Fuzzy Cognitive Map Applications in Medicine over the Last Two Decades: A Review Study
Abstract
1. Introduction
2. Review Methodology
2.1. Research Questions
- What are the leading medical entities covered by applications of FMCs?
- Over the analysis period, which medical problems or diseases have FCMs been associated with?
- What are the current state-of-the-art advancements and the limitations?
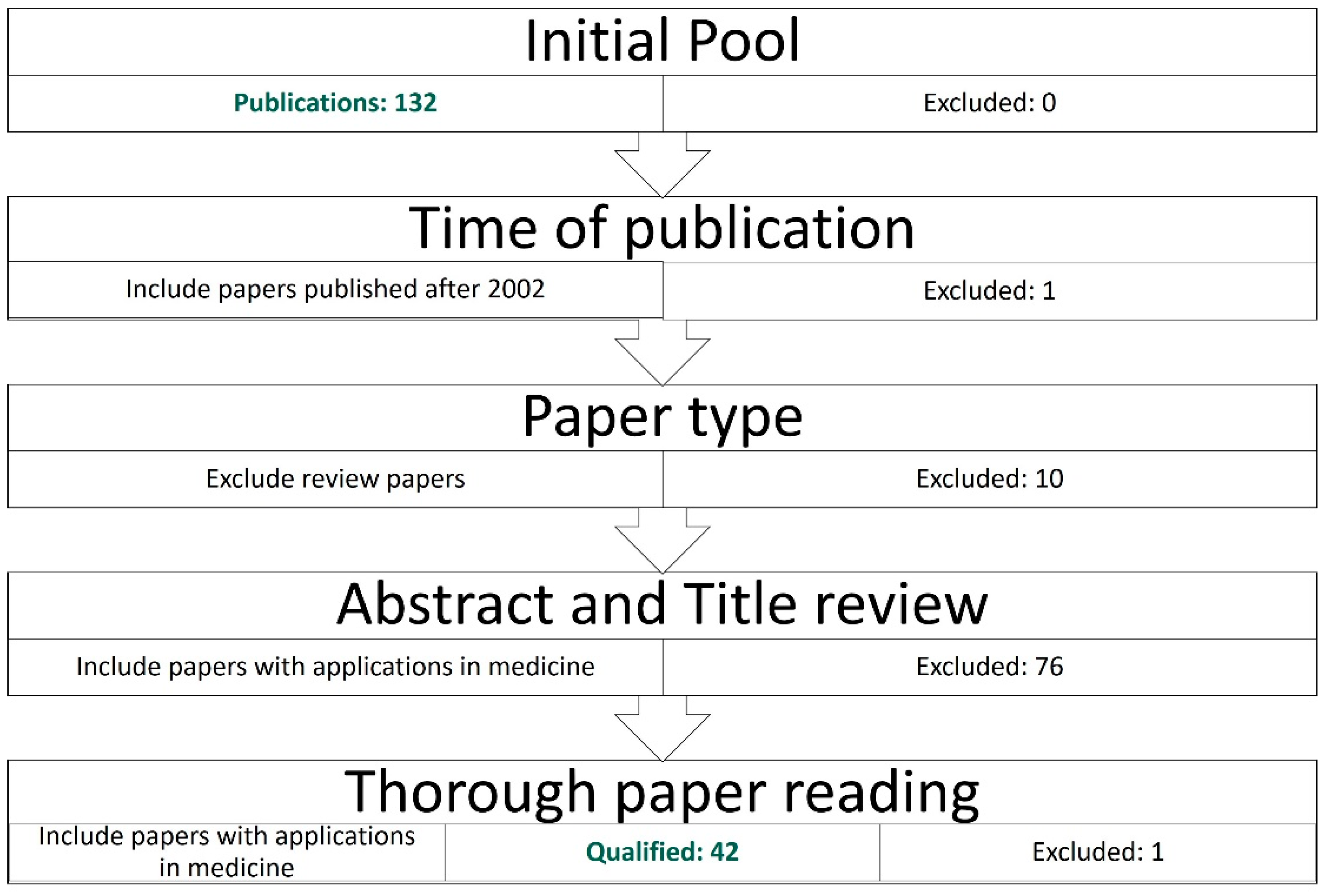
2.2. Review Protocol
Source, Terms, Inclusion, and Exclusion
- Articles published after the end of the year 2002.
- Original research articles (journals and conferences)
- Articles using any FCM, but not solely fuzzy logic.
- Articles demonstrating the use of the proposed methodology to solve a medical problem.
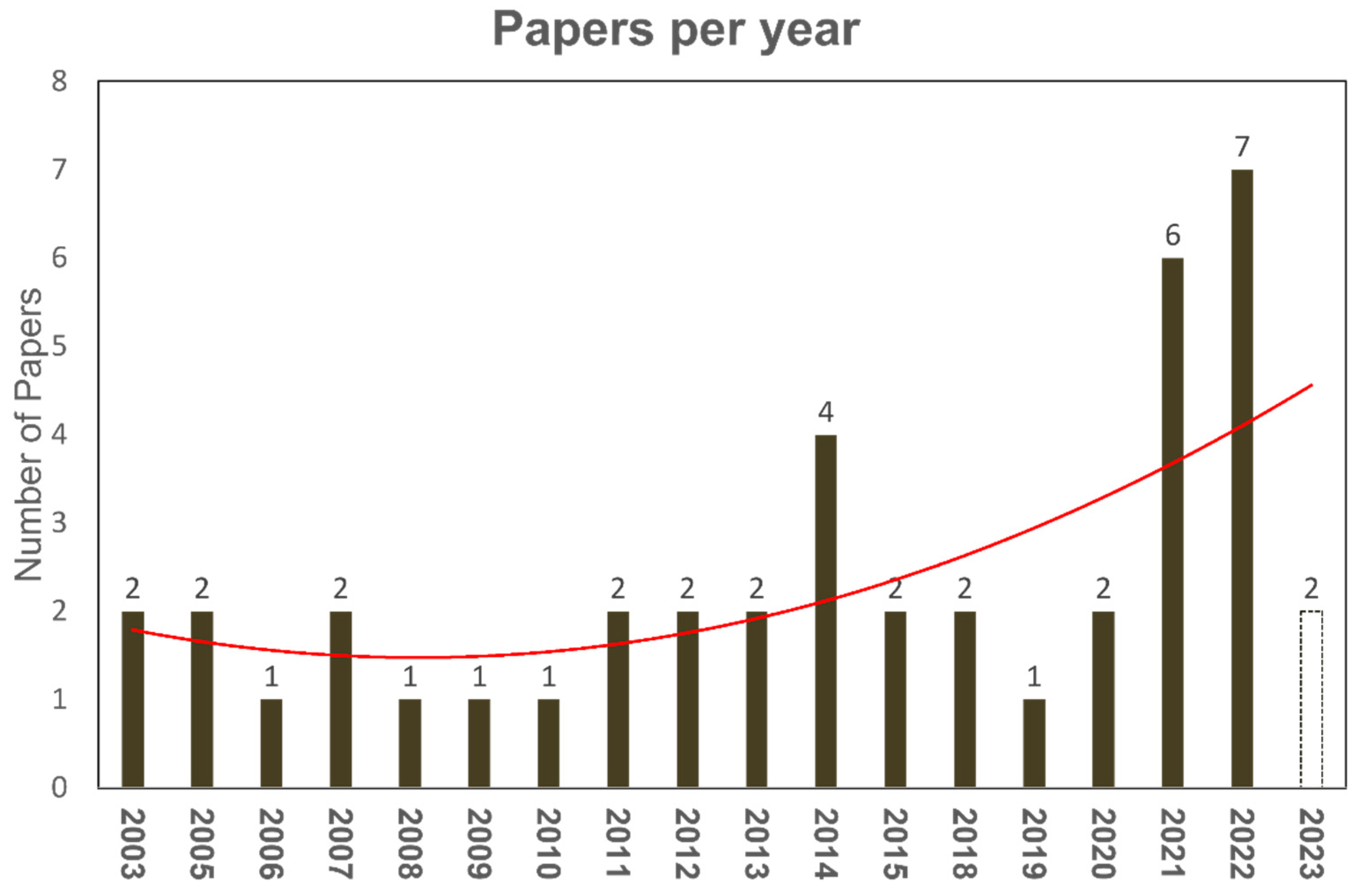
2.3. Literature Collection
3. Fuzzy Cognitive Maps
3.1. Fundamentals of Fuzzy Cognitive Maps
Mathematical Explanation
- w(“temperature”, “crop yields”) = “strong positive”
- w(“sea level”, “coastal infrastructure”) = “moderate negative”
- w(“energy consumption”, “economic growth”) = “weak positive”
3.2. Recent Progress in FCMs
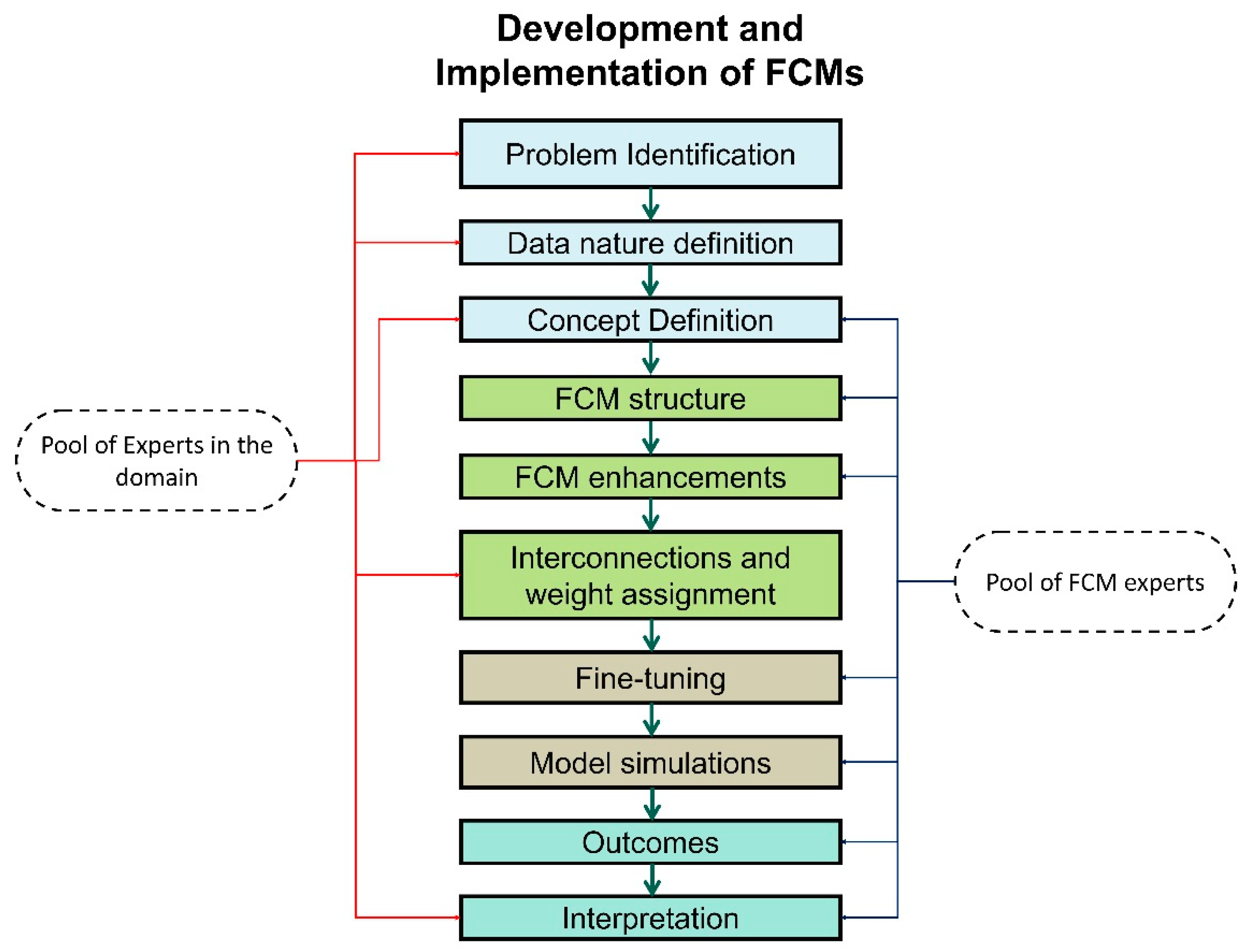
3.3. From Theory to Real-World Scenarios
- Clearly define the problem or system to be modelled using FCMs.
- Identify the fundamental concepts or variables that influence the system’s behaviour.
- Understand the causal relationships between these concepts, including positive and negative influences.
- Select a manageable number of concepts to avoid complexity and over-parameterisation.
- Gather knowledge and expertise from domain experts or data sources to understand the causal relationships between concepts.
