Feasibility of High-Cellular-Resolution Full-Field, Artificial-Intelligence-Assisted, Real-Time Optical Coherence Tomography in the Evaluation of Vitiligo: A Prospective Longitudinal Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Recruitment
2.2. OCT Imaging Protocol
2.3. In Vivo High-Cellular-Resolution Full-Field Optical Coherence Tomography (OCT)
2.4. Artificial-Intelligence-Enhanced Optical Coherence Tomography for Vitiligo Assessment
2.5. Statistical Analysis
3. Results
3.1. Demographic Data and Vitiligo Index Scores
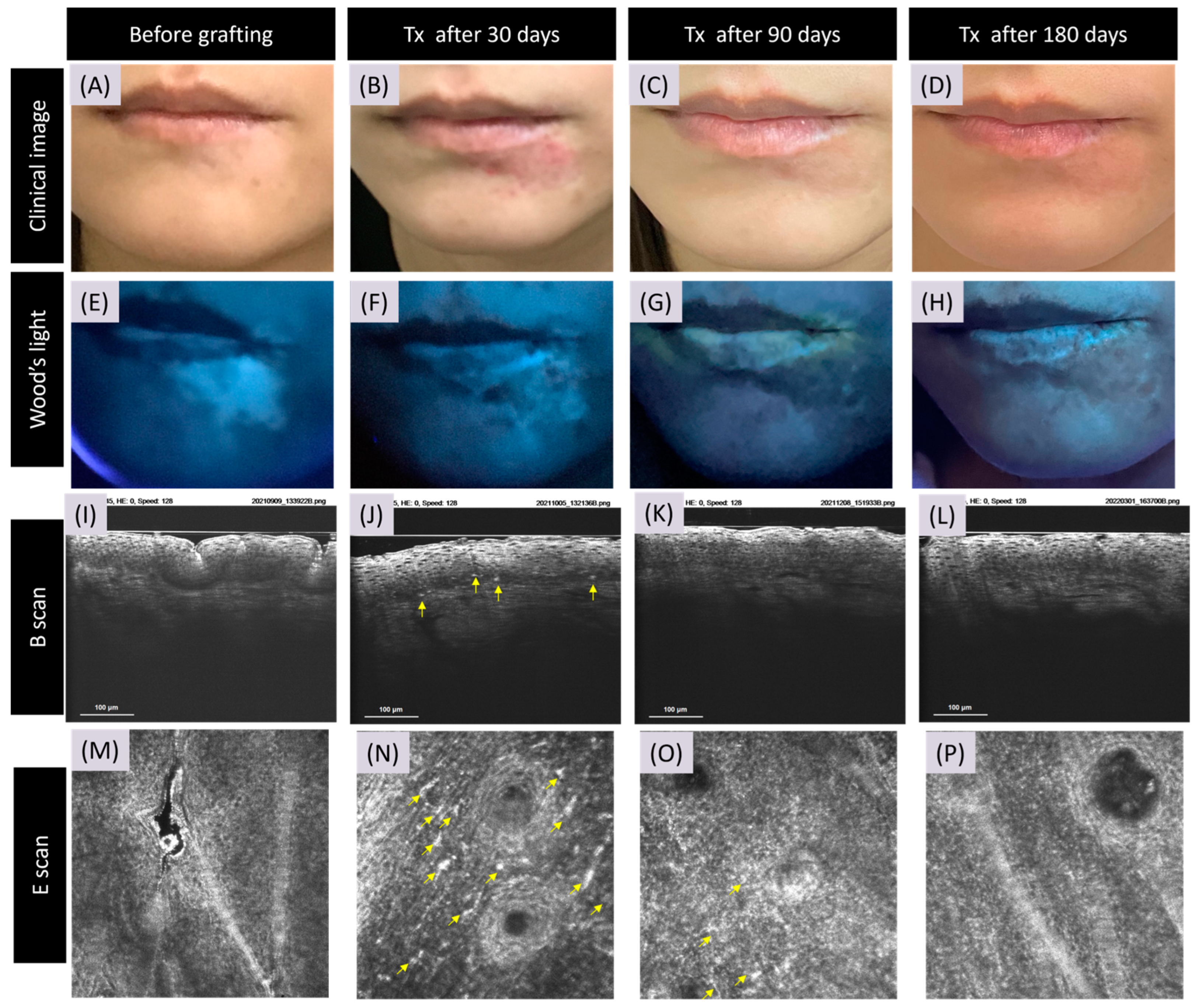
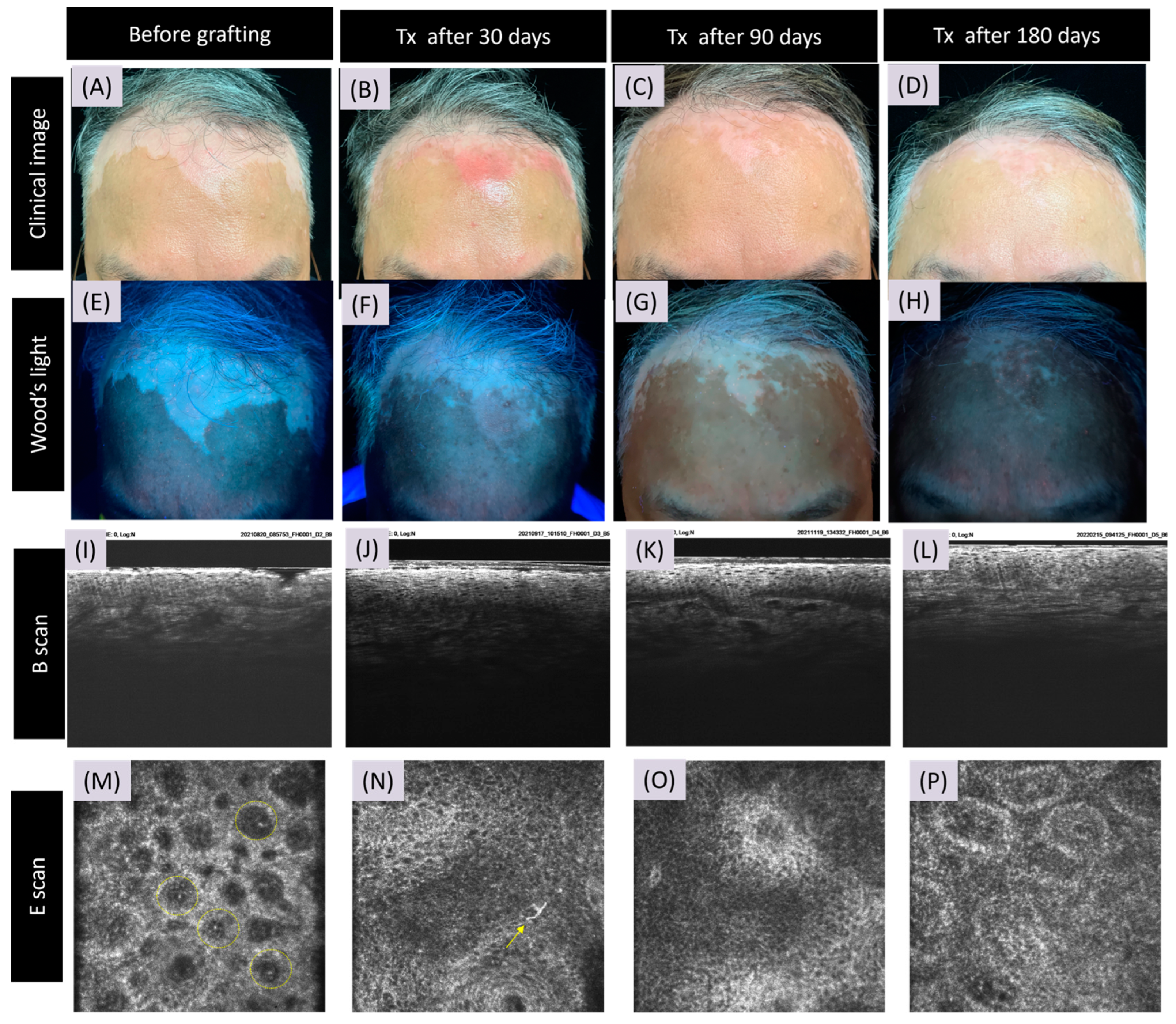
3.2. CRFF-OCT Features of Vitiligo
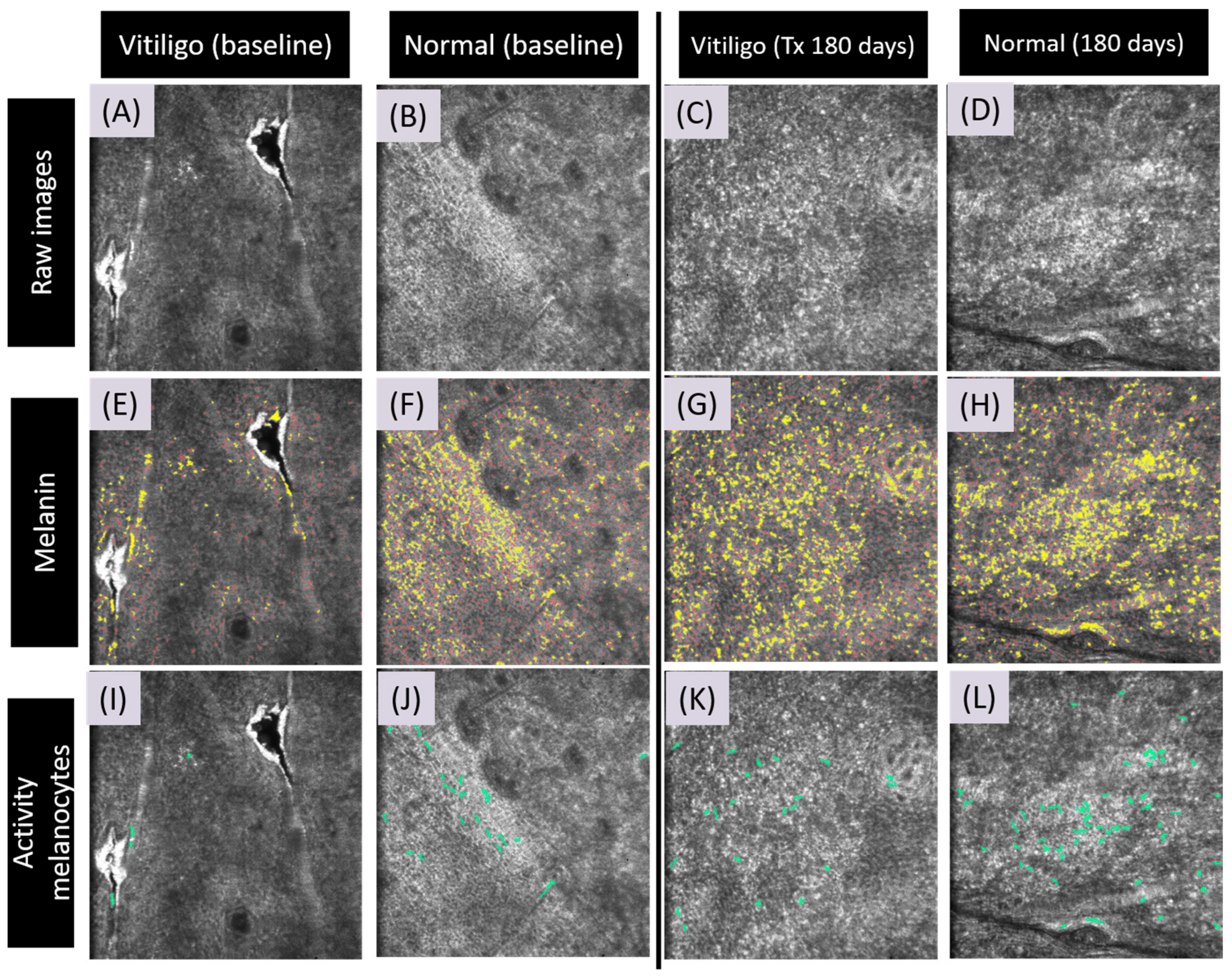
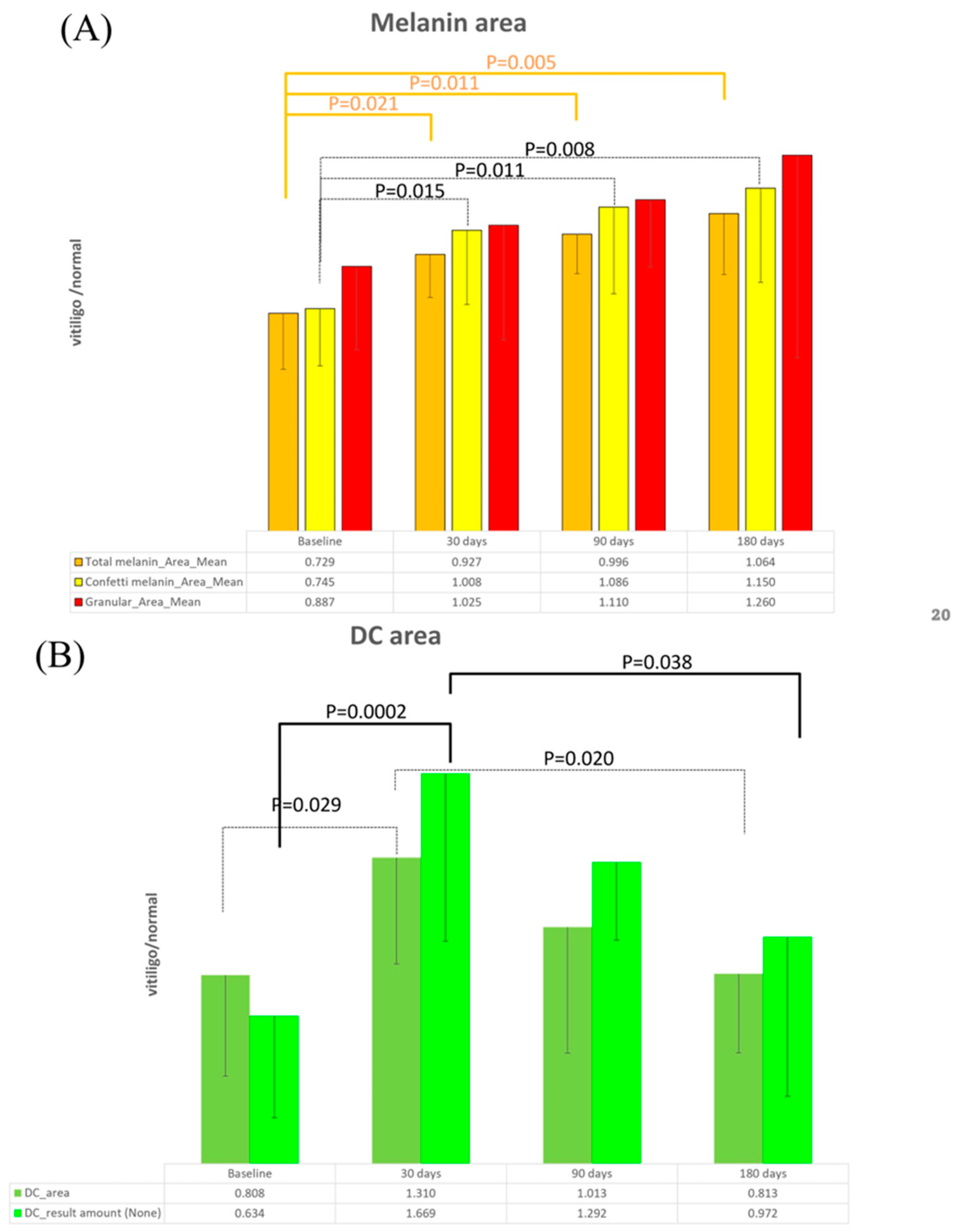
3.3. Artificial Intelligence Program for Melanin and Melanocyte Dendritic Cell Detection
3.4. Detection of Meanin and Melanocyte Dendritic Cells Using Artificial Intelligence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alghamdi, K.M.; Kumar, A.; Taieb, A.; Ezzedine, K. Assessment methods for the evaluation of vitiligo. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1463–1471. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef]
- Hamzavi, I.; Jain, H.; McLean, D.; Shapiro, J.; Zeng, H.; Lui, H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: The Vitiligo Area Scoring Index. Arch. Dermatol. 2004, 140, 677–683. [Google Scholar] [CrossRef]
- van Geel, N.; Hamzavi, I.; Kohli, I.; Wolkerstorfer, A.; Lim, H.W.; Bae, J.M.; Lui, H.; Harris, J.E.; Pandya, A.G.; Thng Tien Guan, S.; et al. Standardizing serial photography for assessing and monitoring vitiligo: A core set of international recommendations for essential clinical and technical specifications. J. Am. Acad. Dermatol. 