on HeLa Cells. J. Drug Deliv. Sci. Technol. 2019, 51, 83–90. [Google Scholar] [CrossRef]
- Srećković, N.Z.; Nedić, Z.P.; Liberti, D.; Monti, D.M.; Mihailović, N.R.; Katanić Stanković, J.S.; Dimitrijević, S.; Mihailović, V.B. Application Potential of Biogenically Synthesized Silver Nanoparticles Using: Lythrum salicaria L. Extracts as Pharmaceuticals and Catalysts for Organic Pollutant Degradation. RSC Adv. 2021, 11, 35585–35599. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of Silver Nanoparticles from Melia Azedarach: Enhancement of Antibacterial, Wound Healing, Antidiabetic and Antioxidant Activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from Leaf Extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Korkmaz, N.; Ceylan, Y.; Hamid, A.; Karadağ, A.; Bülbül, A.S.; Aftab, M.N.; Çevik, Ö.; Şen, F. Biogenic Silver Nanoparticles Synthesized via Mimusops elengi Fruit Extract, a Study on Antibiofilm, Antibacterial, and Anticancer Activities. J. Drug Deliv. Sci. Technol. 2020, 59, 101864. [Google Scholar] [CrossRef]
- Aisida, S.O.; Ugwu, K.; Akpa, P.A.; Nwanya, A.C.; Nwankwo, U.; Bashir, A.K.H.; Madiba, I.G.; Ahmed, I.; Ezema, F.I. Synthesis and Characterization of Iron Oxide Nanoparticles Capped with Moringa Oleifera: The Mechanisms of Formation Effects on the Optical, Structural, Magnetic and Morphological Properties. Mater. Today Proc. 2019, 36, 214–218. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica Fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Cudalbeanu, M.; Peitinho, D.; Silva, F.; Marques, R.; Pinheiro, T.; Ferreira, A.C.; Marques, F.; Paulo, A.; Soeiro, C.F.; Sousa, S.A. Sono-Biosynthesis and Characterization of AuNPs from Danube Delta Nymphaea Alba Root Extracts and Their Biological Properties. Nanomaterials 2021, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Narendra Kumar, H.K.; Chandra Mohana, N.; Nuthan, B.R.; Ramesha, K.P.; Rakshith, D.; Geetha, N.; Satish, S. Phyto-Mediated Synthesis of Zinc Oxide Nanoparticles Using Aqueous Plant Extract of Ocimum americanum and Evaluation of Its Bioactivity. SN Appl. Sci. 2019, 1, 651. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Farouk, W.M.; Gouda, S.M.; Safwat, A.; Hakim, T.A.; El-Shibiny, A. Utilization of Ocimum Basilicum Extracts for Zinc Oxide Nanoparticles Synthesis and Their Antibacterial Activity after a Novel Combination with Phage. Mater. Lett. 2022, 309, 131344. [Google Scholar] [CrossRef]
- Kumar, D.; Arora, S.; Danish, M. ScienceDirect Plant Based Synthesis of Silver Nanoparticles from Ougeinia oojeinensis Leaves Extract and Their Membrane Stabilizing, Antioxidant and Antimicrobial Activities. Mater. Today Proc. 2019, 17, 313–320. [Google Scholar] [CrossRef]
- Alahdal, F.A.M.; Qashqoosh, M.T.A.; Manea, Y.K.; Salem, M.A.S.; Khan, R.H.; Naqvi, S. Ultrafast Fluorescent Detection of Hexavalent Chromium Ions, Catalytic Efficacy and Antioxidant Activity of Green Synthesized Silver Nanoparticles Using Leaf Extract of P. Austroarabica. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100665. [Google Scholar] [CrossRef]
- Owaid, M.N.; Zaidan, T.A.; Muslim, R.F.; Hammood, M.A. Biosynthesis, Characterization and Cytotoxicity of Zinc Nanoparticles Using Panax Ginseng Roots, Araliaceae. Acta Pharm. Sci. 2019, 57, 19–32. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Ali Alharbi, S.; Joshi, D.; Lenin, H. Anticancer and Free Radical Scavenging Competence of Zinc Oxide Nanoparticles Synthesized by Aqueous Leaf Extract of Phyllanthus acidus. Bioinorg. Chem. Appl. 2022, 2022, 9493816. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A.; Fekete, G.; Singh, T. Cytotoxic and Radiosensitizing Potential of Silver Nanoparticles against HepG-2 Cells Prepared by Biosynthetic Route Using Picrasma quassioides Leaf Extract. J. Drug Deliv. Sci. Technol. 2020, 55, 101479. [Google Scholar] [CrossRef]
- Zayed, M.F.; Mahfoze, R.A.; El-kousy, S.M.; Al-Ashkar, E.A. In-Vitro Antioxidant and Antimicrobial Activities of Metal Nanoparticles Biosynthesized Using Optimized Pimpinella anisum Extract. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124167. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Gupta, N.; Kumar, A.; Singh, M. Green Synthesis, Characterization and Antifungal and Photocatalytic Activity of Pithecellobium dulce Peel–Mediated ZnO Nanoparticles. J. Phys. Chem. Solids 2019, 127, 43–51. [Google Scholar] [CrossRef]
- Anwar, A.; Ting, E.L.S.; Anwar, A.; Faizi, S.; Shah, M.R.; Khan, N.A.; Siddiqui, R. Antiamoebic Activity of Plant-Based Natural Products and Their Conjugated Silver Nanoparticles against Acanthamoeba castellanii (ATCC 50492). AMB Express 2020, 10, 24. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Sharma, N.; Gupta, N.; Kumar, A.; Chatterjee, S.; Nimesh, S. Catalytic, Antibacterial and Antibiofilm Efficacy of Biosynthesised Silver Nanoparticles Using Prosopis juliflora Leaf Extract along with Their Wound Healing Potential. J. Photochem. Photobiol. B 2019, 190, 50–58. [Google Scholar] [CrossRef]
- Eze, F.N.; Ovatlarnporn, C.; Nalinbenjapun, S.; Sripetthong, S. Ultra-Fast Sustainable Synthesis, Optimization and Characterization of Guava Phenolic Extract Functionalized Nanosilver with Enhanced Biomimetic Attributes. Arab. J. Chem. 2022, 15, 104167. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Yao, L.; Peng, X.; Li, Y.; Jiang, T.; Kuang, H. Synthesis of ZnO Nanoparticles Using Radish Root Extract for Effective Wound Dressing Agents for Diabetic Foot Ulcers in Nursing Care. J. Drug Deliv. Sci. Technol. 2020, 55, 101364. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of Copper Oxide Nanoparticles by Chemical and Biogenic Methods: Photocatalytic Degradation and in Vitro Antioxidant Activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Hu, D.; Yang, X.; Chen, W.; Feng, Z.; Hu, C.; Yan, F.; Chen, X.; Qu, D.; Chen, Z. Rhodiola Rosea Rhizome Extract-Mediated Green Synthesis of Silver Nanoparticles and Evaluation of Their Potential Antioxidant and Catalytic Reduction Activities. ACS Omega 2021, 6, 24450–24461. [Google Scholar] [CrossRef]
- Shobha, N.; Nanda, N.; Giresha, A.S.; Manjappa, P.; Sophiya, P.; Dharmappa, K.K.; Nagabhushana, B.M. Synthesis and Characterization of Zinc Oxide Nanoparticles Utilizing Seed Source of Ricinus communis and Study of Its Antioxidant, Antifungal and Anticancer Activity. Mater. Sci. Eng. C 2019, 97, 842–850. [Google Scholar] [CrossRef]
- Kaur, J.; Anwer, M.K.; Sartaj, A.; Panda, B.P.; Ali, A.; Zafar, A.; Kumar, V.; Gilani, S.J.; Kala, C.; Taleuzzaman, M. ZnO Nanoparticles of Rubia Cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 1450. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.N.; Nagaraja, G.K.; Prabhu, A.; Navada, K.M.; Kouser, S.; Manasa, D.J. Sauropus androgynus (L.) Leaf Phytochemical Activated Biocompatible Zinc Oxide Nanoparticles: An Antineoplastic Agent against Human Triple Negative Breast Cancer and a Potent Nanocatalyst for Dye Degradation. Appl. Surf. Sci. 2021, 552, 149429. [Google Scholar] [CrossRef]
- Parvataneni, R. Biogenic Synthesis and Characterization of Silver Nanoparticles Using Aqueous Leaf Extract of Scoparia dulcis L. and Assessment of Their Antimicrobial Property. Drug Chem. Toxicol. 2020, 43, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green Synthesis of Gold Nanoparticles from Scutellaria Barbata and Its Anticancer Activity in Pancreatic Cancer Cell (PANC-1). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Parthasarathy, V.; Kumar, P.S. Antimicrobial Activity and Cytotoxicity Effect of the Prepared Bioactive Ag-NPs Using Senna Alata Leaf Extract on MCF-7 Cancer Cell Line and Brine Shrimp. J. Solgel Sci. Technol. 2022, 103, 766–776. [Google Scholar] [CrossRef]
- Priya, D.D.; Rajesh, T.P.; Sundar, R.S.; Narendhar, C. Iron Oxide Nanoparticles–Characterization and Antimicrobial Studies. Int. J. Appl. Pharm. Sci. Res. 2021, 6, 27–32. [Google Scholar] [CrossRef]