- Represent these relationships as directed links in the FCM, using arrows to indicate the direction of influence.
- Assign weights to the links to represent the strength of the causal relationship.
- Assign initial states to each concept, representing their starting values or conditions.
- These initial states can be based on historical data, expert knowledge, or assumed values.
- Define linguistic rules that capture the qualitative relationships between concepts; these rules should express the conditional logic of the system’s behaviour.
- Use fuzzy logic to represent linguistic variables and their relationships.
- Build the FCM network by connecting nodes based on the identified causal relationships.
- Assign weights to the links, representing the strength of the causal influences.
- Implement the linguistic rules into the FCM’s structure.
- Choose an appropriate learning algorithm to update the FCM’s node states and weights.
- Adjust the learning parameters to ensure convergence and accurate model behaviour.
- Train the FCM using historical data or simulation scenarios.
- Monitor the model’s performance during training, evaluating its accuracy and stability.
- Simulate the FCM using the learned weights to predict future states or make decisions.
- Interpret the model’s output by analysing the changes in node states and the propagated causal influences.
- Consider the strength of causal links and the qualitative nature of linguistic rules for meaningful interpretation.
- Based on the training and validation results, refine the FCM’s structure, weights, and learning parameters.
- Adapt the model to incorporate new data, changing conditions, or emerging knowledge.