2020, 83, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Thatte, S.S.; Khopkar, U.S. The utility of dermoscopy in the diagnosis of evolving lesions of vitiligo. Indian. J. Dermatol. Venereol. Leprol. 2014, 80, 505–508. [Google Scholar] [CrossRef]
- Hou, Y.; Wei, Z.; Jiang, Q.; Chen, H.; Chen, L.; Wu, J. In-depth study of Wood’s lamp examination combined with reflective confocal laser scanning microscopy for the guidance of vitiligo staging and treatment. J. Cosmet. Dermatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Uitentuis, S.E.; Heilmann, M.N.; Verdaasdonk, R.M.; Bae, J.M.; Luiten, R.M.; Wolkerstorfer, A.; Bekkenk, M.W. Ultraviolet photography in vitiligo: Image quality, validity and reliability. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1590–1594. [Google Scholar] [CrossRef]
- Brazzelli, V.; Muzio, F.; Antoninetti, M.; Villani, S.; Donadini, F.; Altomare, A.; Borroni, G. The perilesional skin in vitiligo: A colorimetric in vivo study of 25 patients. Photodermatol. Photoimmunol. Photomed. 2008, 24, 314–317. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Xu, A.E. Role of in vivo reflectance confocal microscopy in determining stability in vitiligo: A preliminary study. Indian. J. Dermatol. 2013, 58, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Chuah, S.Y.; Thng, S.T.G. The role of in vivo reflectance confocal microscopy in assessing the stability of vitiligo vulgaris prior to cellular grafting. Skin. Res. Technol. 2019, 25, 245–247. [Google Scholar] [CrossRef]
- Lai, L.G.; Xu, A.E. In vivo reflectance confocal microscopy imaging of vitiligo, nevus depigmentosus and nevus anemicus. Skin. Res. Technol. 2011, 17, 404–410. [Google Scholar] [CrossRef]