- Ramesh, A.M.; Pal, K.; Kodandaram, A.; Manjula, B.L.; Ravishankar, D.K.; Gowtham, H.G.; Murali, M.; Rahdar, A.; Kyzas, G.Z. Antioxidant and Photocatalytic Properties of Zinc Oxide Nanoparticles Phyto-Fabricated Using the Aqueous Leaf Extract of Sida Acuta. Green Process. Synth. 2022, 11, 857–867. [Google Scholar] [CrossRef]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Synthesis of Bio-Mediated Silver Nanoparticles from Silybum marianum and Their Biological and Clinical Activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef]
- Babu, N.; Pathak, V.M.; Singh, A.; Navneet, A. Sonchus Asper Leaves Aqueous Extract Mediated Synthesis of Titanium Dioxide Nanoparticles. Pharma Innov. J. 2019, 8, 817–822. [Google Scholar]
- Manasa, D.J.; Chandrashekar, K.R.; Pavan Kumar, M.A.; Suresh, D.; Madhu Kumar, D.J.; Ravikumar, C.R.; Bhattacharya, T.; Ananda Murthy, H.C. Proficient Synthesis of Zinc Oxide Nanoparticles from Tabernaemontana heyneana Wall. via Green Combustion Method: Antioxidant, Anti-Inflammatory, Antidiabetic, Anticancer and Photocatalytic Activities. Results Chem. 2021, 3, 100178. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sibuyi, N.R.S.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced Anti-Bacterial Activity of Biogenic Silver Nanoparticles Synthesized from Terminalia Mantaly Extracts. Int. J. Nanomed. 2019, 14, 9031–9046. [Google Scholar] [CrossRef] [PubMed]
- Parmanik, A.; Bose, A.; Ghosh, B.; Paul, M.; Itoo, A.; Biswas, S.; Arakha, M. Development of Triphala Churna Extract Mediated Iron Oxide Nanoparticles as Novel Treatment Strategy for Triple Negative Breast Cancer. J. Drug Deliv. Sci. Technol. 2022, 76, 103735. [Google Scholar] [CrossRef]
- Bayrami, A.; Alioghli, S.; Rahim Pouran, S.; Habibi-Yangjeh, A.; Khataee, A.; Ramesh, S. A Facile Ultrasonic-Aided Biosynthesis of ZnO Nanoparticles Using Vaccinium arctostaphylos L. Leaf Extract and Its Antidiabetic, Antibacterial, and Oxidative Activity Evaluation. Ultrason. Sonochem 2019, 55, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaraj, S.; Suriyakala, G.; Gandhi, A.D.; Saranya, S.; Santhoshkumar, M.; Kavitha, P.; Babujanarthanam, R. Green Biosynthesis of Silver Nanoparticles Using Vallarai Chooranam and Their Potential Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4709–4719. [Google Scholar] [CrossRef]
- Tripathi, D.; Modi, A.; Narayan, G.; Rai, S.P. Green and Cost Effective Synthesis of Silver Nanoparticles from Endangered Medicinal Plant Withania coagulans and Their Potential Biomedical Properties. Mater. Sci. Eng. C 2019, 100, 152–164. [Google Scholar] [CrossRef]
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green Synthesis of Iron Oxide Nanorods Using Withania coagulans Extract Improved Photocatalytic Degradation and Antimicrobial Activity. J. Photochem. Photobiol. B 2020, 204, 111784. [Google Scholar] [CrossRef]
- Rajkumar, T.; Sapi, A.; Das, G.; Debnath, T.; Ansari, A.Z.; Patra, J.K. Biosynthesis of Silver Nanoparticle Using Extract of Zea mays (Corn Flour) and Investigation of Its Cytotoxicity Effect and Radical Scavenging Potential. J. Photochem. Photobiol. B 2019, 193, 1–7. [Google Scholar] [CrossRef]
- Mohapatra, B.; Kumar, D.; Sharma, N.; Mohapatra, S. Morphological, Plasmonic and Enhanced Antibacterial Properties of Ag Nanoparticles Prepared Using Zingiber Officinale Extract. J. Phys. Chem. Solids 2019, 126, 257–266. [Google Scholar] [CrossRef]
- Bachheti, R.K.; Abate, L.; Bachheti, A.; Madhusudhan, A.; Husen, A. Algae-, Fungi-, and Yeast-Mediated Biological Synthesis of Nanoparticles and Their Various Biomedical Applications. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 701–734. [Google Scholar]
- Zhang, Y.; Zhao, Q.; Chen, B. Reduction and Removal of Cr (VI) in Water Using Biosynthesized Palladium Nanoparticles Loaded Shewanella Oneidensis MR-1. Sci. Total Environ. 2022, 805, 150336. [Google Scholar] [CrossRef]
- Banik, A.; Vadivel, M.; Mondal, M.; Sakthivel, N. Molecular Mechanisms That Mediate Microbial Synthesis of Metal Nanoparticles. In Microbial Metabolism of Metals and Metalloids; Springer: Berlin/Heidelberg, Germany, 2022; pp. 135–166. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A Review on Biosynthesis of Metal Nanoparticles and Its Environmental Applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Zhang, C.; Zhang, D.; Yao, C.; Meng, Q.; Zhao, R.; Wei, Z. Speciation, Toxicity Mechanism and Remediation Ways of Heavy Metals during Composting: A Novel Theoretical Microbial Remediation Method Is Proposed. J. Environ. Manag. 2020, 272, 111109. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, C.M.; Paul, E.; Saagar, E.V.; Ali, P.P.M. Mycosynthesis of Zinc Oxide Nanoparticles Using Pleurotus Floridanus and Optimization of Process Parameters. Ceram. Int. 2021, 47, 12375–12380. [Google Scholar] [CrossRef]
- Manimaran, K.; Loganathan, S.; Prakash, D.G.; Natarajan, D.; Alasmary, F.A.; Karami, A.M.; Govindasamy, M. Mycosynthesis and Biochemical Characterization of Hypsizygusulmarius Derived ZnO Nanoparticles and Test Its Biomedical Applications. Biomass Convers. Biorefin 2022. [Google Scholar] [CrossRef]
- Gharieb, M.M.; Soliman, A.M.; Omara, M.S. Biosynthesis of Selenium Nanoparticles by Potential Endophytic Fungi Penicillium Citrinum and Rhizopus arrhizus: Characterization and Maximization. Biomass Convers. Biorefin 2023, 1–10. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.Y. Biosynthesis of Inorganic Nanomaterials Using Microbial Cells and Bacteriophages. Nat. Rev. Chem. 2020, 4, 638–656. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Salvadori, M.R.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Exploration of Microbial Factories for Synthesis of Nanoparticles—A Sustainable Approach for Bioremediation of Environmental Contaminants. Front. Microbiol. 2021, 12, 658294. [Google Scholar] [CrossRef]
- da Silva, C.A.; Ribeiro, B.M.; do Valle Trotta, C.; Perina, F.C.; Martins, R.; de Souza Abessa, D.M.; Barbieri, E.; Simões, M.F.; Ottoni, C.A. Effects of Mycogenic Silver Nanoparticles on Organisms of Different Trophic Levels. Chemosphere 2022, 308, 136540. [Google Scholar] [CrossRef]
- Sriramulu, M.; Shanmugam, S.; Ponnusamy, V.K. Agaricus Bisporus Mediated Biosynthesis of Copper Nanoparticles and Its Biological Effects: An in-Vitro Study. Colloids Interface Sci. Commun. 2020, 35, 100254. [Google Scholar] [CrossRef]
- Ameen, F. Optimization of the Synthesis of Fungus-Mediated Bi-Metallic Ag-Cu Nanoparticles. Appl. Sci. 2022, 12, 1384. [Google Scholar] [CrossRef]
- Al-Zubaidi, S.; Al-Ayafi, A.; Abdelkader, H. Biosynthesis, Characterization and Antifungal Activity of Silver Nanoparticles by Aspergillus Niger Isolate. J. Nanotechnol. Res. 2019, 1, 23–36. [Google Scholar] [CrossRef]
- Danagoudar, A.; Pratap, G.K.; Shantaram, M.; Ghosh, K.; Kanade, S.R.; Sannegowda, L.K.; Joshi, C.G. Antioxidant, Cytotoxic and Anti-Choline Esterase Activity of Green Silver Nanoparticles Synthesized Using Aspergillus austroafricanus CGJ-B3 (Endophytic Fungus). Anal. Chem. Lett. 2021, 11, 15–28. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal Mediated Synthesis of Silver Nanoparticles and Evaluation of Antibacterial Activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Azam, Z.; Ayaz, A.; Younas, M.; Qureshi, Z.; Arshad, B.; Zaman, W.; Ullah, F.; Nasar, M.Q.; Bahadur, S.; Irfan, M.M.; et al. Microbial Synthesized Cadmium Oxide Nanoparticles Induce Oxidative Stress and Protein Leakage in Bacterial Cells: Microbial Synthesis of Cadmium Oxide Nanoparticles. Microb. Pathog. 2020, 144, 104188. [Google Scholar] [CrossRef] [PubMed]
- Almaary, K.S.; Sayed, S.R.M.; Abd-Elkader, O.H.; Dawoud, T.M.; El Orabi, N.F.; Elgorban, A.M. Complete Green Synthesis of Silver-Nanoparticles Applying Seed-Borne Penicillium duclauxii. Saudi J. Biol. Sci. 2020, 27, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.; Habib, S.; Zaman, W.; Hussain, S.; Ali, H.; Saqib, S. Synthesis and Characterization of Microbial Mediated Cadmium Oxide Nanoparticles. Microsc. Res. Tech. 2020, 83, 1574–1584. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public. Health 2021, 18, 10536. [Google Scholar] [CrossRef]