3.3.1. Advantages and Limitations
3.3.2. The Role of FCMs in Explainable Artificial Intelligence
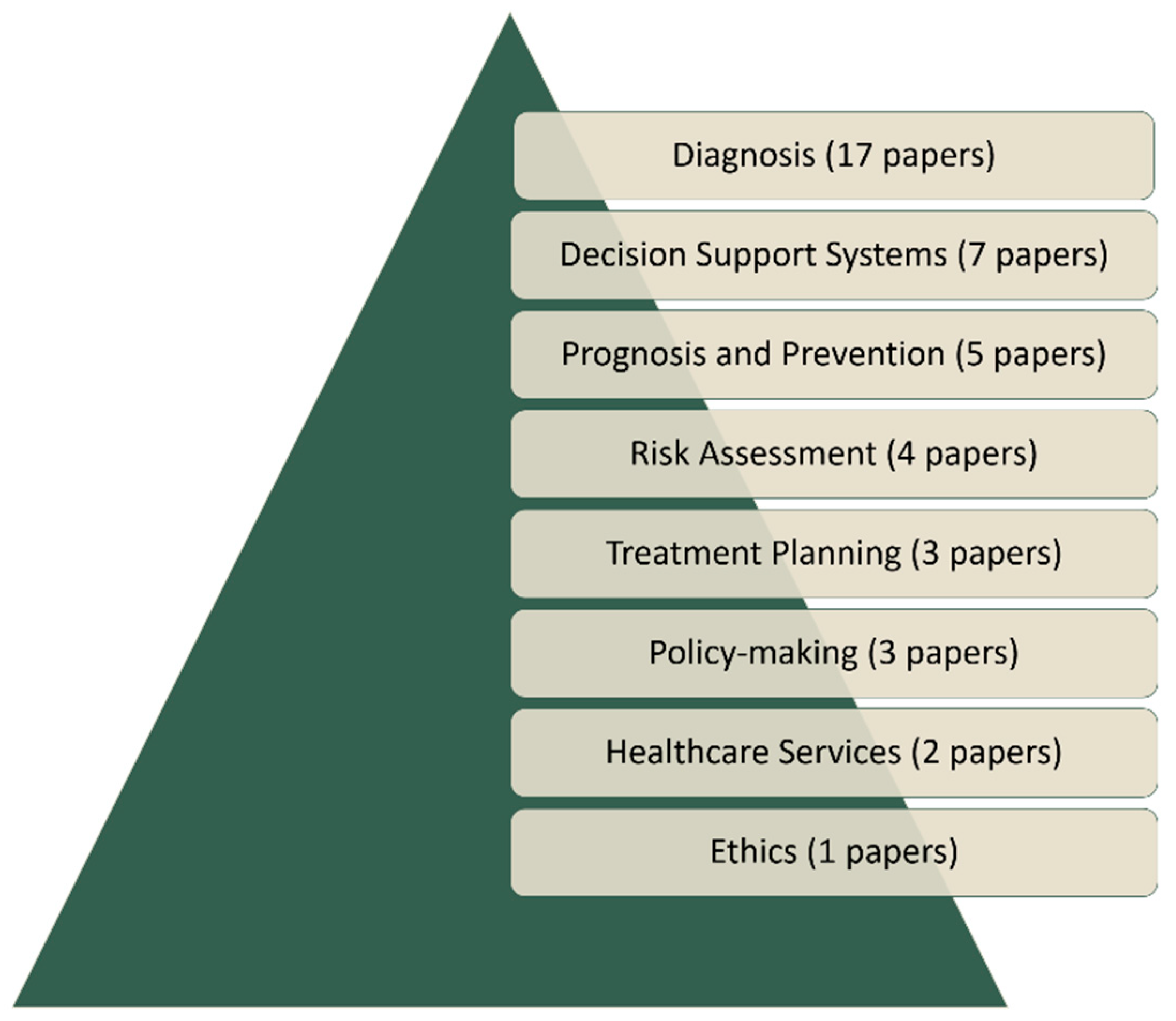
4. Applications of FCMs in Medicine
4.1. Medical Diagnosis
4.2. Medical Decision Support Systems
4.3. Prognosis and Prevention
4.4. Risk Assessment
4.5. Treatment Planning
4.6. Policymaking
4.7. Services and Ethics
| Publication | Year | Domain | Associated Medical Entity |
|---|---|---|---|
| Feleki et al. [24] | 2023 | Diagnosis | Coronary Artery Disease |
| Feleki et al. [29] | 2023 | Diagnosis | Coronary Artery Disease |
| Al-Halabi et al. [23] | 2022 | Diagnosis | Plastic Surgery and Aesthetics |
| Hoyos et al. [25] | 2022 | Diagnosis | Dengue |
| Sovatzidi et al. [26] | 2022 | Diagnosis | Depression |
| Sarmiento et al. [45] | 2021 | Diagnosis | - |
| Apostolopoulos et al. [10] | 2021 | Diagnosis | Coronary Artery Disease |
| Apostolopoulos et al. [46] | 2020 | Diagnosis | Coronary Artery Disease |
| Amirkhani et al. [22] | 2018 | Diagnosis | Protein-DNA |
| Lucchiari et al. [47] | 2014 | Diagnosis | Seizures |
| Douali et al. [48] | 2014 | Diagnosis | Urinary Tract Infection |
| Lee et al. [49] | 2012 | Diagnosis | Pulmonary Infections |
| Georgopoulos et al. [27] | 2009 | Diagnosis | Language Impairment |
| Nguyen et al. [50] | 2008 | Diagnosis | Protein Functions |
| Papageorgiou et al. [28] | 2006 | Diagnosis | Tumour grading |
| Georgopoulos et al. [51] | 2005 | Diagnosis | Dysarthria and Apraxia of Speech |
| Georgopoulos et al. [52] | 2003 | Diagnosis | Language Impairment |
| Meier et al. [44] | 2022 | Ethics | - |
| Poleto et al. [43] | 2021 | Healthcare Services | - |
| Papageorgiou et al. [53] | 2011 | Healthcare Services | Pulmonary Infections |
| Georgopoulos et al. [30] | 2014 | MDSS | Labor |
| Papageorgiou et al. [31] | 2013 | MDSS | Urinary Tract Infection |
| Lucchiari et al. [32] | 2011 | MDSS | - |
| Stylios et al. [15] | 2010 | MDSS | Obstetrics |
| Papageorgiou et al. [54] | 2007 | MDSS | - |
| Papageorgiou et al. [55] | 2006 | MDSS | Bladder Tumor |
| John et al. [56] | 2005 | MDSS | Flu |
| Babroudi et al. [41] | 2021 | Policymaking | COVID-19 |
| Groumpos et al. [57] | 2021 | Policymaking | COVID-19 |
| Dogu et al. [42] | 2021 | Policymaking | Chronic Obstructive Pulmonary Disease |
| Saul et al. [58] | 2022 | Prevention | Healthy Habits |
| Wu et al. [33] | 2022 | Prevention | - |
| Khodadadi et al. [34] | 2019 | Prevention | Stroke |
| Najafi et al. [35] | 2018 | Prevention | Esophageal cancer |
| Billis et al. [59] | 2014 | Prevention | Geriatric depression |
| Mahmoodi et al. [37] | 2020 | Risk assessment | Gastric Cancer |
| Subramanian et al. [36] | 2015 | Risk assessment | Breast Cancer |
| Papageorgiou et al. [38] | 2015 | Risk assessment | Breast Cancer |
| de Brito et al. [60] | 2013 | Risk assessment | Body Dysmorphic Disorder |
| Papageorgiou et al. [40] | 2012 | Treatment Planning | Urinary Tract Infection |
| Giles et al. [39] | 2007 | Treatment Planning | Diabetes |
| Papageorgiou et al. [61] | 2003 | Treatment Planning | Radiation Therapy |
5. Discussion and Conclusions
5.1. Summary of Findings
5.2. FCM Contributions in the Medical Domain
5.3. Performance Metrics
5.4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodman, B.; Flaxman, S. European Union Regulations on Algorithmic Decision-Making and a “Right to Explanation". AIMag 2017, 38, 50–57. [Google Scholar] [CrossRef]
- Dilsizian, S.E.; Siegel, E.L. Artificial intelligence in medicine and cardiac imaging: Harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr. Cardiol. Rep. 2014, 16, 441. [Google Scholar] [CrossRef] [PubMed]
- Domingues, I.; Pereira, G.; Martins, P.; Duarte, H.; Santos, J.; Abreu, P.H. Using Deep Learning Techniques in Medical Imaging: A Systematic Review of Applications on CT and PET. Artif. Intell. Rev. 2020, 53, 4093–4160. [Google Scholar] [CrossRef]
- Amisha; Malik, P.; Pathania, M.; Rathaur, V. Overview of Artificial Intelligence in Medicine. J. Fam. Med. Prim. Care 2019, 8, 2328. [Google Scholar] [CrossRef] [PubMed]
- Aljaloud, S.; Alshudukhi, J.; Alhamazani, K.T.; Belay, A. Comparative Study of Artificial Intelligence Techniques for the Diagnosis of Chronic Nerve Diseases. Comput. Math. Methods Med. 2022, 2022, 3522510. [Google Scholar] [CrossRef] [PubMed]
- Arabi, H.; AkhavanAllaf, A.; Sanaat, A.; Shiri, I.; Zaidi, H. The promise of artificial intelligence and deep learning in PET and SPECT imaging. Phys. Medica 2021, 83, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Clement, T.; Kemmerzell, N.; Abdelaal, M.; Amberg, M. XAIR: A Systematic Metareview of Explainable AI (XAI) Aligned to the Software Development Process. Mach. Learn. Knowl. Extr. 2023, 5, 78–108. [Google Scholar] [CrossRef]
- Kosko, B. Fuzzy cognitive maps. Int. J. Man-Mach. Stud. 1986, 24, 65–75. [Google Scholar] [CrossRef]
- Stylios, C.D.; Groumpos, P.P. Fuzzy Cognitive Maps in Modeling Supervisory Control Systems. J. Intell. Fuzzy Syst. 2000, 8, 83–98. [Google Scholar]
- Apostolopoulos, I.D.; Groumpos, P.P.; Apostolopoulos, D.J. Advanced fuzzy cognitive maps: State-space and rule-based methodology for coronary artery disease detection. Biomed. Phys. Eng. Express 2021, 7, 045007. [Google Scholar] [CrossRef]
- Felix, G.; Nápoles, G.; Falcon, R.; Froelich, W.; Vanhoof, K.; Bello, R. A review on methods and software for fuzzy cognitive maps. Artif. Intell. Rev. 2019, 52, 1707–1737. [Google Scholar] [CrossRef]
- Dickerson, J.A.; Kosko, B. Virtual Worlds as Fuzzy Cognitive Maps. Presence Teleoperators Virtual Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Stylios, C.D.; Groumpos, P.P. Modeling Complex Systems Using Fuzzy Cognitive Maps. IEEE Trans. Syst. Man. Cybern. A 2004, 34, 155–162. [Google Scholar] [CrossRef]
- Mpelogianni, V.; Groumpos, P.P. Re-approaching fuzzy cognitive maps to increase the knowledge of a system. Ai Soc. 2018, 33, 175–188. [Google Scholar] [CrossRef]
- Stylios, C.S.; Georgopoulos, V.C. Fuzzy Cognitive Maps for Medical Decision Support. A Paradigm from Obstetrics. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1174–1177. [Google Scholar]
- Mpelogianni, V.; Arvanitakis, I.; Groumpos, P.P. State Feedback of Complex Systems Using Fuzzy Cognitive Maps. Int. J. Bus. Technol. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Bakhtavar, E.; Valipour, M.; Yousefi, S.; Sadiq, R.; Hewage, K. Fuzzy Cognitive Maps in Systems Risk Analysis: A Comprehensive Review. Complex Intell. Syst. 2021, 7, 621–637. [Google Scholar] [CrossRef]
- Orang, O.; De Lima E Silva, P.C.; Guimarães, F.G. Time Series Forecasting Using Fuzzy Cognitive Maps: A Survey. Artif. Intell. Rev. 2023, 56, 7733–7794. [Google Scholar] [CrossRef]
- Concepcion, L.; Napoles, G.; Falcon, R.; Vanhoof, K.; Bello, R. Unveiling the Dynamic Behavior of Fuzzy Cognitive Maps. IEEE Trans. Fuzzy Syst. 2021, 29, 1252–1261. [Google Scholar] [CrossRef]
- Apostolopoulos, I.D.; Groumpos, P.P. Fuzzy Cognitive Maps: Their Role in Explainable Artificial Intelligence. Appl. Sci. 2023, 13, 3412. [Google Scholar] [CrossRef]
- Nápoles, G.; Ranković, N.; Salgueiro, Y. On the Interpretability of Fuzzy Cognitive Maps. Knowl.-Based Syst. 2023, 281, 111078. [Google Scholar] [CrossRef]
