Enhancing Antibody-Specific Productivity: Unraveling the Impact of XBP1s Overexpression and Glutamine Availability in SP2/0 Cells
Abstract
:1. Introduction
2. Results
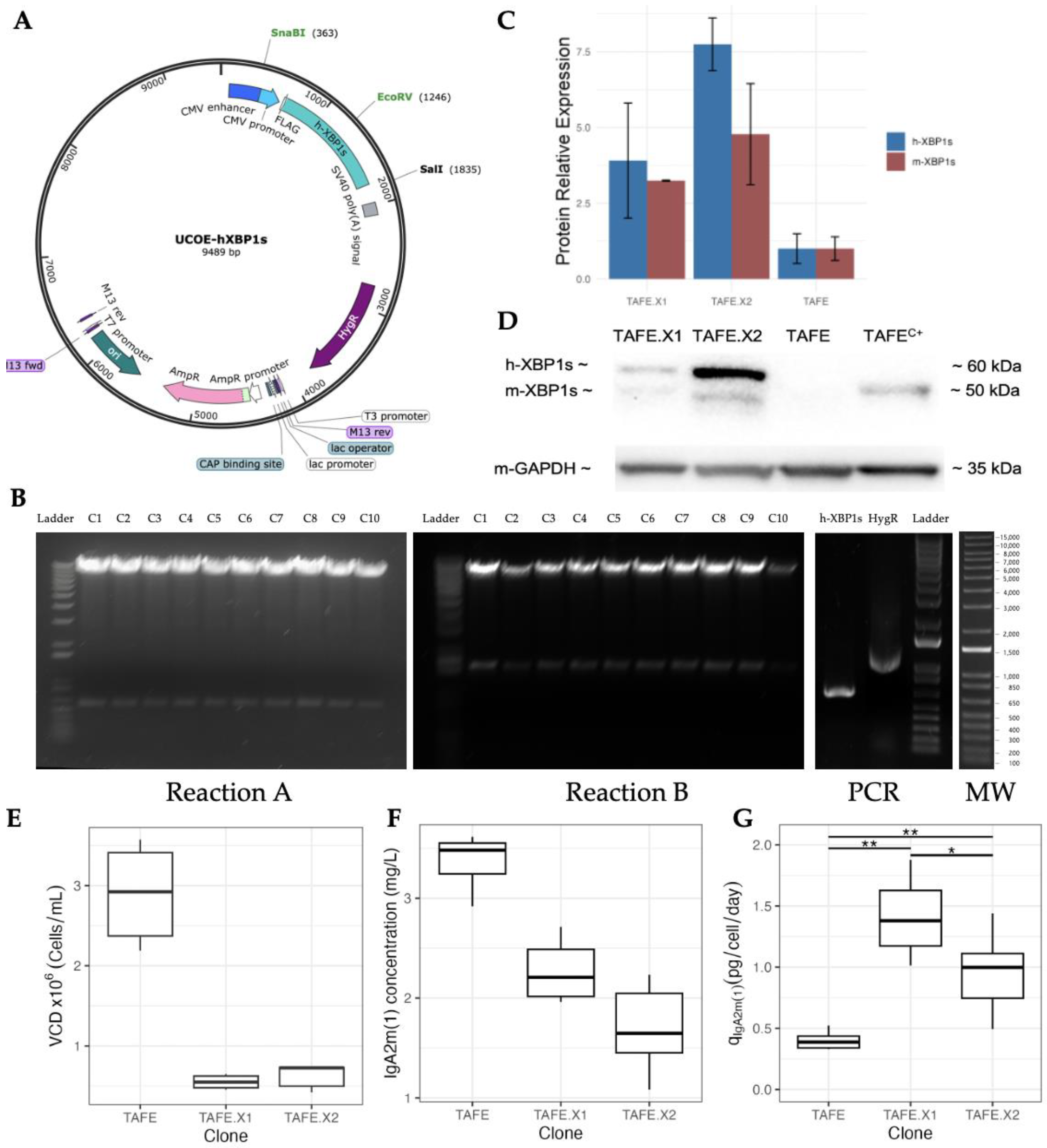
2.1. Establishing a Stable Cell Line Overexpressing h-XBP1s
2.1.1. Cloning Vector Construction
2.1.2. Identification and Characterization of Optimal h-XBP1s Overexpressor Clones
2.2. Adapting the Cells to Stirring and Low Serum Media
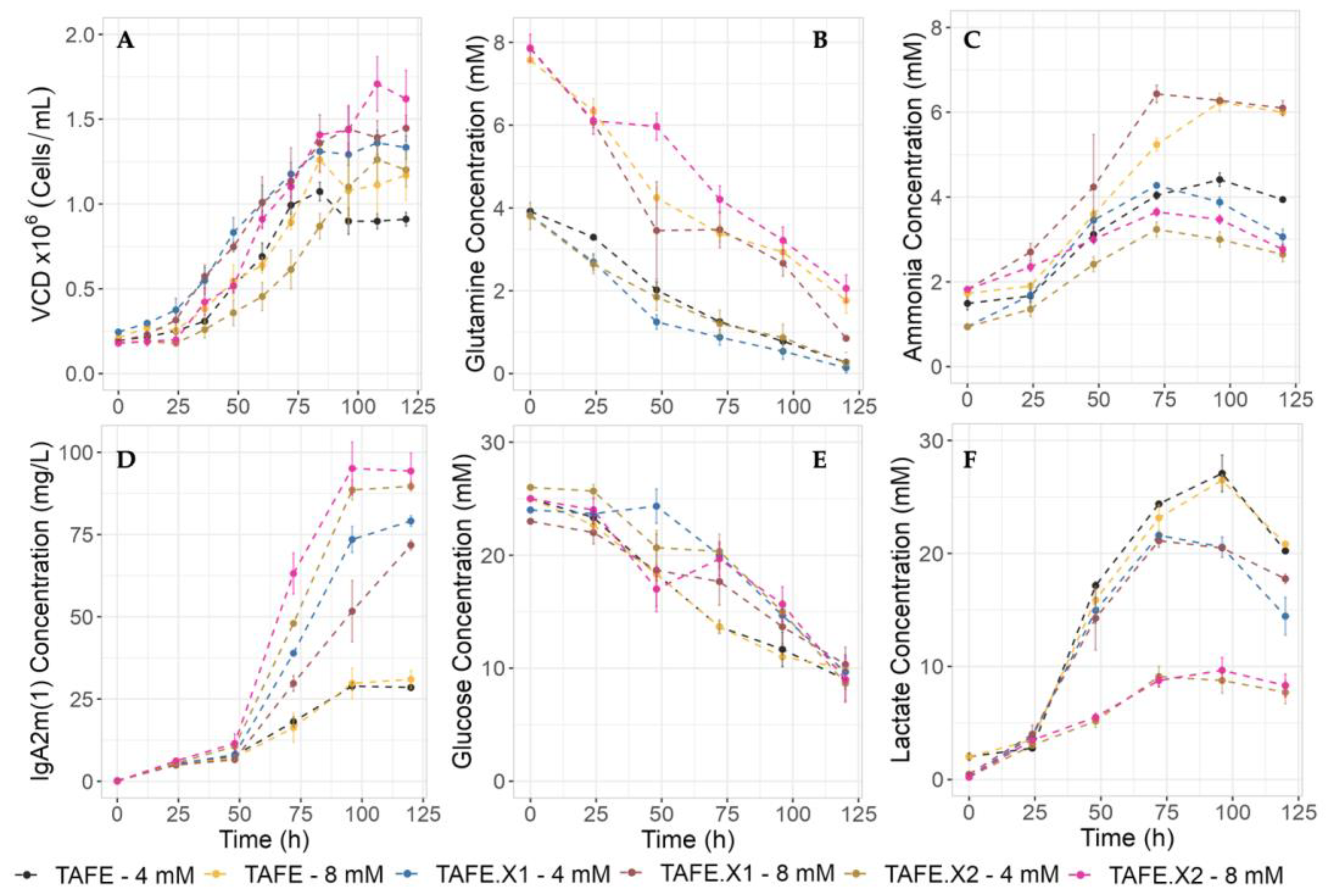
2.3. Assessing Kinetics of h-XBP1s Overexpression in High Glutamine Environments
2.3.1. Cell Growth and IgA2m(1) Production
2.3.2. Glutamine Consumption and Ammonia Production
2.3.3. Glucose Consumption and Lactate Production
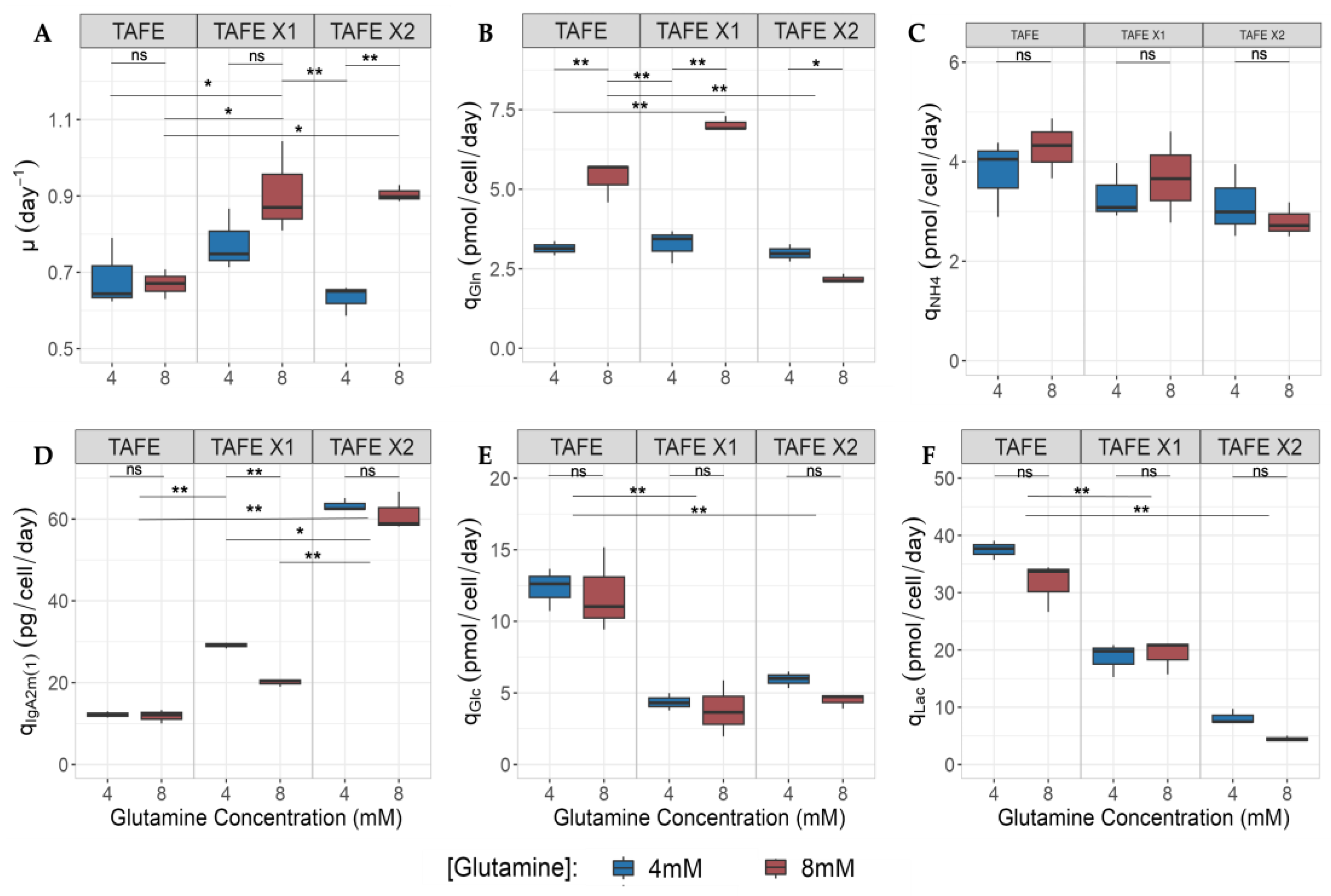
2.3.4. Impact of h-XBP1s Overexpression and Glutamine over Kinetic Parameter
3. Discussion
4. Limitations
5. Materials and Methods
5.1. Cell Culture and Media
5.2. Construction of the h-XBP1s Cloning Vector
5.3. E. coli Transformation, Purification, and Characterization of the UCOE-hXBP1s Cloning Vector
5.4. Stable Lipotransfection of IgA2m(1) Producer SP2/0 Cells with the UCOE-hXBP1s Plasmid
5.5. h-XBP1s Relative Expression: Western Blot
5.6. IgA2m(1) Quantification: ELISA
5.7. Cell Culture Platform for Agitation and Low Serum
5.8. Batch Culture of h-XBP1s Overexpression Cells
5.9. Metabolic Analysis of Lactate, Ammonia, and Glucose
5.10. Specific Rate Calculation
5.11. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perše, M.; Večerić-Haler, Ž. The Role of IgA in the Pathogenesis of IgA Nephropathy. Int. J. Mol. Sci. 2019, 20, 6199. [Google Scholar] [CrossRef]