- Dias, C.; Ayyanar, M.; Amalraj, S.; Khanal, P.; Subramaniyan, V.; Das, S.; Gandhale, P.; Biswa, V.; Ali, R.; Gurav, N. Biogenic Synthesis of Zinc Oxide Nanoparticles Using Mushroom Fungus Cordyceps Militaris: Characterization and Mechanistic Insights of Therapeutic Investigation. J. Drug Deliv. Sci. Technol. 2022, 73, 103444. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Starch/Agar-Based Functional Films Integrated with Enoki Mushroom-Mediated Silver Nanoparticles for Active Packaging Applications. Food Biosci. 2022, 49, 101867. [Google Scholar] [CrossRef]
- Faisal, S.; Khan, M.A.; Jan, H.; Shah, S.A.; Shah, S.; Rizwan, M.; Akbar, M.T. Edible Mushroom (Flammulina velutipes) as Biosource for Silver Nanoparticles: From Synthesis to Diverse Biomedical and Environmental Applications. Nanotechnology 2020, 32, 065101. [Google Scholar] [CrossRef]
- Dandapat, S.; Kumar, M.; Ranjan, R.; Sinha, M.P. Ganoderma applanatum Extract Mediated Synthesis of Silver Nanoparticles. Braz. J. Pharm. Sci. 2022, 58, e19173. [Google Scholar] [CrossRef]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria Acuta and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Bohara, R.A.; Rai, S.N.; Aleya, L.; Singh, M.P. Two Birds with One Stone: Oyster Mushroom Mediated Bimetallic Au-Pt Nanoparticles for Agro-Waste Management and Anticancer Activity. Sci. Pollut. Res. 2021, 28, 13761–13775. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Abd Ellah, N.H.; Bohara, R.A.; Rehan, I.F.; Marraiki, N.; Batiha, G.E.S.; Hetta, H.F.; Singh, M.P. Pleurotus Sajor-Caju-Mediated Synthesis of Silver and Gold Nanoparticles Active against Colon Cancer Cell Lines: A New Era of Herbonanoceutics. Molecules 2020, 25, 3091. [Google Scholar] [CrossRef] [PubMed]
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Şen, F. Synthesis and Characterization of Reishi Mushroom-Mediated Green Synthesis of Silver Nanoparticles for the Biochemical Applications. J. Pharm. Biomed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-Mediated Green Synthesis of Nano-Silver Using Aspergillus Sydowii and Its Antifungal/Antiproliferative Activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sagar, A.; Rana, J.; Rani, R. Green Synthesis of Silver Nanoparticles and Its Antibacterial Activity Using Fungus Talaromyces Purpureogenus Isolated from Taxus baccata Linn. Micro Nano Syst. Lett. 2022, 10, 2. [Google Scholar] [CrossRef]
- Manimaran, K.; Murugesan, S.; Ragavendran, C.; Balasubramani, G.; Natarajan, D.; Ganesan, A.; Seedevi, P. Biosynthesis of TiO2 Nanoparticles Using Edible Mushroom (Pleurotus djamor) Extract: Mosquito Larvicidal, Histopathological, Antibacterial and Anticancer Effect. J. Clust. Sci. 2021, 32, 1229–1240. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Mythili Gnanamangai, B.; Heela Prabha, T.; Poornima, S.; Maruthupandy, M.; Alharbi, N.S.; Kadaikunnan, S.; Li, W.J. Biosynthesized Zinc Oxide Nanoparticles (ZnO NPs) Using Actinomycetes Enhance the Anti-Bacterial Efficacy against K. Pneumoniae. J. King Saud. Univ. Sci. 2022, 34, 101731. [Google Scholar] [CrossRef]
- Li, J.F.; Rupa, E.J.; Hurh, J.; Huo, Y.; Chen, L.; Han, Y.; Ahn, J.c.; Park, J.K.; Lee, H.A.; Mathiyalagan, R.; et al. Cordyceps Militaris Fungus Mediated Zinc Oxide Nanoparticles for the Photocatalytic Degradation of Methylene Blue Dye. Optik 2019, 183, 691–697. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Fu, P.C. Fungus- (Alternaria Sp.) Mediated Silver Nanoparticles Synthesis, Characterization, and Screening of Antifungal Activity against Some Phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus Megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Danish, M.; Mohamad Ibrahim, M.N.; Sekeri, S.H.; Ansari, M.T.; Nanda, A.; Ahmad, G. Bacteria Mediated Synthesis of Iron Oxide Nanoparticles and Their Antibacterial, Antioxidant, Cytocompatibility Properties. J. Clust. Sci. 2021, 32, 1083–1094. [Google Scholar] [CrossRef]
- Shunmugam, R.; Renukadevi Balusamy, S.; Kumar, V.; Menon, S.; Lakshmi, T.; Perumalsamy, H. Biosynthesis of Gold Nanoparticles Using Marine Microbe (Vibrio alginolyticus) and Its Anticancer and Antioxidant Analysis. J. King Saud. Univ. Sci. 2021, 33, 101260. [Google Scholar] [CrossRef]
- Matei, A.; Matei, S.; Matei, G.M.; Cogąlniceanu, G.; Cornea, C.P. Biosynthesis of Silver Nanoparticles Mediated by Culture Filtrate of Lactic Acid Bacteria, Characterization and Antifungal Activity. Eurobiotech J. 2020, 4, 97–103. [Google Scholar] [CrossRef]
- Nandhini, J.T.; Ezhilarasan, D.; Rajeshkumar, S. An Ecofriendly Synthesized Gold Nanoparticles Induces Cytotoxicity via Apoptosis in HepG2 Cells. Environ. Toxicol. 2021, 36, 24–32. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C.; et al. Silver Nanoparticles Synthesized by Using Bacillus Cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of Silver Nanoparticles Using Endophytic Bacteria and Their Role in Inhibition of Rice Pathogenic Bacteria and Plant Growth Promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Ameen, F.; AlYahya, S.; Govarthanan, M.; ALjahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alshehri, W.A.; Alwakeel, S.S.; Alharbi, S.A. Soil Bacteria Cupriavidus Sp. Mediates the Extracellular Synthesis of Antibacterial Silver Nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Ahmed, T.; Wu, Z.; Jiang, H.; Luo, J.; Noman, M.; Shahid, M.; Manzoor, I.; Allemailem, K.S.; Alrumaihi, F.; Li, B. Bioinspired Green Synthesis of Zinc Oxide Nanoparticles from a Native Bacillus cereus Strain Rnt6: Characterization and Antibacterial Activity against Rice Panicle Blight Pathogens Burkholderia Glumae and B. Gladioli. Nanomaterials 2021, 11, 884. [Google Scholar] [CrossRef]
- Abdo, A.M.; Fouda, A.; Eid, A.M.; Fahmy, N.M.; Elsayed, A.M.; Khalil, A.M.A.; Alzahrani, O.M.; Ahmed, A.F.; Soliman, A.M. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and Their Activity against Pathogenic Microbes and Common House Mosquito, Culex pipiens. Materials 2021, 14, 6983. [Google Scholar] [CrossRef] [PubMed]
- Al-Kordy, H.M.H.; Sabry, S.A.; Mabrouk, M.E.M. Statistical Optimization of Experimental Parameters for Extracellular Synthesis of Zinc Oxide Nanoparticles by a Novel Haloalaliphilic Alkalibacillus sp.W7. Sci. Rep. 2021, 11, 10924. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, J.; Mani, G.; Krishnan, N.; Pernabas, J.; Devadoss, J.M.; Jang, H.T. Green Biogenic Synthesis of Zinc Oxide Nanoparticles Using Pseudomonas putida Culture and Its In Vitro Antibacterial and Anti-Biofilm Activity. Biocatal. Agric. Biotechnol. 2019, 21, 101327. [Google Scholar] [CrossRef]
- Faisal, S.; Abdullah; Rizwan, M.; Ullah, R.; Alotaibi, A.; Khattak, A.; Bibi, N.; Idrees, M. Paraclostridium Benzoelyticum Bacterium-Mediated Zinc Oxide Nanoparticles and Their In Vivo Multiple Biological Applications. Oxid. Med. Cell Longev. 2022, 2022, 5994033. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kang, M.j.; Niyonizigiye, I.; Singh, A.; Kim, J.O.; Seo, Y.B.; Kim, G. Do Extracellular Synthesis of Gold Nanoparticles Using the Marine Bacterium Paracoccus Haeundaensis BC74171T and Evaluation of Their Antioxidant Activity and Antiproliferative Effect on Normal and Cancer Cell Lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef]
- Abdulwahid, K.E.; Dwaish, A.S.; Dakhil, O.A. Green synthesis and characterization of zinc oxide nanoparticles from Cladophora glomerata and its antifungal activity against some fungal isolates. Plant Arch. 2019, 19, 3527–3532. [Google Scholar]
- Ramakrishnan, G.; Razack, S.A.; Ravi, L.; Sahadevan, R. Fabrication of Phyco-Functionalized Zinc Oxide Nanoparticles and Their in Vitro Evaluation against Bacteria and Cancer Cell Line. Indian. J. Biochem. Biophys. 2023, 60, 770–778. [Google Scholar] [CrossRef]
- Anjali, K.P.; Sangeetha, B.M.; Raghunathan, R.; Devi, G.; Dutta, S. Seaweed Mediated Fabrication of Zinc Oxide Nanoparticles and Their Antibacterial, Antifungal and Anticancer Applications. ChemistrySelect 2021, 6, 647–656. [Google Scholar] [CrossRef]