- Amirkhani, A.; Kolahdoozi, M.; Wang, C.; Kurgan, L. Prediction of DNA-Binding Residues in Local Segments of Protein Sequences with Fuzzy Cognitive Maps. IEEE/ACM Trans. Comput. Biol. Bioinf. 2018, 17, 1372–1382. [Google Scholar] [CrossRef]
- Al-Halabi, B.; Vassiliou, M.; Gilardino, M. Teaching and Assessing Cognitive Competencies in Aesthetic and Plastic Surgery. Plast. Reconstr. Surg. 2022, 150, 455e–464e. [Google Scholar] [CrossRef] [PubMed]
- Feleki, A.; Apostolopoulos, I.D.; Moustakidis, S.; Papageorgiou, E.I.; Papathanasiou, N.; Apostolopoulos, D.; Papandrianos, N. Explainable Deep Fuzzy Cognitive Map Diagnosis of Coronary Artery Disease: Integrating Myocardial Perfusion Imaging, Clinical Data, and Natural Language Insights. Appl. Sci. 2023, 13, 11953. [Google Scholar] [CrossRef]
- Hoyos, W.; Aguilar, J.; Toro, M. A Clinical Decision-Support System for Dengue Based on Fuzzy Cognitive Maps. Health Care Manag. Sci. 2022, 25, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Sovatzidi, G.; Vasilakakis, M.; Iakovidis, D.K. Constructive Fuzzy Cognitive Map for Depression Severity Estimation. In Studies in Health Technology and Informatics; Séroussi, B., Weber, P., Dhombres, F., Grouin, C., Liebe, J.-D., Pelayo, S., Pinna, A., Rance, B., Sacchi, L., Ugon, A., Eds.; IOS Press: Clifton, VA, USA, 2022; ISBN 978-1-64368-284-6. [Google Scholar]
- Georgopoulos, V.C.; Stylios, C.D. Diagnosis Support Using Fuzzy Cognitive Maps Combined with Genetic Algorithms. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 6226–6229. [Google Scholar]
- Papageorgiou, E.I.; Spyridonos, P.P.; Stylios, C.D.; Ravazoula, P.; Groumpos, P.P.; Nikiforidis, G.N. Advanced soft computing diagnosis method for tumour grading. Artif. Intell. Med. 2006, 36, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Feleki, A.; Apostolopoulos, I.D.; Papageorgiou, K.; Papageorgiou, E.I.; Apostolopoulos, D.J.; Papandrianos, N.I. A Fuzzy Cognitive Map Learning Approach for Coronary Artery Disease Diagnosis in Nuclear Medicine; Springer: Palma, Spain, 2023. [Google Scholar]
- Georgopoulos, V.C.; Chouliara, S.; Stylios, C.D. Fuzzy Cognitive Map Scenario-Based Medical Decision Support Systems for Education. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1813–1816. [Google Scholar]
- Papageorgiou, E.I.; Huszka, C.; De Roo, J.; Douali, N.; Jaulent, M.-C.; Colaert, D. Application of Probabilistic and Fuzzy Cognitive Approaches in Semantic Web Framework for Medical Decision Support. Comput. Methods Programs Biomed. 2013, 112, 580–598. [Google Scholar] [CrossRef] [PubMed]
- Lucchiari, C.; Pravettoni, G. Cognitive Balanced Model: A Conceptual Scheme of Diagnostic Decision Making. Eval. Clin. Pract. 2012, 18, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Y.; Wang, Z.; Hu, G.; Chen, C. Probabilistic Linguistic Fuzzy Cognitive Maps: Applications to the Critical Factors Affecting the Health of Rural Older Adults. BMC Med. Inf. Decis. Mak. 2022, 22, 299. [Google Scholar] [CrossRef]
- Khodadadi, M.; Shayanfar, H.; Maghooli, K.; Hooshang Mazinan, A. Fuzzy Cognitive Map Based Approach for Determining the Risk of Ischemic Stroke. IET Syst. Biol. 2019, 13, 297–304. [Google Scholar] [CrossRef]
- Najafi, A. Effects of Food Insecurity on the Women Esophageal Cancer in the Zanjan Province. J. Cancer Res. Ther. 2018, 14, 490–494. [Google Scholar] [CrossRef]
- Subramanian, J.; Karmegam, A.; Papageorgiou, E.; Papandrianos, N.; Vasukie, A. An Integrated Breast Cancer Risk Assessment and Management Model Based on Fuzzy Cognitive Maps. Comput. Methods Programs Biomed. 2015, 118, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.A.; Mirzaie, K.; Mahmoodi, M.S.; Mahmoudi, S.M. A Medical Decision Support System to Assess Risk Factors for Gastric Cancer Based on Fuzzy Cognitive Map. Comput. Math. Methods Med. 2020, 2020, 1016284. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, E.I.; Subramanian, J.; Karmegam, A.; Papandrianos, N. A Risk Management Model for Familial Breast Cancer: A New Application Using Fuzzy Cognitive Map Method. Comput. Methods Programs Biomed. 2015, 122, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Giles, B.G.; Findlay, C.S.; Haas, G.; LaFrance, B.; Laughing, W.; Pembleton, S. Integrating Conventional Science and Aboriginal Perspectives on Diabetes Using Fuzzy Cognitive Maps. Soc. Sci. Med. 2007, 64, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, E.I. Fuzzy Cognitive Map Software Tool for Treatment Management of Uncomplicated Urinary Tract Infection. Comput. Methods Programs Biomed. 2012, 105, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Babroudi, N.E.P.; Sabri-Laghaie, K.; Ghoushchi, N.G. Re-Evaluation of the Healthcare Service Quality Criteria for the COVID-19 Pandemic: Z-Number Fuzzy Cognitive Map. Appl. Soft Comput. 2021, 112, 107775. [Google Scholar] [CrossRef]
- Dogu, E.; Albayrak, Y.E.; Tuncay, E. Length of Hospital Stay Prediction with an Integrated Approach of Statistical-Based Fuzzy Cognitive Maps and Artificial Neural Networks. Med. Biol. Eng. Comput. 2021, 59, 483–496. [Google Scholar] [CrossRef]
- Poleto, T.; Carvalho, V.D.H.D.; Silva, A.L.B.D.; Clemente, T.R.N.; Silva, M.M.; Gusmão, A.P.H.D.; Costa, A.P.C.S.; Nepomuceno, T.C.C. Fuzzy Cognitive Scenario Mapping for Causes of Cybersecurity in Telehealth Services. Healthcare 2021, 9, 1504. [Google Scholar] [CrossRef]
- Meier, L.J.; Hein, A.; Diepold, K.; Buyx, A. Algorithms for Ethical Decision-Making in the Clinic: A Proof of Concept. Am. J. Bioeth. 2022, 22, 4–20. [Google Scholar] [CrossRef]
- Sarmiento, I.; Ansari, U.; Omer, K.; Gidado, Y.; Baba, M.C.; Gamawa, A.I.; Andersson, N.; Cockcroft, A. Causes of Short Birth Interval (Kunika) in Bauchi State, Nigeria: Systematizing Local Knowledge with Fuzzy Cognitive Mapping. Reprod. Health 2021, 18, 74. [Google Scholar] [CrossRef]
- Apostolopoulos, I.D.; Groumpos, P.P. Non Invasive Modelling Methodology for the Diagnosis of Coronary Artery Disease Using Fuzzy Cognitive Maps. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 879–887. [Google Scholar] [CrossRef]
- Lucchiari, C.; Folgieri, R.; Pravettoni, G. Fuzzy Cognitive Maps: A Tool to Improve Diagnostic Decisions. Diagnosis 2014, 1, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Douali, N.; Csaba, H.; De Roo, J.; Papageorgiou, E.I.; Jaulent, M.-C. Diagnosis Support System Based on Clinical Guidelines: Comparison between Case-Based Fuzzy Cognitive Maps and Bayesian Networks. Comput. Methods Programs Biomed. 2014, 113, 133–143. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, H.S.; Cho, H. Design of Activation Functions for Inference of Fuzzy Cognitive Maps: Application to Clinical Decision Making in Diagnosis of Pulmonary Infection. Health Inf. Res. 2012, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Mannino, M.; Gardiner, K.; Cios, K.J. ClusFCM: An algorithm for predicting protein functions using homologies and protein interactions. J. Bioinform. Comput. Biol. 2008, 06, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, V.C.; Malandraki, G.A. A Fuzzy Cognitive Map Hierarchical Model for Differential Diagnosis of Dysarthrias and Apraxia of Speech. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 2409–2412. [Google Scholar]
- Georgopoulos, V.C.; Malandraki, G.A.; Stylios, C.D. A Fuzzy Cognitive Map Approach to Differential Diagnosis of Specific Language Impairment. Artif. Intell. Med. 2003, 29, 261–278. [Google Scholar] [CrossRef]
