Cybersickness in People with Multiple Sclerosis Exposed to Immersive Virtual Reality
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
- Low disability (EDSS in the range 0–2, n = 34).
- Mild–moderate disability (EDSS in the range 2.5–6.5, n = 22).
2.2. General Outline of the Study
2.3. The Immersive VR System
2.4. Postural Sway Measurement
- Sway area (mm2, 95% confidence ellipse);
- COP path length (mm, the overall length of the trajectory followed by the COP during the trial);
- COP maximum displacements in antero–posterior (AP) and medio–lateral (ML) direction (mm, the difference between the maximum and the minimum coordinate assumed by the COP during the trial);
- COP velocities in the antero–posterior (AP) and medio–lateral (ML) directions (mm/s).
2.5. Subjective Rating of Cybersickness: The Simulator Sickness Questionnaire (SSQ)
2.6. Statistical Analyses
3. Results
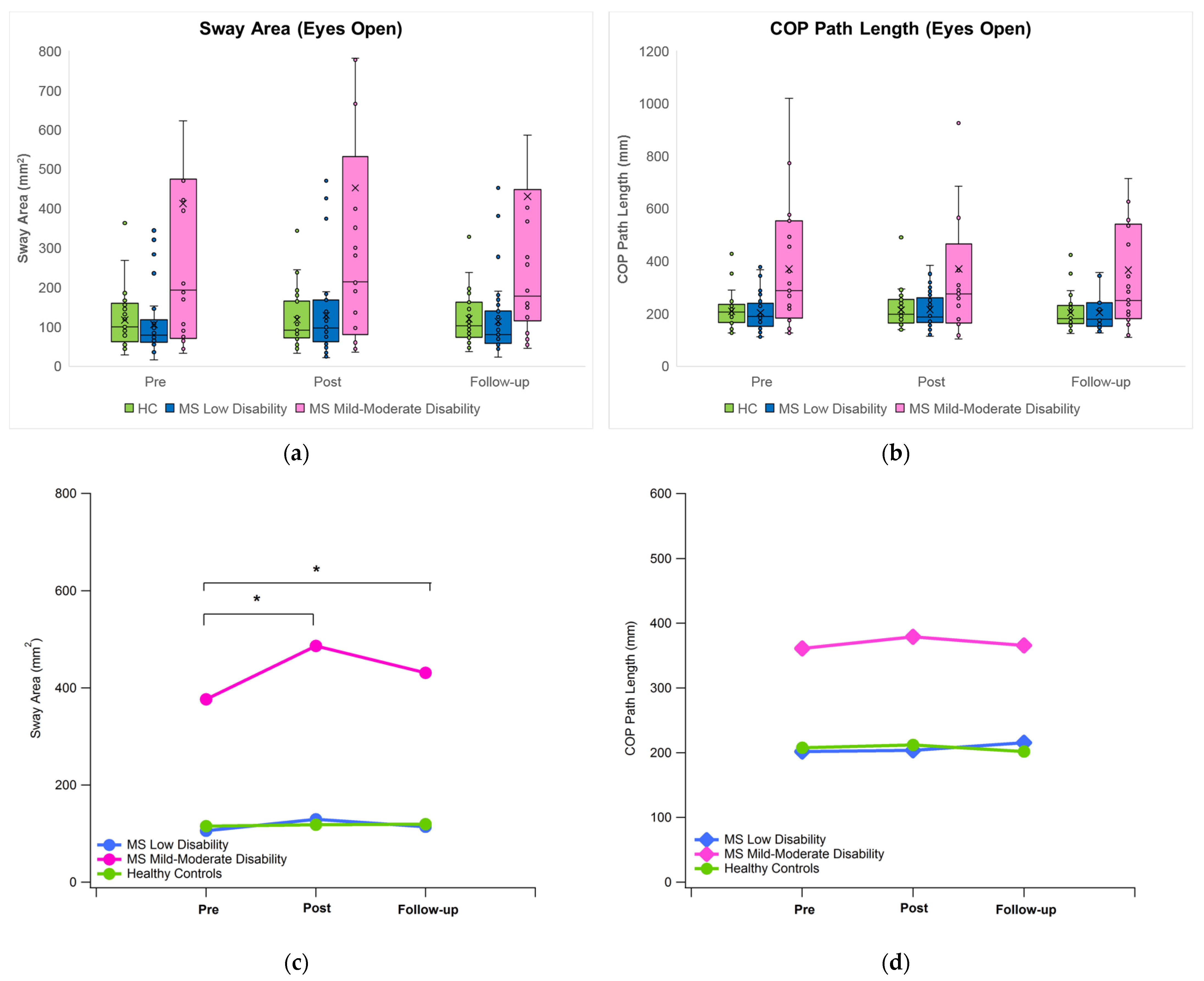
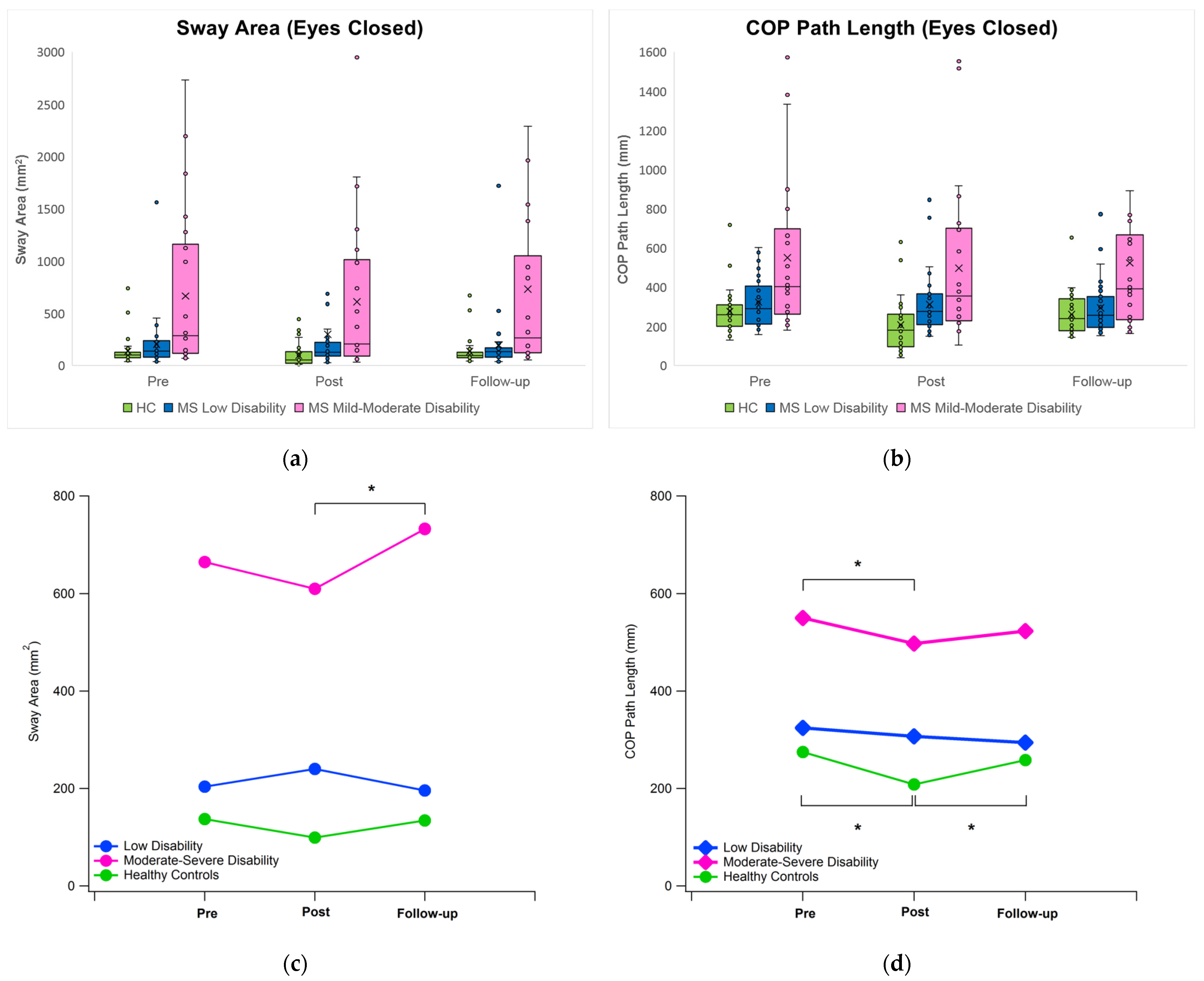
3.1. Postural Sway
3.2. Sickness Simulator Sickness Questionnaire
4. Discussion
4.1. General Remarks and Effect of Immersive VR on Postural Sway under Eyes Open Condition
4.2. Effect of Immersive VR on Postural Sway under Eyes Closed Condition
4.3. Effect of Immersive VR on Subjective Perception of Cybersickness
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bown, J.; White, E.; Boopalan, A. Chapter 12—Looking for the Ultimate Display: A Brief History of Virtual Reality. In Boundaries of Self and Reality Online; Gackenbach, J., Bown, J., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 239–259. ISBN 978-0-12-804157-4. [Google Scholar]
- Fisher, S.S. The Nasa Ames Viewlab Project—A Brief History. Presence Teleoper. Virtual Environ. 2016, 24, 339–348. [Google Scholar] [CrossRef]
- Voinescu, A.; Sui, J.; Stanton Fraser, D. Virtual Reality in Neurorehabilitation: An Umbrella Review of Meta-Analyses. J. Clin. Med. 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors 2021, 21, 7389. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.S.; Fagundes, C.V.; Mendes, F.A.D.S.; Leal, J.C. Effectiveness of Virtual Reality Rehabilitation in Persons with Multiple Sclerosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mult. Scler. Relat. Disord. 2021, 54, 103128. [Google Scholar] [CrossRef]