- Ardigo, M.; Malizewsky, I.; Dell’anna, M.L.; Berardesca, E.; Picardo, M. Preliminary evaluation of vitiligo using in vivo reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1344–1350. [Google Scholar] [CrossRef]
- Cortelazzi, C.; Pellacani, G.; Raposio, E.; Di Nuzzo, S. Vitiligo management: Combination of surgical treatment and phototherapy under reflectance confocal microscopy monitoring. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7366–7371. [Google Scholar] [CrossRef]
- Olsen, J.; Holmes, J.; Jemec, G.B. Advances in optical coherence tomography in dermatology-a review. J. Biomed. Opt. 2018, 23, 1–10. [Google Scholar] [CrossRef]
- Chen, I.L.; Wang, Y.J.; Chang, C.C.; Wu, Y.H.; Lu, C.W.; Shen, J.W.; Huang, L.; Lin, B.S.; Chiang, H.M. Computer-Aided Detection (CADe) System with Optical Coherent Tomography for Melanin Morphology Quantification in Melasma Patients. Diagnostics 2021, 11, 1498. [Google Scholar] [CrossRef]
- Rajabi-Estarabadi, A.; Bittar, J.M.; Zheng, C.; Nascimento, V.; Camacho, I.; Feun, L.G.; Nasiriavanaki, M.; Kunz, M.; Nouri, K. Optical coherence tomography imaging of melanoma skin cancer. Lasers Med. Sci. 2019, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Csuka, E.A.; Ward, S.C.; Ekelem, C.; Csuka, D.A.; Ardigo, M.; Mesinkovska, N.A. Reflectance Confocal Microscopy, Optical Coherence Tomography, and Multiphoton Microscopy in Inflammatory Skin Disease Diagnosis. Lasers Surg. Med. 2021, 53, 776–797. [Google Scholar] [CrossRef]