- Papageorgiou, E.I.; Froelich, W. Application of Evolutionary Fuzzy Cognitive Maps for Prediction of Pulmonary Infections. IEEE Trans. Inform. Technol. Biomed. 2012, 16, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, E.; Stylios, C.; Groumpos, P. Novel Architecture for Supporting Medical Decision Making of Different Data Types Based on Fuzzy Cognitive Map Framework. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 1192–1195. [Google Scholar]
- Papageorgiou, E.; Stylios, C.; Groumpos, P. A Combined Fuzzy Cognitive Map and Decision Trees Model for Medical Decision Making. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 6117–6120. [Google Scholar]
- John, R.I.; Innocent, P.R. Modeling Uncertainty in Clinical Diagnosis Using Fuzzy Logic. IEEE Trans. Syst., Man. Cybern. B 2005, 35, 1340–1350. [Google Scholar] [CrossRef]
- Groumpos, P.P.; Apostolopoulos, I.D. Modeling the Spread of Dangerous Pandemics with the Utilization of a Hybrid-Statistical–Advanced-Fuzzy-Cognitive-Map Algorithm: The Example of COVID-19. Res. Biomed. Eng. 2021, 37, 749–764. [Google Scholar] [CrossRef]
- Saúl, L.A.; Sanfeliciano, A.; Botella, L.; Perea, R.; Gonzalez-Puerto, J.A. Fuzzy Cognitive Maps as a Tool for Identifying Cognitive Conflicts That Hinder the Adoption of Healthy Habits. Int. J. Environ. Res. Public Health 2022, 19, 1411. [Google Scholar] [CrossRef]
- Billis, A.S.; Papageorgiou, E.I.; Frantzidis, C.A.; Tsatali, M.S.; Tsolaki, A.C.; Bamidis, P.D. A Decision-Support Framework for Promoting Independent Living and Ageing Well. IEEE J. Biomed. Health Inform. 2015, 19, 199–209. [Google Scholar] [CrossRef] [PubMed]
- De Brito, M.J.A.; Nahas, F.X.; Ortega, N.R.S.; Cordás, T.A.; Dini, G.M.; Neto, M.S.; Ferreira, L.M. Support System for Decision Making in the Identification of Risk for Body Dysmorphic Disorder: A Fuzzy Model. Int. J. Med. Inform. 2013, 82, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, E.I.; Stylios, C.D.; Groumpos, P.P. An integrated two-level hierarchical system for decision making in radiation therapy based on fuzzy cognitive maps. IEEE Trans. Biomed. Eng. 2003, 50, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Liu, J. Port Resilience Analysis Based on the HHM-FCM Approach under COVID-19. Ocean. Coast. Manag. 2023, 243, 106741. [Google Scholar] [CrossRef]
- Ameli, M.; Shams Esfandabadi, Z.; Sadeghi, S.; Ranjbari, M.; Zanetti, M.C. COVID-19 and Sustainable Development Goals (SDGs): Scenario Analysis through Fuzzy Cognitive Map Modeling. Gondwana Res. 2023, 114, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.Q.; Lin, C.-T.; Zhu, L.-M.; Tang, Z.R.; Jie, Y.-W.; Zhou, G.-R. Fatigue Detection of Pilots’ Brain Through Brains Cognitive Map and Multilayer Latent Incremental Learning Model. IEEE Trans. Cybern. 2022, 52, 12302–12314. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.B.; Thampi, S.M.; Berretti, S. A Psychologically Inspired Fuzzy Cognitive Deep Learning Framework to Predict Crowd Behavior. IEEE Trans. Affect. Comput. 2022, 13, 1005–1022. [Google Scholar] [CrossRef]
- Goswami, R.; Roy, K.; Dutta, S.; Ray, K.; Sarkar, S.; Brahmachari, K.; Nanda, M.K.; Mainuddin, M.; Banerjee, H.; Timsina, J.; et al. Multi-Faceted Impact and Outcome of COVID-19 on Smallholder Agricultural Systems: Integrating Qualitative Research and Fuzzy Cognitive Mapping to Explore Resilient Strategies. Agric. Syst. 2021, 189, 103051. [Google Scholar] [CrossRef]
- Feng, G.; Lu, W.; Pedrycz, W.; Yang, J.; Liu, X. The Learning of Fuzzy Cognitive Maps With Noisy Data: A Rapid and Robust Learning Method With Maximum Entropy. IEEE Trans. Cybern. 2021, 51, 2080–2092. [Google Scholar] [CrossRef]
- Poomagal, S.; Sujatha, R.; Kumar, P.S.; Vo, D.-V.N. A Fuzzy Cognitive Map Approach to Predict the Hazardous Effects of Malathion to Environment (Air, Water and Soil). Chemosphere 2021, 263, 127926. [Google Scholar] [CrossRef]
- Mourhir, A. Scoping Review of the Potentials of Fuzzy Cognitive Maps as a Modeling Approach for Integrated Environmental Assessment and Management. Environ. Model. Softw. 2021, 135, 104891. [Google Scholar] [CrossRef]
- Bamakan, S.M.H.; Malekinejad, P.; Ziaeian, M.; Motavali, A. Bullwhip Effect Reduction Map for COVID-19 Vaccine Supply Chain. Sustain. Oper. Comput. 2021, 2, 139–148. [Google Scholar] [CrossRef]
- Assunção, E.R.G.T.R.; Ferreira, F.A.F.; Meidutė-Kavaliauskienė, I.; Zopounidis, C.; Pereira, L.F.; Correia, R.J.C. Rethinking Urban Sustainability Using Fuzzy Cognitive Mapping and System Dynamics. Int. J. Sustain. Dev. World Ecol. 2020, 27, 261–275. [Google Scholar] [CrossRef]
- Radhika, K.; Anbalagan, S.; Alexander, C. Symptoms of Lung Cancer Using Fuzzy Cognitive MAPs(FCMs)-An Analysis. Malaya J. Mat. 2020, 8, 709–711. [Google Scholar] [CrossRef]
- Martin, N.; Aleeswari, A.; Lilly Merline, W. Risk Factors of Lifestyle Diseases—Analysis by Decagonal Linguistic Neutrosophic Fuzzy Cognitive Map. Mater. Today Proc. 2020, 24, 1939–1943. [Google Scholar] [CrossRef]
- Aguilar, J. A Survey about Fuzzy Cognitive Maps Papers. Int. J. Comput. Cogn. 2005, 3, 27–33. [Google Scholar]
- Alipour, M.; Hafezi, R.; Papageorgiou, E.; Hafezi, M.; Alipour, M. Characteristics and Scenarios of Solar Energy Development in Iran: Fuzzy Cognitive Map-Based Approach. Renew. Sustain. Energy Rev. 2019, 116, 109410. [Google Scholar] [CrossRef]
- Akinnuwesi, B.A.; Adegbite, B.A.; Adelowo, F.; Ima-Edomwonyi, U.; Fashoto, G.; Amumeji, O.T. Decision Support System for Diagnosing Rheumatic-Musculoskeletal Disease Using Fuzzy Cognitive Map Technique. Inform. Med. Unlocked 2020, 18, 100279. [Google Scholar] [CrossRef]
- Sperry, R.C.; Jetter, A.J. A Systems Approach to Project Stakeholder Management: Fuzzy Cognitive Map Modeling. Proj. Manag. J. 2019, 50, 699–715. [Google Scholar] [CrossRef]
- Sánchez, H.; Aguilar, J.; Terán, O.; Gutiérrez De Mesa, J. Modeling the Process of Shaping the Public Opinion through Multilevel Fuzzy Cognitive Maps. Appl. Soft Comput. 2019, 85, 105756. [Google Scholar] [CrossRef]
- Dabbagh, R.; Yousefi, S. A Hybrid Decision-Making Approach Based on FCM and MOORA for Occupational Health and Safety Risk Analysis. J. Saf. Res. 2019, 71, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Reckien, D.; Van Maarseveen, M.F.A.M. A Generalised Fuzzy Cognitive Mapping Approach for Modelling Complex Systems. Appl. Soft Comput. 2019, 84, 105754. [Google Scholar] [CrossRef]
- Arji, G.; Ahmadi, H.; Nilashi, M.; Rashid, T.A.; Hassan Ahmed, O.; Aljojo, N.; Zainol, A. Fuzzy Logic Approach for Infectious Disease Diagnosis: A Methodical Evaluation, Literature and Classification. Biocybern. Biomed. Eng. 2019, 39, 937–955. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Akram, M. Medical Decision Support Systems Based on Fuzzy Cognitive Maps. Int. J. Biomath. 2019, 12, 1950069. [Google Scholar] [CrossRef]
- Begicheva, S. Fuzzy Model for Evaluating the Quality of Medical Care. In Proceedings of the 2019 IEEE 21st Conference on Business Informatics (CBI), Moscow, Russia, 15–17 July 2019; pp. 5–8. [Google Scholar]