- Johansen, F.; Braathen, R.; Brandtzaeg, P. Role of J Chain in Secretory Immunoglobulin Formation. Scand. J. Immunol. 2000, 52, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.M.; Chintalacharuvu, K.R.; Penichet, M.L.; Morrison, S.L. Myeloma expression systems. J. Immunol. Methods 2002, 261, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, A.E.; Bjorkman, P.J.; Herr, A.B. Structural insights into antibody-mediated mucosal immunity. Curr. Top. Microbiol. Immunol. 2006, 308, 173–204. [Google Scholar] [PubMed]
- Torres, M.; Akhtar, S.; McKenzie, E.A.; Dickson, A.J. Temperature Down-Shift Modifies Expression of UPR-/ERAD-Related Genes and Enhances Production of a Chimeric Fusion Protein in CHO Cells. Biotechnol. J. 2020, 16, e2000081. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.B.; Roberts, L.S.; Chandra, V.; Rivera, I.G.; Nomura, D.K.; Forbes, D.J.; Niwa, M. The UPR Activator ATF6 Responds to Proteotoxic and Lipotoxic Stress by Distinct Mechanisms Arvin. Dev. Cell 2018, 46, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Pour Savizi, I.S.; Motamedian, E.; Lewis, N.E.; del Val, I.J.; Shojaosadati, S.A. An integrated modular framework for modeling the effect of ammonium on the sialylation process of monoclonal antibodies produced by CHO cells. Biotechnol. J. 2021, 16, 2100019. [Google Scholar] [CrossRef]
- Slivac, I.; Blajić, V.; Radošević, K.; Kniewald, Z.; Srček, V.G. Influence of different ammonium, lactate and glutamine concentrations on CCO cell growth. Cytotechnology 2010, 62, 585–594. [Google Scholar] [CrossRef]