- Kislevitz, M.; Akgul, Y.; Wamsley, C.; Hoopman, J.; Kenkel, J. Use of Optical Coherence Tomography (OCT) in Aesthetic Skin Assessment-A Short Review. Lasers Surg. Med. 2020, 52, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Whigham, B.T.; Al-Aswad, L.A. Artificial Intelligence and Optical Coherence Tomography Imaging. Asia Pac. J. Ophthalmol. 2019, 8, 187–194. [Google Scholar] [CrossRef]
- Dahrouj, M.; Miller, J.B. Artificial Intelligence (AI) and Retinal Optical Coherence Tomography (OCT). Semin. Ophthalmol. 2021, 36, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Liu, T.Y.A.; Jones, C. Optical Coherence Tomography Angiography, Artificial Intelligence, and the Missing Capillaries. JAMA Ophthalmol. 2023, 141, 649–650. [Google Scholar] [CrossRef]
- Tsai, M.R.; Ho, T.S.; Wu, Y.H.; Lu, C.W. In Vivo dual-mode full-field optical coherence tomography for differentiation of types of melanocytic nevi. J. Biomed. Opt. 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, J.Y.; Wu, Y.H. Application of Cellular Resolution Full-Field Optical Coherence Tomography in vivo for the Diagnosis of Skin Tumours and Inflammatory Skin Diseases: A Pilot Study. Dermatology 2022, 238, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Zuiderveld, K. VIII.5.—Contrast Limited Adaptive Histogram Equalization. In Graphics Gems; Heckbert, P.S., Ed.; Academic Press: Cambridge, MA, USA, 1994; pp. 474–485. [Google Scholar]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Cambridge, MA, USA, 11–13 October 1998. [Google Scholar]
- Boone, M.A.; Marneffe, A.; Suppa, M.; Miyamoto, M.; Alarcon, I.; Hofmann-Wellenhof, R.; Malvehy, J.; Pellacani, G.; Del Marmol, V. High-definition optical coherence tomography algorithm for the discrimination of actinic keratosis from normal skin and from squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Wahrlich, C.; Alawi, S.A.; Batz, S.; Fluhr, J.W.; Lademann, J.; Ulrich, M. Assessment