- Poczeta, K.; Kubuś, Ł.; Yastrebov, A. Analysis of an Evolutionary Algorithm for Complex Fuzzy Cognitive Map Learning Based on Graph Theory Metrics and Output Concepts. Biosystems 2019, 179, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Puerto, E.; Aguilar, J.; López, C.; Chávez, D. Using Multilayer Fuzzy Cognitive Maps to Diagnose Autism Spectrum Disorder. Appl. Soft Comput. 2019, 75, 58–71. [Google Scholar] [CrossRef]
- Guo, K.; Chai, R.; Candra, H.; Guo, Y.; Song, R.; Nguyen, H.; Su, S. A Hybrid Fuzzy Cognitive Map/Support Vector Machine Approach for EEG-Based Emotion Classification Using Compressed Sensing. Int. J. Fuzzy Syst. 2019, 21, 263–273. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J. Learning of Fuzzy Cognitive Maps Using a Niching-Based Multi-Modal Multi-Agent Genetic Algorithm. Appl. Soft Comput. 2019, 74, 356–367. [Google Scholar] [CrossRef]
- Morone, P.; Falcone, P.M.; Lopolito, A. How to Promote a New and Sustainable Food Consumption Model: A Fuzzy Cognitive Map Study. J. Clean. Prod. 2019, 208, 563–574. [Google Scholar] [CrossRef]
- Azar, A.; Mostafaee Dolatabad, K. A Method for Modelling Operational Risk with Fuzzy Cognitive Maps and Bayesian Belief Networks. Expert. Syst. Appl. 2019, 115, 607–617. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhang, S.; Liu, J. A Novel Approach to Fuzzy Cognitive Map Based on Hesitant Fuzzy Sets for Modeling Risk Impact on Electric Power System. Int. J. Comput. Intell. Syst. 2019, 12, 842. [Google Scholar] [CrossRef]
- Jahangoshai Rezaee, M.; Yousefi, S.; Hayati, J. A Decision System Using Fuzzy Cognitive Map and Multi-Group Data Envelopment Analysis to Estimate Hospitals’ Outputs Level. Neural Comput. Applic 2018, 29, 761–777. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Ciarapica, F.E.; Mazzuto, G. Fuzzy Cognitive Maps for Adverse Drug Event Risk Management. Saf. Sci. 2018, 102, 194–210. [Google Scholar] [CrossRef]
- Anninou, A.P.; Groumpos, P.P.; Poulios, P.; Gkliatis, I. A New Approach of Dynamic Fuzzy Cognitive Knowledge Networks in Modelling Diagnosing Process of Meniscus. IFAC-PapersOnLine 2017, 50, 5861–5866. [Google Scholar] [CrossRef]
- Obiedat, M.; Samarasinghe, S. A Novel Semi-Quantitative Fuzzy Cognitive Map Model for Complex Systems for Addressing Challenging Participatory Real Life Problems. Appl. Soft Comput. 2016, 48, 91–110. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, J.; Gao, J. Improving Performance Evaluation of Health, Safety and Environment Management System by Combining Fuzzy Cognitive Maps and Relative Degree Analysis. Saf. Sci. 2016, 87, 92–100. [Google Scholar] [CrossRef]
- Büyükavcu, A.; Albayrak, Y.E.; Göker, N. A Fuzzy Information-Based Approach for Breast Cancer Risk Factors Assessment. Appl. Soft Comput. 2016, 38, 437–452. [Google Scholar] [CrossRef]
- Gaurav; Kumar, M.; Bhutani, K.; Aggarwal, S. Hybrid Model for Medical Diagnosis Using Neutrosophic Cognitive Maps with Genetic Algorithms. In Proceedings of the 2015 IEEE International Conference on Fuzzy Systems (FUZZ-IEEE), Istanbul, Turkey, 2–5 August 2015; pp. 1–7. [Google Scholar]
- Nguyen, T.; Khosravi, A.; Creighton, D.; Nahavandi, S. Medical Data Classification Using Interval Type-2 Fuzzy Logic System and Wavelets. Appl. Soft Comput. 2015, 30, 812–822. [Google Scholar] [CrossRef]
- Amirkhani, A.; Mosavi, M.R.; Mohammadizadeh, F.; Shokouhi, S.B. Classification of Intraductal Breast Lesions Based on the Fuzzy Cognitive Map. Arab. J. Sci. Eng. 2014, 39, 3723–3732. [Google Scholar] [CrossRef]
- Mei, S.; Zhu, Y.; Qiu, X.; Zhou, X.; Zu, Z.; Boukhanovsky, A.V.; Sloot, P.M.A. Individual Decision Making Can Drive Epidemics: A Fuzzy Cognitive Map Study. IEEE Trans. Fuzzy Syst. 2014, 22, 264–273. [Google Scholar] [CrossRef]
- Baykasoglu, A.; Durmusoglu, Z.D.U. A Hybrid MCDM for Private Primary School Assessment Using DEMATEL Based on ANP and Fuzzy Cognitive Map. Int. J. Comput. Intell. Syst. 2014, 7, 615. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Torsney-Weir, T.; Mago, V.K. A fuzzy cognitive map of the psychosocial determinants of obesity. Appl. Soft Comput. 2012, 12, 3711–3724. [Google Scholar] [CrossRef]
- Groumpos, P.P.; Anninou, A.P. A theoretical mathematical modeling of Parkinson’s disease using Fuzzy Cognitive Maps. In Proceedings of the 2012 IEEE 12th International Conference on Bioinformatics & Bioengineering (BIBE), Larnaca, Cyprus, 11–13 November 2012; pp. 677–682. [Google Scholar]
- Lee, S.; Yang, J.; Han, J. Development of a Decision Making System for Selection of Dental Implant Abutments Based on the Fuzzy Cognitive Map. Expert. Syst. Appl. 2012, 39, 11564–11575. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Ciarapica, F.E.; Mazzuto, G. Analysis of Injury Events with Fuzzy Cognitive Maps. J. Loss Prev. Process Ind. 2012, 25, 677–685. [Google Scholar] [CrossRef]
- Sengan, S.; Kamalam, G.K.; Vellingiri, J.; Gopal, J.; Velayutham, P.; Subramaniyaswamy, V. Medical Information Retrieval Systems for E-Health Care Records Using Fuzzy Based Machine Learning Model. Microprocess. Microsyst. 2020, 103344. [Google Scholar] [CrossRef]
- Raza, K. Fuzzy Logic Based Approaches for Gene Regulatory Network Inference. Artif. Intell. Med. 2019, 97, 189–203. [Google Scholar] [CrossRef]
- Dion, A.; Joseph, L.; Jimenez, V.; Gutierrez, A.C.; Ben Ameur, A.; Robert, E.; Andersson, N. Grounding Evidence in Experience to Support People-Centered Health Services. Int. J. Public. Health 2019, 64, 797–802. [Google Scholar] [CrossRef]
- Ahmadi, H.; Gholamzadeh, M.; Shahmoradi, L.; Nilashi, M.; Rashvand, P. Diseases Diagnosis Using Fuzzy Logic Methods: A Systematic and Meta-Analysis Review. Comput. Methods Programs Biomed. 2018, 161, 145–172. [Google Scholar] [CrossRef]
- Siuly, S.; Zhang, Y. Medical Big Data: Neurological Diseases Diagnosis Through Medical Data Analysis. Data Sci. Eng. 2016, 1, 54–64. [Google Scholar] [CrossRef]
- Miranda, G.H.B.; Felipe, J.C. Computer-Aided Diagnosis System Based on Fuzzy Logic for Breast Cancer Categorization. Comput. Biol. Med. 2015, 64, 334–346. [Google Scholar] [CrossRef]
| Publication | Key Contribution |
|---|---|
| Feleki et al. [24] | Proposed DeepFCM for CAD diagnosis, integrating MPI, clinical data, and natural language insights. Achieved 83.07% accuracy, 86.21% sensitivity, and 79.99% specificity. Enhanced explainability with Grad-CAM, weight disclosure, and GPT 3.5. |
| Feleki et al. [29] | Introduced FCM-PSO and DeepFCM for CAD classification, achieving 77.95% accuracy. The DeepFCM framework combines image and clinical data, providing enhanced explainability for nuclear physicians by revealing meaningful causal relationships between clinical factors in diagnosis. The method outperforms traditional machine learning algorithms, demonstrating efficiency in CAD diagnosis. |
| Al-Halabi et al. [23] | Analyzed mental models in breast augmentation and flexor tendon repair in plastic surgery. Identified five cognitive competency domains: situation awareness, decision-making, task management, leadership, and communication/teamwork. Framework aids teaching and assessment of competencies. |
| Hoyos et al. [25] | Clinical decision-support system for dengue diagnosis utilises a fuzzy cognitive map, achieving 89.4% accuracy. The model, based on signs, symptoms, and laboratory tests, provides an explainable method for evaluating dengue severity. |