- Webster, A.; Poyade, M.; Rooney, S.; Paul, L. Upper Limb Rehabilitation Interventions Using Virtual Reality for People with Multiple Sclerosis: A Systematic Review. Mult. Scler. Relat. Disord. 2021, 47, 102610. [Google Scholar] [CrossRef]
- Bertoni, R.; Mestanza Mattos, F.G.; Porta, M.; Arippa, F.; Cocco, E.; Pau, M.; Cattaneo, D. Effects of Immersive Virtual Reality on Upper Limb Function in Subjects with Multiple Sclerosis: A Cross-over Study. Mult. Scler. Relat. Disord. 2022, 65, 104004. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Porta, M.; Bertoni, R.; Mattos, F.G.M.; Cocco, E.; Cattaneo, D. Effect of Immersive Virtual Reality Training on Hand-to-Mouth Task Performance in People with Multiple Sclerosis: A Quantitative Kinematic Study. Mult. Scler. Relat. Disord. 2023, 69, 104455. [Google Scholar] [CrossRef]
- Hollywood, R.-A.; Poyade, M.; Paul, L.; Webster, A. Proof of Concept for the Use of Immersive Virtual Reality in Upper Limb Rehabilitation of Multiple Sclerosis Patients. Adv. Exp. Med. Biol. 2022, 1356, 73–93. [Google Scholar] [CrossRef]
- Pau, M.; Cocco, E.; Arippa, F.; Casu, G.; Porta, M.; Menascu, S.; Achiron, A.; Kalron, A. An Immersive Virtual Kitchen Training System for People with Multiple Sclerosis: A Development and Validation Study. J. Clin. Med. 2023, 12, 3222. [Google Scholar] [CrossRef]
- Nieto-Escamez, F.; Cortés-Pérez, I.; Obrero-Gaitán, E.; Fusco, A. Virtual Reality Applications in Neurorehabilitation: Current Panorama and Challenges. Brain Sci. 2023, 13, 819. [Google Scholar] [CrossRef]
- Slater, M. Place Illusion and Plausibility Can Lead to Realistic Behaviour in Immersive Virtual Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The Effectiveness of Immersive Virtual Reality in Physical Recovery of Stroke Patients: A Systematic Review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef] [PubMed]
- Saredakis, D.; Szpak, A.; Birckhead, B.; Keage, H.A.D.; Rizzo, A.; Loetscher, T. Factors Associated with Virtual Reality Sickness in Head-Mounted Displays: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2020, 14, 96. [Google Scholar] [CrossRef]