- Lie, S.; Wang, T.; Forbes, B.; Proud, C.G.; Petersen, J. The ability to utilise ammonia as nitrogen source is cell type specific and intricately linked to GDH, AMPK and mTORC1. Sci. Rep. 2019, 9, 1461. [Google Scholar] [CrossRef]
- Blondeel, E.J.; Aucoin, M.G. Supplementing glycosylation: A review of applying nucleotide-sugar precursors to growth medium to affect therapeutic recombinant protein glycoform distributions. Biotechnol. Adv. 2018, 36, 1505–1523. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Harnett, C.C.; Abusneina, A.; Clément, J.; Gauthier, E.R. Inhibition of MCL-1 by obatoclax sensitizes Sp2/0-Ag14 hybridoma cells to glutamine deprivation-induced apoptosis. Cell Biochem. Funct. 2015, 33, 334–340. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022, 21, 115–140. [Google Scholar] [CrossRef]
- Hussain, H.; Maldonado-Agurto, R.; Dickson, A.J. The endoplasmic reticulum and unfolded protein response in the control of mammalian recombinant protein production. Biotechnol. Lett. 2014, 36, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Remondelli, P.; Renna, M. The Endoplasmic Reticulum Unfolded Protein Response in Neurodegenerative Disorders and Its Potential Therapeutic Significance. Front. Mol. Neurosci. 2017, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Qi, L. Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018, 43, 593–605. [Google Scholar] [CrossRef]