- Sonawane, H.; Shelke, D.; Chambhare, M.; Dixit, N.; Math, S.; Sen, S.; Borah, S.N.; Islam, N.F.; Joshi, S.J.; Yousaf, B. Fungi-Derived Agriculturally Important Nanoparticles and Their Application in Crop Stress Management–Prospects and Environmental Risks. Environ. Res. 2022, 212, 113543. [Google Scholar] [CrossRef]
- Mehrotra, R.S.; Aneja, K.R. An Introduction to Mycology; New Age International: Delhi, India, 1990; ISBN 8122400892. [Google Scholar]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Cellular, Genomic and Metabolic Complexity. Biol. Rev. 2020, 95, 1198–1232. [Google Scholar] [CrossRef]
- Segal-Kischinevzky, C.; Romero-Aguilar, L.; Alcaraz, L.D.; López-Ortiz, G.; Martínez-Castillo, B.; Torres-Ramírez, N.; Sandoval, G.; González, J. Yeasts Inhabiting Extreme Environments and Their Biotechnological Applications. Microorganisms 2022, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Akther, T.; Khan, M.S.; Hemalatha, S. Biosynthesis of Silver Nanoparticles via Fungal Cell Filtrate and Their Anti-Quorum Sensing against Pseudomonas aeruginosa. J. Environ. Chem. Eng. 2020, 8, 104365. [Google Scholar] [CrossRef]
- Kumar, R.V.; Vinoth, S.; Baskar, V.; Arun, M.; Gurusaravanan, P. Synthesis of Zinc Oxide Nanoparticles Mediated by Dictyota Dichotoma Endophytic Fungi and Its Photocatalytic Degradation of Fast Green Dye and Antibacterial Applications. South. Afr. J. Bot. 2022, 151, 337–344. [Google Scholar] [CrossRef]
- Ranjani, S.; Karunya, J.R.; Hemalatha, S. Differential Actions of Nanoparticles and Nanoemulsion Synthesized from Colletotrichum siamense on Food Borne Pathogen. LWT 2022, 155, 112995. [Google Scholar]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green Synthesis of Silver Nanoparticles with Algae and the Importance of Capping Agents in the Process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Li, S.-N.; Wang, R.; Ho, S.-H. Algae-Mediated Biosystems for Metallic Nanoparticle Production: From Synthetic Mechanisms to Aquatic Environmental Applications. J. Hazard. Mater. 2021, 420, 126625. [Google Scholar] [CrossRef]
- Yugay, Y.A.; Usoltseva, R.V.; Silant’ev, V.E.; Egorova, A.E.; Karabtsov, A.A.; Kumeiko, V.V.; Ermakova, S.P.; Bulgakov, V.P.; Shkryl, Y.N. Synthesis of Bioactive Silver Nanoparticles Using Alginate, Fucoidan and Laminaran from Brown Algae as a Reducing and Stabilizing Agent. Carbohydr. Polym. 2020, 245, 116547. [Google Scholar] [CrossRef]
- Al-Radadi, N.S.; Hussain, T.; Faisal, S.; Shah, S.A.R. Novel Biosynthesis, Characterization and Bio-Catalytic Potential of Green Algae (Spirogyra hyalina) Mediated Silver Nanomaterials. Saudi J. Biol. Sci. 2022, 29, 411–419. [Google Scholar]
- Doman, K.M.; Gharieb, M.M.; Abd El-Monem, A.M.; Morsi, H.H. Synthesis of Silver and Copper Nanoparticle Using Spirulina platensis and Evaluation of Their Anticancer Activity. Int. J. Environ. Health Res. 2024, 34, 661–673. [Google Scholar] [CrossRef]
- Khandelwal, M.; Choudhary, S.; Harish; Kumawat, A.; Misra, K.P.; Vyas, Y.; Singh, B.; Rathore, D.S.; Soni, K.; Bagaria, A. An Eco-Friendly Synthesis Approach for Enhanced Photocatalytic and Antibacterial Properties of Copper Oxide Nanoparticles Using Coelastrella Terrestris Algal Extract. Int. J. Nanomed. 2024, 19, 4137–4162. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaraj, G.; Vinosha, M.; Sangeetha, D.; Manikandakrishnan, M.; Palanisamy, S.; Sonaimuthu, M.; Manikandan, R.; You, S.; Prabhu, N.M. Bio-Directed Synthesis of Pt-Nanoparticles from Aqueous Extract of Red Algae Halymenia dilatata and Their Biomedical Applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126434. [Google Scholar] [CrossRef]
- Díaz, E.M.; Zamora, J.C.; Ruibal, C.; Divakar, P.K.; González-Benítez, N.; Le Devehat, F.; Chollet, M.; Ferron, S.; Sauvager, A.; Boustie, J. Axenic Culture and Biosynthesis of Secondary Compounds in Lichen Symbiotic Fungi, the Parmeliaceae. Symbiosis 2020, 82, 79–93. [Google Scholar] [CrossRef]
- Nallal, V.U.M.; Devi, K.N.; Razia, M. Biogenic Fabrication and Characterization of Silver Nanoparticles Using High Altitude Lichen Heteroderimia Leucomela Extract and Its Potential Applications. Mater. Today Proc. 2022, 50, 365–370. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and Applications of Flavonoid Metal Complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Jan, S.A.; Shinwari, Z.K.; Maaza, M. Bioinspired Synthesis of Pure Massicot Phase Lead Oxide Nanoparticles and Assessment of Their Biocompatibility, Cytotoxicity and in-Vitro Biological Properties. Arab. J. Chem. 2020, 13, 916–931. [Google Scholar] [CrossRef]
- Clichici, S.; David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Filip, M.; Nagy, A.; Luca, V.; Crivii, C.; Mircea, P.; et al. Hepatoprotective Effects of Silymarin Coated Gold Nanoparticles in Experimental Cholestasis. Mater. Sci. Eng. C 2020, 115, 111117. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Khan, K.A.; Ibrahim, E.H. Biological Activities of Euphorbia peplus Leaves Ethanolic Extract and the Extract Fabricated Gold Nanoparticles (AuNPs). Molecules 2019, 24, 1431. [Google Scholar] [CrossRef]
- Madivoli, E.S.; Kareru, P.G.; Maina, E.G.; Nyabola, A.O.; Wanakai, S.I.; Nyang’au, J.O. Biosynthesis of Iron Nanoparticles Using Ageratum Conyzoides Extracts, Their Antimicrobial and Photocatalytic Activity. SN Appl. Sci. 2019, 1, 500. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Al-Balakocy, N.G.; Hassan, M.M.; Elshafei, A.M. Biosynthesis and Characterization of Silver Nanoparticles Induced by Fungal Proteins and Its Application in Different Biological Activities. J. Genet. Eng. Biotechnol. 2019, 17, 8. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, Z.; Liu, R.; Li, H.; Zhang, Z.; Su, T.; Yang, J.; Liu, H. The Effect of Phospho-Peptide on the Stability of Gold Nanoparticles and Drug Delivery. J. Nanobiotechnology 2019, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.; Zafar, H.; Raza, F.; Sulaiman, M. Factors Influencing the Green Synthesis of Metallic Nanoparticles Using Plant Extracts: A Comprehensive Review. Pharm. Front. 2023, 5, e117–e131. [Google Scholar] [CrossRef]
- Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah, D.H.M.; Al Jaouni, S.K.; Salem, S.S. Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste. J. Funct. Biomater. 2022, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, M.M.; Zangeneh, A. Novel Green Synthesis of Hibiscus Sabdariffa Flower Extract Conjugated Gold Nanoparticles with Excellent Anti-Acute Myeloid Leukemia Effect in Comparison to Daunorubicin in a Leukemic Rodent Model. Appl. Organomet. Chem. 2020, 34, e5271. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Gupta, A.; Kim, B.S. Ultrasound Assisted Green Synthesis of Silver and Iron Oxide Nanoparticles Using Fenugreek Seed Extract and Their Enhanced Antibacterial and Antioxidant Activities. Biomed. Res. Int. 2019, 2019, 1714358. [Google Scholar] [CrossRef]
- Keerthiga, N.; Anitha, R.; Rajeshkumar, S.; Lakshmi, T. Antioxidant Activity of Cumin Oil Mediated Silver Nanoparticles. Pharmacogn. J. 2019, 11, 787–789. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Divya, M.; Vaseeharan, B.; Chen, J.; Biruntha, M.; Silva, L.P.; Durán-Lara, E.F.; Shreema, K.; Ranjan, S.; Dasgupta, N. Biological Compound Capping of Silver Nanoparticle with the Seed Extracts of Blackcumin (Nigella sativa): A Potential Antibacterial, Antidiabetic, Anti-Inflammatory, and Antioxidant. J. Inorg. Organomet. Polym. Mater. 2021, 31, 624–635. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pîrjol, T.; Muntean, D.; Danciu, C. Properties of Salvia offcinalis L. and Thymus serpyllum l. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.S.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of Biogenic Gold Nanoparticles from Terminalia Mantaly Extracts and the Evaluation of Their in Vitro Cytotoxic Effects in Cancer Cells. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Jagadeesan Sharavanan, V.; Karaiyagowder Govindarajan, D.; Meganathan, Y.; Devaraj, B.S.; Natesan, S.; Kothandan, R.; Kandaswamy, K. Green Synthesized Silver Nanoparticles Using Aqueous Leaf Extracts of Leucas aspera Exhibits Antimicrobial and Catalytic Dye Degradation Properties. SN Appl. Sci. 2019, 1, 208. [Google Scholar] [CrossRef]