- McCauley, M.; Sharkey, T. Cybersickness: Perception of Self-Motion in Virtual Environment. Presence 1992, 1, 311–318. [Google Scholar] [CrossRef]
- Chang, E.; Kim, H.-T.; Yoo, B. Virtual Reality Sickness: A Review of Causes and Measurements. Int. J. Hum.-Comput. Interact. 2020, 36, 1658–1682. [Google Scholar] [CrossRef]
- Kennedy, R.S.; Lane, N.E.; Berbaum, K.S.; Lilienthal, M.G. Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Arafat, I.; Ferdous, S.M.; Quarles, J. The Effects of Cybersickness on Persons with Multiple Sclerosis. In Proceedings of the 22nd ACM Conference on Virtual Reality Software and Technology, Munich, Germany, 2–4 November 2016; p. 59, ISBN 978-1-4503-4491-3. [Google Scholar]
- Arafat, I.; Ferdous, S.M.; Quarles, J. Cybersickness-Provoking Virtual Reality Alters Brain Signals of Persons with Multiple Sclerosis. In Proceedings of the 2018 IEEE Conference on Virtual Reality and 3D User Interfaces, Tuebingen/Reutlingen, Germany, 18–22 March 2018; p. 120. [Google Scholar]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Ferdous, S.M.; Chowdhury, T.I.; Arafat, I.M.; Quarles, J. Static Rest Frame to Improve Postural Stability in Virtual and Augmented Reality. Front. Virtual Real. 2021, 1, 582169. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- da Silva Marinho, A.; Terton, U.; Jones, C.M. Cybersickness and Postural Stability of First Time VR Users Playing VR Videogames. Appl. Ergon. 2022, 101, 103698. [Google Scholar] [CrossRef]
- Woo, Y.S.; Jang, K.-M.; Nam, S.G.; Kwon, M.; Lim, H.K. Recovery Time from VR Sickness Due to Susceptibility: Objective and Quantitative Evaluation Using Electroencephalography. Heliyon 2023, 9, e14792. [Google Scholar] [CrossRef]
- Dennison, M.S.; Wisti, A.Z.; D’Zmura, M. Use of Physiological Signals to Predict Cybersickness. Displays 2016, 44, 42–52. [Google Scholar] [CrossRef]
- Brown, P.; Spronck, P.; Powell, W. The Simulator Sickness Questionnaire, and the Erroneous Zero Baseline Assumption. Front. Virtual Real. 2022, 3, 118. [Google Scholar] [CrossRef]
- Kapteyn, T.; Bles, W.; Njiokiktjien, C.; Kodde, L.; Massen, C.; Mol, J. Standardisation in Platform Stabilometry Being Part of Posturography. Agressol. Rev. Int. Physio-Biol. Pharmacol. Appl. Eff. L’agression 1983, 24, 321–326. [Google Scholar]