| Sovatzidi et al. [26] | Proposed Constructive FCM framework utilises EEG data for adolescent depression severity estimation. Simplifies FCM creation, reduces expert involvement, ensures interpretability, and demonstrates effectiveness on a recent dataset. |
| Sarmiento et al. [45] | Fuzzy cognitive mapping in northern Nigeria identified frequent sex, lack of contraception use, family dynamics, and lack of male involvement as major causes of short birth intervals (kunika). Cultural dynamics emphasized the need for comprehensive strategies beyond contraception promotion. |
| Apostolopoulos et al. [10] | FCM for CAD detection uses State Space Advanced FCMs with rule-based mechanism. Achieves 85.47% accuracy, an improvement of more than 7% over traditional FCM. |
| Apostolopoulos et al. [46] | MDSS for CAD diagnosis using FCMs achieves 78.2% accuracy, surpassing state-of-the-art classification algorithms. |
| Amirkhani et al. [22] | A novel Fuzzy Cognitive Map (FCM) model predicts DNA-binding residues in protein sequences, achieving AUC = 0.72. Outperforms hybridNAP and various machine learning methods. Enhanced performance attributed to FCM’s intrinsic feature incorporating input feature relations. |
| Lucchiari et al. [47] | Illustrates a differential diagnosis between psychogenic non-epileptic seizures (PNES) and epileptic seizures (ES). In the model, decision-concepts (PNES, ES) and factor-concepts (important distinguishing factors) are represented, demonstrating the FCM’s applicability in ambiguous contexts with incomplete or unreliable information. |
| Douali et al. [48] | Case-based fuzzy cognitive maps proposed for medical diagnosis, evaluated against Bayesian belief networks. Utilised a semantic web framework with a database of 174 patients, demonstrating the approach’s effectiveness through statistical comparison. |
| Lee et al. [49] | Proposed method designs sinusoidal-type and linear activation functions for FCMs in clinical decision-making. Focuses on improving visibility and stability in inference processes, addressing the limitations of sigmoid functions. Applied to pulmonary infections, results indicate appropriateness for clinical decision-making. |
| Georgopoulos et al. [27] | The GAFI-CFCM hybrid methodology, combining genetic algorithms and FCM, successfully applied to a diagnostic support model. Effectively processes patient information, reaching the most probable disorder diagnosis out of three options. |
| Nguyen et al. [50] | Utilises protein homologies and interaction network topology to enhance recall in predictions. Successfully annotates protein functions in Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster. Outperforms four state-of-the-art methods in terms of recall, precision, Matthews correlation coefficient, harmonic mean, and area under the ROC curves. |
| Papageorgiou et al. [28] | Developed an advanced diagnostic method for urinary bladder tumor grading using FCMs augmented with unsupervised active Hebbian learning (AHL) algorithm. Achieved a classification accuracy of 72.5%, 74.42%, and 95.55% for tumor grades I, II, and III, respectively. The method combines soft computing FCMs with specialised histopathological knowledge and AHL, providing a transparent and explainable solution for physicians. |
| Georgopoulos et al. [51] | Proposes a soft computing system for the differential diagnosis of dysarthria and apraxia of speech. Utilises a hierarchical FCM approach based on an established dysarthria classification system. The system aims to provide a “second opinion” or training support for clinicians facing the challenging task of accurate diagnosis of speech disorders. Tested successfully using case studies and real patients. |
| Georgopoulos et al. [52] | FCM-based approach for differentiating Specific Language Impairment (SLI) from dyslexia and autism. Initial phase uses literature-based “experts” for model development, with plans to involve expert specialists for enrichment. |
| Meier et al. [44] | METHAD employs machine intelligence for ethical advisory in medical dilemmas, using FCMs. The framework systematically breaks down medical ethics cases, employing fuzzy cognitive maps for ethical advisory. While the technology exists, ethical concerns arise, emphasizing the importance of human contact and empathy in medicine. |
| Poleto et al. [43] | FCMs, relying on expert knowledge, create intelligible cognitive maps and simulate scenarios for understanding and enhancing cybersecurity strategies in telehealth services. |
| Papageorgiou et al. [53] | A novel evolutionary-based fuzzy cognitive map (FCM) methodology enhances patient state forecasting in pulmonary infections. The research achieves improved prediction efficiency through innovative data fuzzification for observables and optimization of transformation function gain using evolutionary learning. Validation with real patient data from internal care units demonstrates lower prediction errors compared to conventional genetic-based algorithms. |
| Georgopoulos et al. [30] | Scenario-based Fuzzy Cognitive Maps (FCM) in Medical Decision Support Systems (MDSS) aid training by simulating decision-making processes. Illustrated in labor decision-making, this approach enhances medical professional education for improved patient care. |
| Papageorgiou et al. [31] | Bayesian belief networks (BBNs) and fuzzy cognitive maps (FCMs) were employed for medical knowledge formalization in decision support within a semantic web framework. A general-purpose reasoning engine, EYE, facilitated reasoning on these models. Validation using the UTI therapy problem demonstrated the reliability and efficiency of these approaches in semantic web decision support tasks. |
| Lucchiari et al. [32] | Underscores the importance of diagnostic reasoning in clinical practice and critiques the limitations of traditional and solely objective models. It acknowledges the impact of cognitive biases on diagnoses and challenges in implementing debiasing techniques. The proposed solution integrates cognitive understanding with technology, suggesting a conceptual scheme using fuzzy cognitive maps for safer medical practices. |
| Stylios et al. [15] | A hierarchical Fuzzy Cognitive Map (FCM) in Medical Decision Support Systems integrates factors for dynamic decision-making, enhancing maternal safety. Enables strategic planning and timely decisions, minimizing fetal distress and maternal complications. |
| Papageorgiou et al. [54] | The proposed FCM-based architecture integrates various data types, enhancing the model through unsupervised learning. FCMs provide robust reasoning by capturing complex relationships among concepts. The approach synergises fuzzy and neural techniques, incorporating rules from knowledge processing and data mining. It ensures transparency and interpretability in medical decision-making. |
| Papageorgiou et al. [55] | Decision Tree-Fuzzy Cognitive Map hybrid model that enhances decision-making tasks, effectively handling different types of input data, demonstrated within a medical context. |
| John et al. [56] | Matlab prototype tested for influenza diagnosis using fuzzy cognitive maps. Symptom observations considered duration certainty and intensity variations. Results showed support index variation for differential diagnosis, emphasizing parameter tuning and uncertainty consideration for practical applicability. |