- Park, S.-M.; Kang, T.-I.; So, J.-S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Almanza, A.; Mnich, K.; Blomme, A.; Robinson, C.M.; Rodriguez-Blanco, G.; Kierszniowska, S.; McGrath, E.P.; Le Gallo, M.; Pilalis, E.; Swinnen, J.V.; et al. Regulated IRE1α-dependent decay (RIDD)-mediated reprograming of lipid metabolism in cancer. Nat. Commun. 2022, 13, 2493. [Google Scholar] [CrossRef]
- Morotti, M.; Zois, C.E.; El-Ansari, R.; Craze, M.L.; Rakha, E.A.; Fan, S.-J.; Valli, A.; Haider, S.; Goberdhan, D.C.I.; Green, A.R.; et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer. Br. J. Cancer 2021, 124, 494–505. [Google Scholar] [CrossRef]
- Shi, W.; Chen, Z.; Li, L.; Liu, H.; Zhang, R.; Cheng, Q.; Xu, D.; Wu, L. Unravel the molecular mechanism of XBP1 in regulating the biology of cancer cells. J. Cancer 2019, 10, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Sandoval, T.A.; Chae, C.-S.; Chopra, S.; Tan, C.; Rutkowski, M.R.; Raundhal, M.; Chaurio, R.A.; Payne, K.K.; Konrad, C.; et al. IRE1α–XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 2018, 562, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Camera, D.; Jo, E.; Chan, S.M.H.; Herbert, T.P.; Molero, J.C.; Sun, R.-Q.; Zeng, X.-Y.; Ye, J.-M. Endoplasmic reticulum stress up-regulates Nedd4-2 to induce autophagy. FASEB J. 2016, 30, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, S.; Kondo, S.; Ogata, M.; Murakami, T.; Urano, F.; Imaizumi, K. XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem. Biophys. Res. Commun. 