- Bimberg, P.; Weissker, T.; Kulik, A. On the Usage of the Simulator Sickness Questionnaire for Virtual Reality Research. In Proceedings of the 2020 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW), Atlanta, GA, USA, 22–26 March 2020; p. 467. [Google Scholar]
- Kim, A.; Darakjian, N.; Finley, J.M. Walking in Fully Immersive Virtual Environments: An Evaluation of Potential Adverse Effects in Older Adults and Individuals with Parkinson’s Disease. J. Neuroeng. Rehabil. 2017, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Kern, F.; Gall, D.; Latoschik, M.E.; Pauli, P.; Käthner, I. Immersive Virtual Reality during Gait Rehabilitation Increases Walking Speed and Motivation: A Usability Evaluation with Healthy Participants and Patients with Multiple Sclerosis and Stroke. J. Neuroeng. Rehabil. 2021, 18, 68. [Google Scholar] [CrossRef]
- Comber, L.; Sosnoff, J.J.; Galvin, R.; Coote, S. Postural Control Deficits in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Gait Posture 2018, 61, 445–452. [Google Scholar] [CrossRef]
- Kalron, A.; Nitzani, D.; Achiron, A. Static Posturography across the EDSS Scale in People with Multiple Sclerosis: A Cross Sectional Study. BMC Neurol. 2016, 16, 70. [Google Scholar] [CrossRef]
- Oh, H.; Lee, G. Feasibility of Full Immersive Virtual Reality Video Game on Balance and Cybersickness of Healthy Adolescents. Neurosci. Lett. 2021, 760, 136063. [Google Scholar] [CrossRef]
- Reason, J.T. Motion Sickness Adaptation: A Neural Mismatch Model. J. R. Soc. Med. 1978, 71, 819–829. [Google Scholar] [CrossRef]
- Yap, S.M.; Etzelmueller, M.S.; O’Keeffe, C.; Gaughan, M.; Kearney, H.; Tubridy, N.; Reilly, R.B.; McGuigan, C. Postural Stability Is a Valid and Meaningful Disability Metric in Progressive MS with Potential for Use in Neuroprotective Therapy Trials. Mult. Scler. Relat. Disord. 2021, 52, 102946. [Google Scholar] [CrossRef]
- Boyas, S.; Remaud, A.; Rivers, E.; Bilodeau, M. Fatiguing Exercise Intensity Influences the Relationship between Parameters Reflecting Neuromuscular Function and Postural Control Variables. PLoS ONE 2013, 8, e72482. [Google Scholar] [CrossRef] [PubMed]