of a scoring system for Basal Cell Carcinoma with multi-beam optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Greco, M.; Farnetani, F.; Ciardo, S.; De Carvalho, N.; Mandel, V.D.; Starace, M.; Pellacani, G. Acne: Morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Aldahan, A.S.; Chen, L.L.; Fertig, R.M.; Holmes, J.; Shah, V.V.; Mlacker, S.; Hsu, V.M.; Nouri, K.; Tosti, A. Vascular Features of Nail Psoriasis Using Dynamic Optical Coherence Tomography. Skin Appendage Disord. 2017, 2, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.; Jemec, G.B.; Del Marmol, V. Differentiating allergic and irritant contact dermatitis by high-definition optical coherence tomography: A pilot study. Arch. Dermatol. Res. 2015, 307, 11–22. [Google Scholar] [CrossRef]
- Kang, H.Y.; le Duff, F.; Passeron, T.; Lacour, J.P.; Ortonne, J.P.; Bahadoran, P. A noninvasive technique, reflectance confocal microscopy, for the characterization of melanocyte loss in untreated and treated vitiligo lesions. J. Am. Acad. Dermatol. 2010, 63, e97–e100. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Yan, F.; Zhang, Z.H.; Zhang, Q.A.; Xiang, L.H.; Zheng, Z.Z. In vivo reflectance confocal microscopy for the differential diagnosis between vitiligo and nevus depigmentosus. Int. J. Dermatol. 2011, 50, 740–745. [Google Scholar] [CrossRef]
| Characteristics | N = 10 |
|---|---|
| Gender (male/female) | 4/6 |
| Age (years; mean + SD) | 34 + 14.9 |
| Recipient sites | |
| - Eyebrow | 2 |
| - Jaw | 3 |
| - Forehead | 2 |
| - Postauricular area | 1 |
| - Periorbital | 2 |
| VIDA score at first visit | |
| - 0 | 2 |
| - −1 | 8 |