- Das, S.K.; Behera, S.; Patra, J.K.; Thatoi, H. Green Synthesis of Sliver Nanoparticles Using Avicennia officinalis and Xylocarpus granatum Extracts and in Vitro Evaluation of Antioxidant, Antidiabetic and Anti-Inflammatory Activities. J. Clust. Sci. 2019, 30, 1103–1113. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of Green Synthesis of Metal Nanoparticles Using Algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant-Mediated Fabrication of Silver Nanoparticles, Process Optimization, and Impact on Tomato Plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C. Light Emitting Diode Irradiation Can Control the Morphology and Optical Properties of Silver Nanoparticles. J. Am. Chem. Soc. 2010, 132, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Prakash, S. Factors Affecting the Geometry of Silver Nanoparticles Synthesis in Chrysosporium tropicum and Fusarium oxysporum. Am. J. Nanotechnol. 2011, 2, 112–121. [Google Scholar]
- Singh, A.K.; Srivastava, O.N. One-Step Green Synthesis of Gold Nanoparticles Using Black Cardamom and Effect of PH on Its Synthesis. Nanoscale Res. Lett. 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Yazdani, S.; Daneshkhah, A.; Diwate, A.; Patel, H.; Smith, J.; Reul, O.; Cheng, R.; Izadian, A.; Hajrasouliha, A.R. Model for Gold Nanoparticle Synthesis: Effect of PH and Reaction Time. ACS Omega 2021, 6, 16847–16853. [Google Scholar] [CrossRef]
- Kim, H.; Seo, Y.S.; Kim, K.; Han, J.W.; Park, Y.; Cho, S. Concentration Effect of Reducing Agents on Green Synthesis of Gold Nanoparticles: Size, Morphology, and Growth Mechanism. Nanoscale Res. Lett. 2016, 11, 230. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.u.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnology 2020, 18, 172. [Google Scholar] [CrossRef]
- Gulati, S.; Sachdeva, M.; Bhasin, K.K. Capping Agents in Nanoparticle Synthesis: Surfactant and Solvent System. In Proceedings of the AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2018; Volume 1953. [Google Scholar]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A. Green Synthesis and Characterization of Gold Nanoparticles Using Endophytic Fungi Fusarium Solani and Its In-Vitro Anticancer and Biomedical Applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2015, 2014, 219. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2020, 121, 834–881. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Nobakht, V. Encapsulation of Metal Nanoparticles (MNPs) as Catalyst. In Nanocomposite Materials for Biomedical and Energy Storage Applications; IntechOpen: London, UK, 2022; ISBN 1803556196. [Google Scholar]
- Skóra, B.; Szychowski, K.A.; Gmiński, J. A Concise Review of Metallic Nanoparticles Encapsulation Methods and Their Potential Use in Anticancer Therapy and Medicine. Eur. J. Pharm. Biopharm. 2020, 154, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Singh, S.P. Drug Encapsulating Polysaccharide-loaded Metal Nanoparticles: A Perspective Drug Delivery System. Drug Dev. Res. 2021, 82, 145–148. [Google Scholar] [CrossRef]
- Chiozzi, V.; Rossi, F. Inorganic–Organic Core/Shell Nanoparticles: Progress and Applications. Nanoscale Adv. 2020, 2, 5090–5105. [Google Scholar] [CrossRef]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan Based-Nanoparticles and Nanocapsules: Overview, Physicochemical Features, Applications of a Nanofibrous Scaffold, and Bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, D.; Stenzel, M.H. Polymer-Functionalized Upconversion Nanoparticles for Light/Imaging-Guided Drug Delivery. Biomacromolecules 2021, 22, 3168–3201. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sousa, R.; Silva, C.J.R. Multifunctional and Smart Organic–Inorganic Hybrid Sol–Gel Coatings for Corrosion Protection Applications. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–97. [Google Scholar]
- Choi, I.; Lee, H.K.; Lee, G.W.; Kim, J.; Joo, J.B. Inorganic Shell Nanostructures to Enhance Performance and Stability of Metal Nanoparticles in Catalytic Applications. Rare Met. 2020, 39, 767–783. [Google Scholar] [CrossRef]
- Campani, V.; Giarra, S.; De Rosa, G. Lipid-Based Core-Shell Nanoparticles: Evolution and Potentialities in Drug Delivery. OpenNano 2018, 3, 5–17. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and Its Biomedical Applications in Health and Diseases: Special Focus on Drug Delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives. Adv. Healthc. Mater. 2019, 8, 1800939. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; De, G. Sol-Gel Synthesis of Metal Nanoparticle Incorporated Oxide Films on Glass. In Glass Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 145–163. [Google Scholar]
- Gittins, D.I.; Caruso, F. Tailoring the Polyelectrolyte Coating of Metal Nanoparticles. J. Phys. Chem. B 2001, 105, 6846–6852. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, M.; Zhao, M.; Xu, T.; Guo, M.; Wang, C.; Li, Y.; Zhu, B.; Liu, H. Doxorubicin-Loaded Functionalized Selenium Nanoparticles for Enhanced Antitumor Efficacy in Cervical Carcinoma Therapy. Mater. Sci. Eng. C 2020, 106, 110100. [Google Scholar] [CrossRef] [PubMed]
- Aboeita, N.M.; Fahmy, S.A.; El-Sayed, M.M.H.; Azzazy, H.M.E.S.; Shoeib, T. Enhanced Anticancer Activity of Nedaplatin Loaded onto Copper Nanoparticles Synthesized Using Red Algae. Pharmaceutics 2022, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, F.; Gholizadeh, M.; Saljooghi, A.S. Deferasirox Loaded on Fumed Silica Nanoparticles Used in Cancer Treatment. New J. Chem. 2016, 40, 2696–2703. [Google Scholar] [CrossRef]
- Hu, X.; Ahmeda, A.; Zangeneh, M.M. Chemical Characterization and Evaluation of Antimicrobial and Cutaneous Wound Healing Potentials of Gold Nanoparticles Using Allium saralicum RM Fritsch. Appl. Organomet. Chem. 2020, 34, e5484. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L. Antibacterial Activity and Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas Aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Aqeel, S.; Gupta, A.; Singh, L. A Review on Unknown Repercussions Associated with Metallic Nanoparticles and Their Rectification Techniques. Curr. Nanomater. 2022, 7, 181–192. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Laurenzana, A.; Scavone, F.; Frediani, E.; Fibbi, G.; Fanelli, F.; Sibillano, T.; Giannini, C.; Fini, P. The “End Life” of the Grape Pomace Waste Become the New Beginning: The Development of a Virtuous Cycle for the Green Synthesis of Gold Nanoparticles and Removal of Emerging Contaminants from Water. Antioxidants 2022, 11, 994. [Google Scholar] [CrossRef]
- Ciccarese, F.; Raimondi, V.; Sharova, E.; Silic-Benussi, M.; Ciminale, V. Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells. Antioxidants 2020, 9, 211. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical Applications of Reactive Oxygen Species Generation by Metal Nanoparticles. Materials 2020, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Miaskowski, A.; Jenkins, S.I.; Lim, J.; Dobson, J. Remote Manipulation of Magnetic Nanoparticles Using Magnetic Field Gradient to Promote Cancer Cell Death. Appl. Phys. A 2019, 125, 226. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In Vitro Antioxidant and Antidiabetic Activities of Zinc Oxide Nanoparticles Synthesized Using Different Plant Extracts. Bioprocess. Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, D.; Mahalingam, G. Green Synthesized Metal Nanoparticles, Characterization and Its Antidiabetic Activities-a Review. Res. J. Pharm. Technol. 2020, 13, 468–474. [Google Scholar] [CrossRef]
- Kanmani, R.; IrudayaIrin, S.P. Antioxidant and Antidiabetic Activities of Biologically Synthesized Silver Nanoparticles Using Linumusitatissimum Extract. Orient. J. Chem. 2021, 37, 1235. [Google Scholar]
- Manam, D.V.K. Biosynthesis and Characterization of Silver Nanoparticles from Marine Macroscopic Brown Seaweed Colpomenia sinuosa (Mertens Ex Roth) Derbes and Solier. J. Adv. Chem. Sci. 2020. Available online: https://ssrn.com/abstract=3591081 (accessed on 19 September 2024).