- Soffel, F.; Zank, M.; Kunz, A. Postural Stability Analysis in Virtual Reality Using the HTC Vive. In Proceedings of the 22nd ACM Conference on Virtual Reality Software and Technology, Munich, Germany, 2–4 November 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 351–352. [Google Scholar]
- Ciążyńska, J.; Maciaszek, J. Effects of Low-Immersive vs. High-Immersive Exercise Environment on Postural Stability and Reaction and Motor Time of Healthy Young Adults. J. Clin. Med. 2023, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.S.; Drexler, J.; Kennedy, R.C. Research in Visually Induced Motion Sickness. Appl. Ergon. 2010, 41, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, B.; Ramkhalawansingh, R.; Haycock, B.; Shahab, S.; Campos, J. Comparing Simulator Sickness in Younger and Older Adults during Simulated Driving under Different Multisensory Conditions. Transp. Res. Part F Traffic Psychol. Behav. 2018, 54, 47–62. [Google Scholar] [CrossRef]
- Soltani, P.; Andrade, R. The Influence of Virtual Reality Head-Mounted Displays on Balance Outcomes and Training Paradigms: A Systematic Review. Front. Sports Act. Living 2020, 2, 531535. [Google Scholar] [CrossRef]
- Fransson, P.-A.; Patel, M.; Jensen, H.; Lundberg, M.; Tjernström, F.; Magnusson, M.; Ekvall Hansson, E. Postural Instability in an Immersive Virtual Reality Adapts with Repetition and Includes Directional and Gender Specific Effects. Sci. Rep. 2019, 9, 3168. [Google Scholar] [CrossRef]

| Domain | |||
|---|---|---|---|
| Symptom | Nausea | Oculomotor Disturbance | Disorientation |
| General Discomfort | X | X | |
| Fatigue | X | ||
| Headache | X | ||
| Eyestrain | X | ||
| Difficulty Focusing | X | X | |
| Increased Salivation | X | ||
| Sweating | X | ||
| Nausea | X | X | |
| Difficulty Concentrating | X | X | |
| Fullness of Head | X | ||
| Blurred Vision | X | X | |
| Dizzy (eyes open) | X | ||
| Dizzy (eyes closed) | X | ||
| Vertigo | X | ||
| Stomach Awareness | X | ||
| Burping | X | ||
| Healthy Controls | All MS | MS Low Disability (EDSS 0–2.0) | MS Mild–Moderate Disability (EDSS 2.5–6.5) | |
|---|---|---|---|---|
| Participants | 33 (29 F, 4 M) | 56 (46 F, 10 M) | 34 (28 F, 6 M) | 22 (18 F, 4 M) |
| Age (years) | 47.6 (10.5) | 45.3 (13.1) | 41.8 (13.4) * | 50.7 (10.7) |