| Average duration of active vitiligo (years; mean + SD) | 1.65 ± 1.1 |
| VASI score at first visit | |
| - 100% (complete depigmentation) | 7 |
| - 90% (specks of pigment present) | 3 |
| Vitiligo PRI score at first visit | |
| - Type A * | 1 |
| - Type C * | 9 |
| Sex/Age | Last Time of Active Vitiligo | Grafting Area | VASI Score * | ||||
|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | ||||
| 1 | F/37 | >1 year | Eyebrow and forehead | 100% | 25% | 25% | 10% |
| 2 | F/20 | 1 year ago | Jaw | 90% | 25% | 25% | 10% |
| 3 | F/55 | 1 year ago | Scalp | 100% | 25% | 25% | 25% |
| 4 | M/24 | 1 year ago | Postauricular area | 100% | 25% | 25% | 10% |
| 5 | F/65 | >1 year | Forehead | 90% | 75% | 10% | 25% |
| 6 | M/24 | 2 years | Eyebrow | 100% | 25% | 25% | 10% |
| 7 | M/39 | 2 years | Jaw | 100% | 50% | 10% | 10% |
| 8 | F/33 | 4–5 years | Jaw | 90% | 50% | 10% | 10% |
| 9 | M/44 | 2 years | Periorbital | 100% | 10% | 10% | 10% |
| 10 | F/35 | >1 year | Periorbital | 100% | 10% | 10% | 10% |
| Reflectance Confocal Microscopy (RCM) | Optical Coherence Tomography (OCT) | |
|---|---|---|
| Devices and specifications | Lucid Vivascope 1500® (Henrietta, New York, NY, USA) | Apollo Medical Optics Inc. |
| Advantages |
|
|
| Imaging section plane |
|
|
| Limitations |
|
|
| Score index and definition | RCM scoring: (1) Pigmentation status: +1 for presence of remaining pigment −1 for complete loss of pigment (2) Border status of vitiligo lesions: +1 for indistinct border −1 for clear border (3) Inflammatory cell infiltrate: +1 for presence of inflammatory cells −1 for absence of inflammatory cells (4) Melanocyte regeneration −1: if dendritic melanocytes appear in vitiligo lesion Total score interpretation:
| OCT scoring: OCT melanin grading is determined by the pigment area ratio score at the junction of the dermis and epidermis, categorized as follows:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.-Y.; Chen, Y.-T.; Chen, I.-L.; Shih, Y.-C.; Liu, R.T.-L.; Lai, Y.-J.; Ng, C.Y. Feasibility of High-Cellular-Resolution Full-Field, Artificial-Intelligence-Assisted, Real-Time Optical Coherence Tomography in the Evaluation of Vitiligo: A Prospective Longitudinal Follow-Up Study. Bioengineering 2024, 11, 196. https://doi.org/10.3390/bioengineering11020196
Lu L-Y, Chen Y-T, Chen I-L, Shih Y-C, Liu RT-L, Lai Y-J, Ng CY. Feasibility of High-Cellular-Resolution Full-Field, Artificial-Intelligence-Assisted, Real-Time Optical Coherence Tomography in the Evaluation of Vitiligo: A Prospective Longitudinal Follow-Up Study. Bioengineering. 2024; 11(2):196. https://doi.org/10.3390/bioengineering11020196
Chicago/Turabian StyleLu, Lai-Ying, Yi-Ting Chen, I-Ling Chen, Yu-Chang Shih, Rosalie Tzu-Li Liu, Yi-Jing Lai, and Chau Yee Ng. 2024. "Feasibility of High-Cellular-Resolution Full-Field, Artificial-Intelligence-Assisted, Real-Time Optical Coherence Tomography in the Evaluation of Vitiligo: A Prospective Longitudinal Follow-Up Study" Bioengineering 11, no. 2: 196. https://doi.org/10.3390/bioengineering11020196
APA StyleLu, L.-Y., Chen, Y.-T., Chen, I.-L., Shih, Y.-C., Liu, R. T.-L., Lai, Y.-J., & Ng, C. Y. (2024). Feasibility of High-Cellular-Resolution Full-Field, Artificial-Intelligence-Assisted, Real-Time Optical Coherence Tomography in the Evaluation of Vitiligo: A Prospective Longitudinal Follow-Up Study. Bioengineering, 11(2), 196. https://doi.org/10.3390/bioengineering11020196