- Ullah, A.; Lim, S.I. Plant Extract-based Synthesis of Metallic Nanomaterials, Their Applications, and Safety Concerns. Biotechnol. Bioeng. 2022, 119, 2273–2304. [Google Scholar] [CrossRef]
- Laird, N.Z.; Acri, T.M.; Chakka, J.L.; Quarterman, J.C.; Malkawi, W.I.; Elangovan, S.; Salem, A.K. Applications of Nanotechnology in 3D Printed Tissue Engineering Scaffolds. Eur. J. Pharm. Biopharm. 2021, 161, 15–28. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y.; Xie, K. AgNPs-Decorated 3D Printed PEEK Implant for Infection Control and Bone Repair. Colloids Surf. B Biointerfaces 2017, 160, 483–492. [Google Scholar] [CrossRef]
- Liu, X.; Mao, Y.; Huang, S.; Li, W.; Zhang, W.; An, J.; Jin, Y.; Guan, J.; Wu, L.; Zhou, P. Selenium Nanoparticles Derived from Proteus Mirabilis YC801 Alleviate Oxidative Stress and Inflammatory Response to Promote Nerve Repair in Rats with Spinal Cord Injury. Regen. Biomater. 2022, 9, rbac042. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Zahed Nasab, S.; Zare, I.; Dahri, M.; Moein Sadeghi, M.; Koohi, M.; Tan, Y.N. Biomimetic Metallic Nanostructures for Biomedical Applications, Catalysis, and Beyond. Ind. Eng. Chem. Res. 2022, 61, 7547–7593. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-Modified Gold-Palladium Nanoparticles as a Potential Autophagy Inducer for the Treatment of Alzheimer’s Disease. J. Colloid. Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.; Shetaia, A.A.; Eid, N.; Abd El-Wahed, A.A.; Abolibda, T.Z.; El Omri, A.; Yu, Q.; Shenashen, M.A.; Hussain, H.; Salem, M.F.; et al. Green Innovation and Synthesis of Honeybee Products-Mediated Nanoparticles: Potential Approaches and Wide Applications. Bioengineering 2024, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef]
- Taavoni-Gilan, A. Chemical Synthesis of MnFe2O4/Chitosan Nanocomposites for Controlled Release of Ofloxacin Drug. J. Chin. Chem. Soc. 2019, 66, 600–607. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Cavalcanti, I.D.L.; Cajuba de Britto Lira Nogueira, M. Pharmaceutical Nanotechnology: Which Products Are Been Designed against COVID-19? J. Nanopart. Res. 2020, 22, 276. [Google Scholar] [CrossRef]
- Kaur, M.; Gautam, A.; Guleria, P.; Singh, K.; Kumar, V. Green Synthesis of Metal Nanoparticles and Their Environmental Applications. Curr. Opin. Environ. Sci. Health 2022, 29, 100390. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Torbati, M.; Mousavi, M.-M.; de la Guardia, M.; Dolatabadi, J.E.N. Nanomaterial-Based Molecularly Imprinted Polymers for Pesticides Detection: Recent Trends and Future Prospects. TrAC Trends Anal. Chem. 2020, 129, 115943. [Google Scholar] [CrossRef]