| Height (cm) | 161.7 (7.5) | 163.0 (7.7) | 164.2 (7.1) | 161.2 (7.1) |
| Body Mass (kg) | 63.3 (12.9) | 62.9 (12.6) | 64.7 (12.5) | 60.1 (12.5) |
| EDSS score | - | 2.3 (1.4) | 1.3 (0.6) | 3.7 (1.1) |
| Type of MS | - | 44 RR/4 PP/8 SP | 34 RR | 10 RR/4 PP/8 SP |
| Disease duration (years) | - | 6.1 (5.8) | 4.8 (6.2) | 8.2 (6.4) |
| Group | Time | Sway Area | COP Path Length | COP Disp. ML | COP Disp. AP | COP Velocity ML | COP Velocity AP |
|---|---|---|---|---|---|---|---|
| HC | Pre | 115.49 (69.17) | 207.64 (62.88) | 12.88 (5.71) | 17.83 (5.00) | 4.33 (1.30) | 4.76 (1.45) |
| Post | 118.05 (67.17) | 212.04 (68.13) | 12.45 (4.40) | 19.88 (6.19) | 4.32 (1.48) | 4.95 (1.57) | |
| FU | 119.47 (63.57) | 201.86 (63.19) | 13.17 (5.77) | 19.15 (5.50) | 4.16 (1.39) | 4.66 (1.37) | |
| MS Low | Pre | 105.87 (79.03) | 203.61 (68.44) | 12.17 (5.66) | 16.93 (6.15) | 4.25 (1.44) | 4.70 (1.68) |
| Post | 129.29 (105.28) | 215.40 (71.84) | 12.99 (6.12) | 19.16 (6.51) | 4.45 (1.42) | 5.01 (1.84) | |
| FU | 114.03 (93.56) | 204.07 (65.48) | 12.29 (5.75) | 19.03 (7.28) | 4.22 (1.32) | 4.75 (1.62) | |
| MS Mod | Pre | 376.24 (479.08) †‡ | 361.06 (244.10) †‡ | 23.90 (19.84) †‡ | 25.77 (13.93) †‡ | 7.66 (5.63) †‡ | 8.05 (5.09) †‡ |
| Post | 486.40 (674.65) *†‡ | 379.08 (318.98) †‡ | 25.99 (21.39) †‡ | 30.62 (20.05) †‡ | 8.04 (7.19) †‡ | 8.47 (6.76) †‡ | |
| FU | 430.94 (587.54) *†‡ | 365.76 (297.36) †‡ | 23.54 (17.38) †‡ | 29.63 (17.19) †‡ | 7.81 (7.02) †‡ | 8.12 (6.10) †‡ |
| Group | Time | Sway Area | COP Path Length | COP Disp. ML | COP Disp. AP | COP Velocity ML | COP Velocity AP |
|---|---|---|---|---|---|---|---|
| HC | Pre | 137.09 (135.84) | 274.98 (110.39) | 14.37 (7.55) | 20.02 (6.35) | 5.15 (1.84) | 6.81 (3.00) |
| Post | 99.09 (107.12) | 208.09 (131.16) * | 13.73 (7.32) | 17.42 (8.26) | 6.78 (2.83) * | 5.88 (2.66) | |
| FU | 134.12 (131.17) | 258.19 (104.52) ** | 14.00 (7.60) | 19.78 (8.23) | 4.90 (1.95) ** | 6.33 (2.66) | |
| MS Low | Pre | 203.64 (262.64) | 324.41 (131.17) | 15.58 (7.88) | 25.01 (9.53) | 6.11 (2.61) | 8.05 (3.25) |
| Post | 240.24 (425.12) | 306.84 (138.88) | 16.88 (11.74) | 25.49 (12.67) | 5.68 (2.67) | 7.70 (3.43) | |
| FU | 195.97 (291.44) | 294.04 (135.57) | 14.25 (9.90) | 24.13 (10.26) | 5.47 (2.65) | 7.36 (3.35) | |