| Babroudi et al. [41] | Findings highlight hospital reliability, hygiene, and completeness as top influential factors in improving health service quality during infectious disease circumstances. |
| Groumpos et al. [57] | Advanced Fuzzy Cognitive Maps (AFCM) model to predict the COVID-19 pandemic’s spread. Unlike statistical models, AFCM captures dynamic cause-and-effect relationships among predefined factors. The model, evaluated using data from Greece, South Korea, and Germany, achieved high accuracy in predicting confirmed cases, with coefficients of determination and Pearson’s correlation coefficients demonstrating its effectiveness. |
| Dogu et al. [42] | The study combines statistical-based fuzzy cognitive maps (SBFCM) and artificial neural networks (ANN) for predicting hospital stay length in COPD patients. SBFCM provides statistical analysis and gathers expert opinions to define input variables for the ANN model. The integrated approach outperforms other methods with 79.95% accuracy, emphasizing the value of expert opinions in medical decision support for enhanced hospital management and better predictions in COPD care. |
| Saul et al. [58] | Presents FCMs to explore an individual’s personal construction system, identifying barriers to behavior change. Illustrated using a simulated case on healthy habit adoption, it suggests the potential for targeted psychological interventions. |
| Wu et al. [33] | Extended probabilistic linguistic fuzzy cognitive map model addresses the complexities of rural elderly health in China. Education is identified as the most critical factor influencing rural elderly health, followed by occupational history, psychology, and physical exercise. Intergenerational relationships gain prominence. |
| Khodadadi et al. [34] | An FCM approach is proposed for ischemic stroke risk diagnosis, employing non-linear Hebbian learning. Neurologists’ opinions determine individual risk rates, achieving a high accuracy of 93.6 ± 4.5% in testing using 110 real cases, outperforming support vector machine and K-nearest neighbors models. |
| Najafi et al. [35] | A cross-sectional study in Zanjan Province, Iran, explores the hypothesis that food insecurity contributes to esophageal cancer among women. Utilizing fuzzy cognitive maps (FCMs) for analysis, the research involves 580 women, revealing a 23% and 38% prevalence of hunger and hidden hunger, respectively. Only 39% have secure access to key nutrients. The study suggests an association between food insecurity, body mass index (BMI), and esophageal cancer. Results highlight the impact of food insecurity on nutritional status and its potential role in cancer development. |
| Billis et al. [59] | Modular decision support framework for aging well, including trend analysis, decision support core, and risk prediction. The trend analysis uses personalised sleep models, while the decision support core accurately identifies health states and monitors disease progression. Risk prediction, employing FCM-based approaches, shows promising results, especially in detecting depression. Real-life clinical case testing is crucial for validation. |
| Mahmoodi et al. [37] | Employed FCMs based on the Nonlinear Hebbian Learning (NHL) algorithm for complex system modeling. Utilised data from the medical records of 560 patients and selected 27 effective features with expert opinions. Achieved a prediction accuracy of 95.83%, surpassing other decision-making algorithms like decision trees, Naïve Bayes, and ANN. The proposed system is deemed simple, comprehensive, and effective for healthcare professionals in predicting gastric cancer risk factors in clinical settings. |
| Subramanian et al. [36] | Developed an integrated breast cancer risk prediction model using a two-level fuzzy cognitive map (FCM). Combined demographic factors and screening mammogram findings for enhanced risk assessment. Employed Hebbian-based learning to improve the model’s performance and aid in tumor grading and risk prediction. Demonstrated superior accuracy compared to benchmark machine learning methods. |
| Papageorgiou et al. [38] | Created a familial breast cancer risk assessment model using FCMs, focused on personalised decision-making and incorporating family history and demographic risk factors to identify hidden risks of breast cancer. Utilised Hebbian-based learning capabilities of FCM to enhance modeling and contribute to risk prediction. |
| de Brito et al. [60] | Developed a fuzzy model to quantify body image dissatisfaction. Successfully measured distress levels in cosmetic surgery patients. The model serves as a screening tool for Body Dysmorphic Disorder BDD in cosmetic surgery. Applicable in psychiatric practice for treating BDD patients. |
| Papageorgiou et al. [40] | Proposed fuzzy cognitive maps (FCMs) for modeling uUTI treatment decision-making. Developed a software tool, FCM-uUTI DSS, to assist in uUTI treatment management. Evaluated the tool in 38 patient cases, demonstrating reliability and functionality. Results showed the FCM-uUTI tool provides antibiotic suggestions for uUTI treatment. Highlighted the tool’s potential to make medical knowledge widely available through computer consultation systems. |
| Giles et al. [39] | Used FCMs to compare Aboriginal and conventional science perspectives on diabetes determinants. FCM detailed the complex system of culture, spirituality, and balance in the Aboriginal view. Highlighted tangible stressors and outcomes amenable to management and monitoring. Demonstrated FCM’s potential to integrate diverse perspectives into health management and policy. |
| Papageorgiou et al. [61] | Introduced FCMs for decision-making in radiation therapy. Used FCMs to estimate the final dose delivered to the target volume. Proposed a two-level integrated hierarchical structure for supervision and evaluation. Applied the methodology to two clinical case studies for testing and evaluation. Discussed the usefulness of the hierarchical structure and suggested future research directions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolopoulos, I.D.; Papandrianos, N.I.; Papathanasiou, N.D.; Papageorgiou, E.I. Fuzzy Cognitive Map Applications in Medicine over the Last Two Decades: A Review Study. Bioengineering 2024, 11, 139. https://doi.org/10.3390/bioengineering11020139
Apostolopoulos ID, Papandrianos NI, Papathanasiou ND, Papageorgiou EI. Fuzzy Cognitive Map Applications in Medicine over the Last Two Decades: A Review Study. Bioengineering. 2024; 11(2):139. https://doi.org/10.3390/bioengineering11020139
Chicago/Turabian StyleApostolopoulos, Ioannis D., Nikolaos I. Papandrianos, Nikolaos D. Papathanasiou, and Elpiniki I. Papageorgiou. 2024. "Fuzzy Cognitive Map Applications in Medicine over the Last Two Decades: A Review Study" Bioengineering 11, no. 2: 139. https://doi.org/10.3390/bioengineering11020139
APA StyleApostolopoulos, I. D., Papandrianos, N. I., Papathanasiou, N. D., & Papageorgiou, E. I. (2024). Fuzzy Cognitive Map Applications in Medicine over the Last Two Decades: A Review Study. Bioengineering, 11(2), 139. https://doi.org/10.3390/bioengineering11020139