- Constantinou, M.; Hadjigeorgiou, K.; Abalde-Cela, S.; Andreou, C. Label-Free Sensing with Metal Nanostructure-Based Surface-Enhanced Raman Spectroscopy for Cancer Diagnosis. ACS Appl. Nano Mater. 2022, 5, 12276–12299. [Google Scholar] [CrossRef]
- Varghese Alex, K.; Tamil Pavai, P.; Rugmini, R.; Shiva Prasad, M.; Kamakshi, K.; Sekhar, K.C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5, 13123–13129. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Alvarado-Hidalgo, F.; Robles-Chaves, D.; Sáenz-Arce, G.; Avendano-Soto, E.D.; Sánchez-Kopper, A.; Starbird-Perez, R. Electrochemical Characterization of Mancozeb Degradation for Wastewater Treatment Using a Sensor Based on Poly (3, 4-Ethylenedioxythiophene) (PEDOT) Modified with Carbon Nanotubes and Gold Nanoparticles. Polymers 2019, 11, 1449. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.W.Y.; Lieu, Z.Z.; Cao, X.; Yong, X.E.; Wong, J.Z.L.; Cheong, Y.S.; Browder, L.K.; Chin, W.S. Biogenic Synthesis of Silver Nanoparticles with High Antimicrobial and Catalytic Activities Using Sheng Di Huang (Rehmannia glutinosa). Chem. –An. Asian J. 2021, 16, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, S.; Nazir, F.; Abbasi, N.M.; Khan, A.A.; Ahmed, F. Rumex Hastatus Mediated Green Synthesis of AgNPs: An Efficient Nanocatalyst and Colorimetric Probe for Cu2+. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127356. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Roy, D.; Yadav, A.K.; Bharagava, R.N. Green Synthesis of Nanoparticles and Their Applications in Water and Wastewater Treatment. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Springer: Singapore, 2020; pp. 349–379. [Google Scholar]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnology 2021, 19, 86. [Google Scholar] [CrossRef]
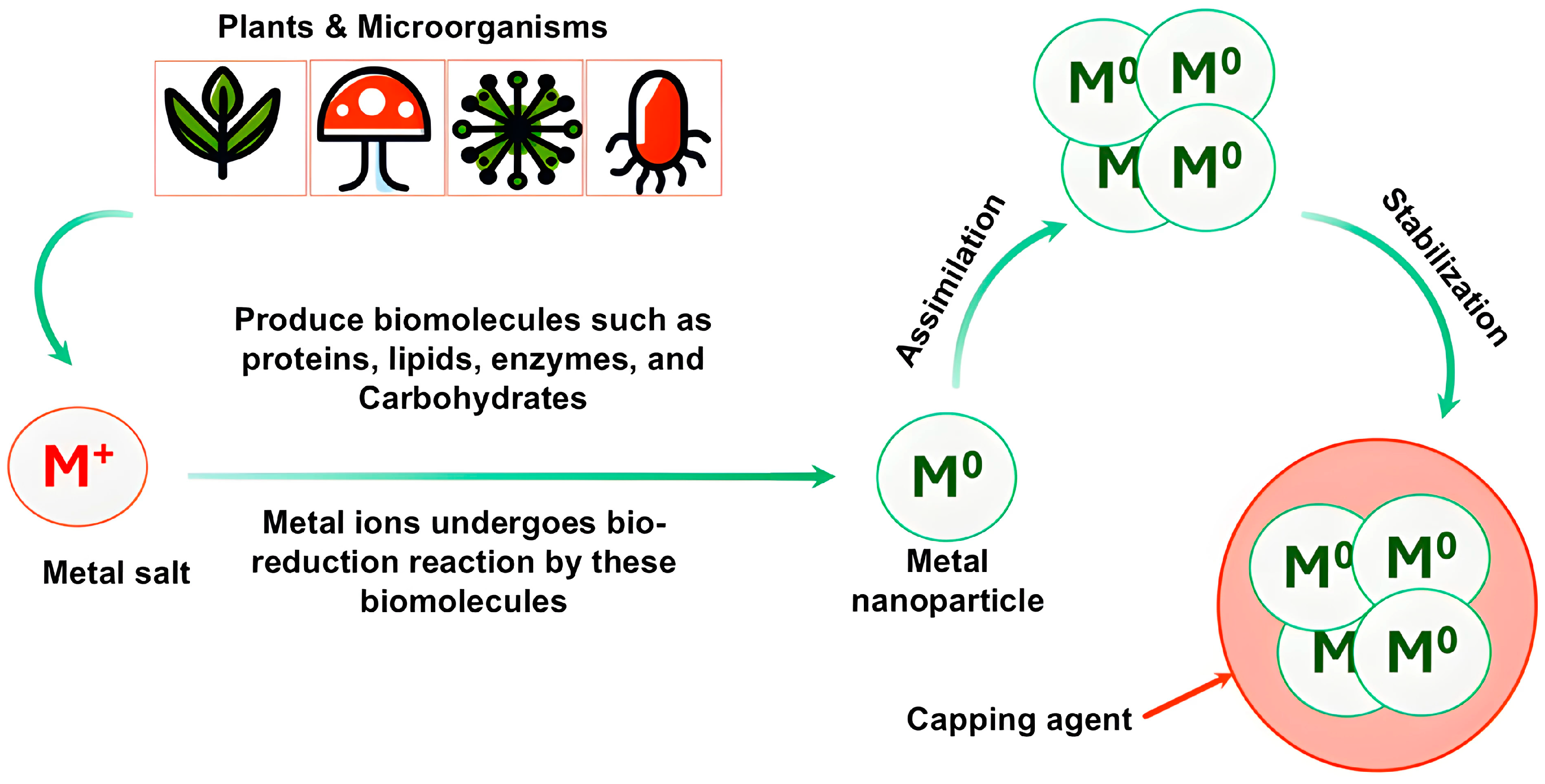
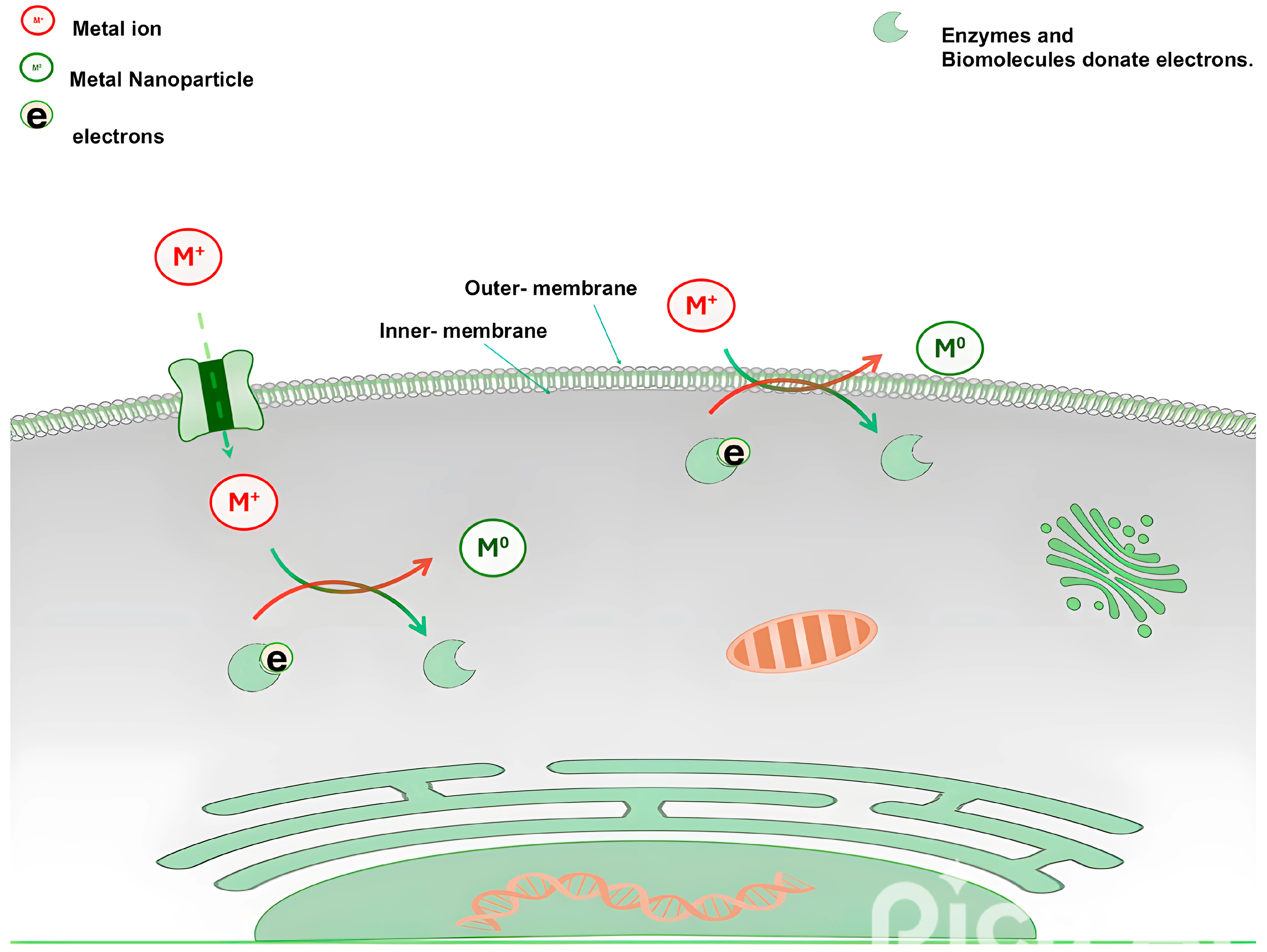
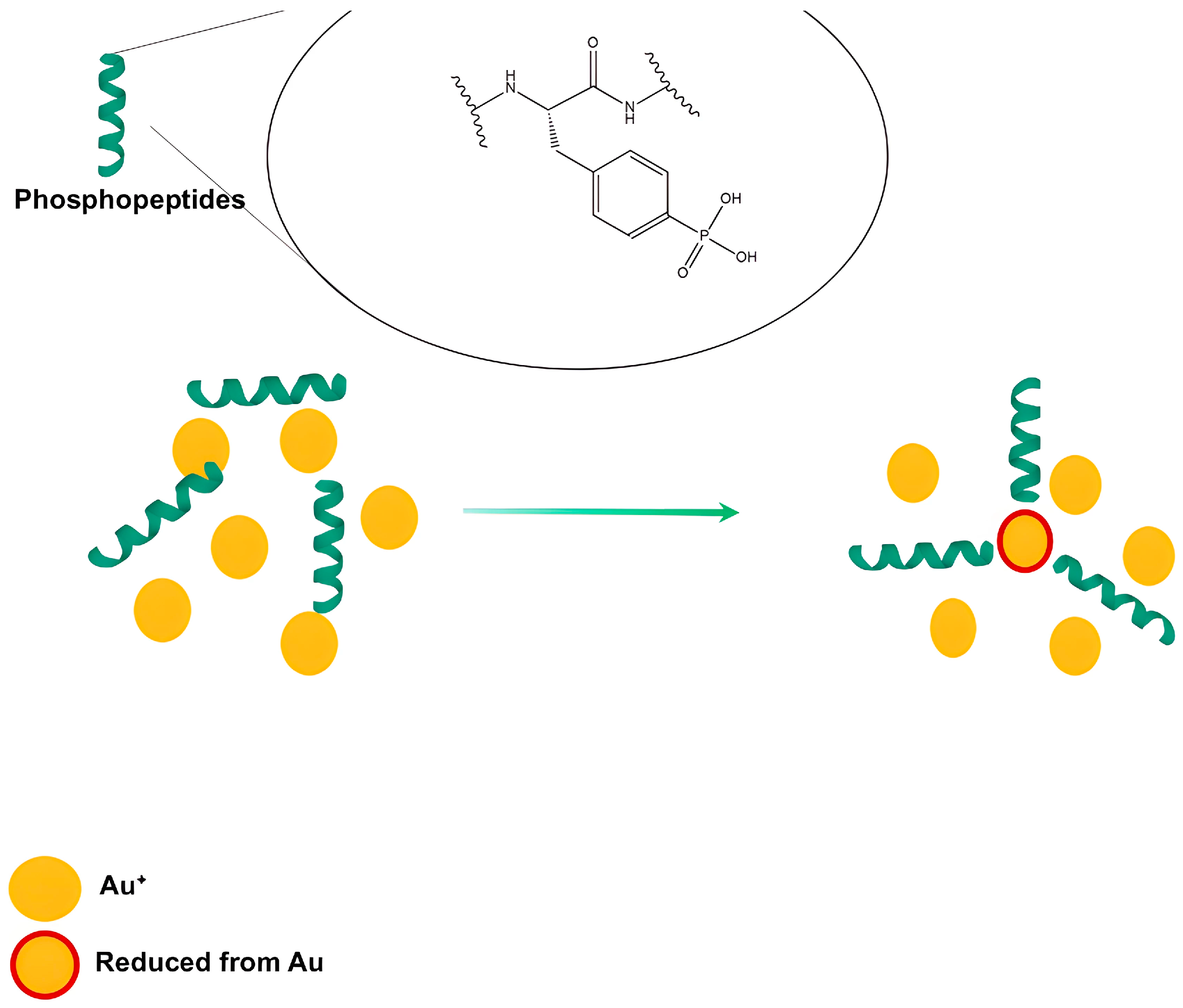
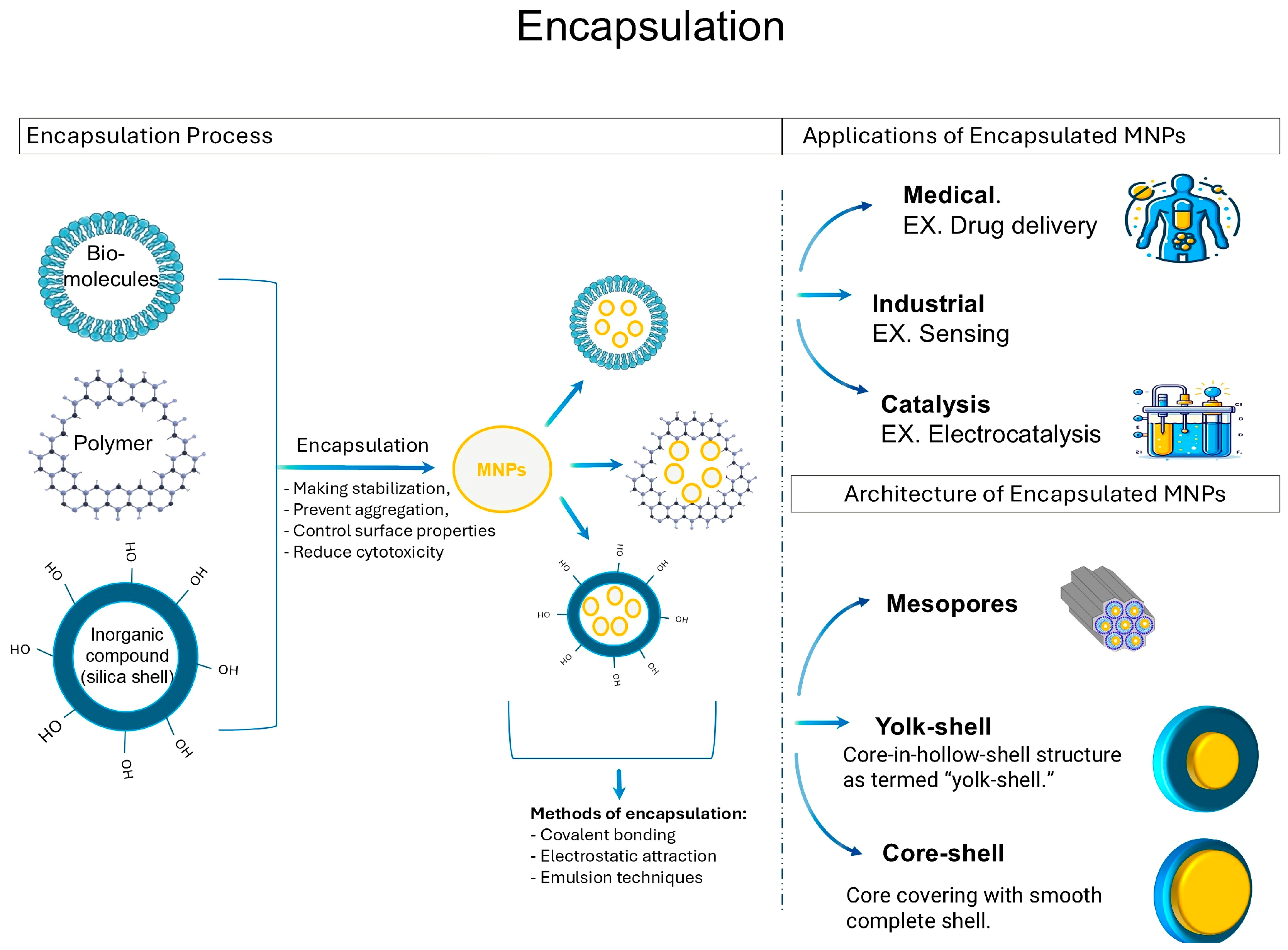
| Plant Species | Metabolites Identified | Class | Metal NPs | Ref. |
|---|---|---|---|---|
| Prickly pear Opuntia (peel extract) | ------------- | Proteins and carbohydrates | Se | [189] |
| Hibiscus sabdariffa | Kaempferol, Quercetin Cryptochlorogenic acid, neocholorogenic acid, caffeoyl shikimic acid | Flavonoid Acid | Au | [190] |
| Fenugreek seed extract | -------------------- | Saponin, proteins, and polyphenols | Ag-Fe3O4 | [191] |
| (Cuminum cyminum) Cumin oil | Quinoid | Phenolic | Ag | [192] |