| MS Mod | Pre | 664.95 (777.82) †‡ | 549.94 (408.54) †‡ | 29.63 (20.40) †‡ | 36.30 (18.95) †‡ | 10.62 (7.88) †‡ | 13.23 (9.94) ‡ |
| Post | 609.80 (754.38) †‡ | 497.37 (405.85) *†‡ | 26.29 (20.42) †‡ | 37.82 (22–18) ‡ | 9.47 (7.77) *‡ | 12.11 (9.95) ‡ | |
| FU | 732.52 (954.22) **†‡ | 523.22 (422.10) †‡ | 29.86 (24.42) †‡ | 38.37 (21.70) ‡ | 10.34 (8.96) †‡ | 12.29 (9.24) ‡ |
| Healthy Controls | MS Low Disability EDSS 0–2.0 | MS Mild–Moderate Disability EDSS 2.5–6.5 | ||||
|---|---|---|---|---|---|---|
| Pre-VR | Post-VR | Pre-VR | Post-VR | Pre-VR | Post-VR | |
| Nausea | 0.00 (0.00) | 28.35 (33.17) * | 1.06 (3.04) | 14.31 (21.21) * | 12.14 (24.13)) †‡ | 22.98 (33.78) |
| Oculomotor | 0.00 (0.00) | 11.48 (18.14) * | 3.58 (7.79) | 9.26 (12.83) * | 18.95 (32.64) †‡ | 22.05 (29.21) ‡ |
| Disorientation | 0.80 (3.28) | 36.19 (46.07) * | 3.48 (10.72) | 28.61 (41.55) * | 24.68 (39.59) | 44.29 (53.07) * |
| Total Score | 0.21 (0.88) | 26.50 (32.50) * | 3.12 (7.12) | 17.87 (23.94) * | 20.74 (34.81) | 31.79 (36.89) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pau, M.; Arippa, F.; Leban, B.; Porta, M.; Casu, G.; Frau, J.; Lorefice, L.; Coghe, G.; Cocco, E. Cybersickness in People with Multiple Sclerosis Exposed to Immersive Virtual Reality. Bioengineering 2024, 11, 115. https://doi.org/10.3390/bioengineering11020115
Pau M, Arippa F, Leban B, Porta M, Casu G, Frau J, Lorefice L, Coghe G, Cocco E. Cybersickness in People with Multiple Sclerosis Exposed to Immersive Virtual Reality. Bioengineering. 2024; 11(2):115. https://doi.org/10.3390/bioengineering11020115
Chicago/Turabian StylePau, Massimiliano, Federico Arippa, Bruno Leban, Micaela Porta, Giulia Casu, Jessica Frau, Lorena Lorefice, Giancarlo Coghe, and Eleonora Cocco. 2024. "Cybersickness in People with Multiple Sclerosis Exposed to Immersive Virtual Reality" Bioengineering 11, no. 2: 115. https://doi.org/10.3390/bioengineering11020115
APA StylePau, M., Arippa, F., Leban, B., Porta, M., Casu, G., Frau, J., Lorefice, L., Coghe, G., & Cocco, E. (2024). Cybersickness in People with Multiple Sclerosis Exposed to Immersive Virtual Reality. Bioengineering, 11(2), 115. https://doi.org/10.3390/bioengineering11020115