| Nigella sativa seed extract | Steroids, tannins, flavonoids, coumarins, cardiac glycosides, saponins, and diterpenes | Ag | [193] | |
| Salvia officinalis and Thymus serpyllum ethanolic and hydroalcoholic extracts | Gallic acid Protocatechuic acid Caftaric acid Chlorogenic acid Caffeic acid trans p-coumaric acid trans ferulic acid Rosmarinic acid Rrutin hydrate Chlorophyll a and b | Acid Flavonoid chlorophyll | Mesopores of silica and titania nanomaterials | [194] |
| Terminalia mantaly ™ extracts of leaf, root and stem/bark | Alkaloids, phenolic content, flavonoids, tannins, triterpenes, glucosides, saponins, anthraquinones, and steroids | Au | [195] | |
| Euphorbia peplus ethanolic leaf extract (EpExt) | Obtusifoliol Cycloartenol Peplusol 24-methylenecycloartanol, lanosterol, 24-methylenelanosterol, and 9-cis-tricosene Angelic acid | Steroids Triterpene acid | Au | [185] |
| Leucas aspera leaf extract | ---------------------------- | Polyphenols and proteins | Ag | [196] |
| Xylocarpus granatum mangrove (bark extracts) and Avicennia officinalis (leaf extract) | -------------- | Phenolic compounds | Ag | [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Seedi, H.R.; Omara, M.S.; Omar, A.H.; Elakshar, M.M.; Shoukhba, Y.M.; Duman, H.; Karav, S.; Rashwan, A.K.; El-Seedi, A.H.; Altaleb, H.A.; et al. Updated Review of Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. Bioengineering 2024, 11, 1095. https://doi.org/10.3390/bioengineering11111095
El-Seedi HR, Omara MS, Omar AH, Elakshar MM, Shoukhba YM, Duman H, Karav S, Rashwan AK, El-Seedi AH, Altaleb HA, et al. Updated Review of Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. Bioengineering. 2024; 11(11):1095. https://doi.org/10.3390/bioengineering11111095
Chicago/Turabian StyleEl-Seedi, Hesham R., Mohamed S. Omara, Abdulrahman H. Omar, Mahmoud M. Elakshar, Yousef M. Shoukhba, Hatice Duman, Sercan Karav, Ahmed K. Rashwan, Awg H. El-Seedi, Hamud A. Altaleb, and et al. 2024. "Updated Review of Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications" Bioengineering 11, no. 11: 1095. https://doi.org/10.3390/bioengineering11111095
APA StyleEl-Seedi, H. R., Omara, M. S., Omar, A. H., Elakshar, M. M., Shoukhba, Y. M., Duman, H., Karav, S., Rashwan, A. K., El-Seedi, A. H., Altaleb, H. A., Gao, H., Saeed, A., Jefri, O. A., Guo, Z., & Khalifa, S. A. M. (2024). Updated Review of Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. Bioengineering, 11(11), 1095. https://doi.org/10.3390/bioengineering11111095