2005, 331, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Reimold, A.M.; Iwakoshi, N.N.; Manis, J.; Vallabhajosyula, P.; Szomolanyi-Tsuda, E.; Gravallese, E.M.; Friend, D.; Grusby, M.J.; Alt, F.; Glimcher, L.H. Plasma cell differentiation requires the transcription factor XBP-1. Nature 2001, 412, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J. Fat Controller. Sci. Signal. 2008, 1, ec228. [Google Scholar] [CrossRef]
- Latorre, Y.; Torres, M.; Vergara, M.; Berrios, J.; Sampayo, M.M.; Gödecke, N.; Wirth, D.; Hauser, H.; Dickson, A.J.; Altamirano, C. Engineering of Chinese hamster ovary cells for co-overexpressing MYC and XBP1s increased cell proliferation and recombinant EPO production. Sci. Rep. 2023, 13, 1482. [Google Scholar] [CrossRef]
- Ku, S.C.; Ng, D.T.; Yap, M.G.; Chao, S. Effects of overexpression of X-box binding protein 1 on recombinant protein production in Chinese hamster ovary and NS0 myeloma cells. Biotechnol. Bioeng. 2007, 99, 155–164. [Google Scholar] [CrossRef]
- Abaandou, L.; Quan, D.; Shiloach, J. Affecting HEK293 Cell Growth and Production Performance by Modifying the Expression of Specific Genes. Cells 2021, 10, 1667. [Google Scholar] [CrossRef]
- Tirosh, B.; Iwakoshi, N.N.; Glimcher, L.H.; Ploegh, H.L. XBP-1 specifically promotes IgM synthesis and secretion, but is dispensable for degradation of glycoproteins in primary B cells. J. Exp. Med. 2005, 202, 505–516. [Google Scholar] [CrossRef]
- Tigges, M.; Fussenegger, M. Xbp1-based engineering of secretory capacity enhances the productivity of Chinese hamster ovary cells. Metab. Eng. 2006, 8, 264–272. [Google Scholar] [CrossRef]
- Fink, E.E.; Moparthy, S.; Bagati, A.; Bianchi-Smiraglia, A.; Lipchick, B.C.; Wolff, D.W.; Roll, M.V.; Wang, J.; Liu, S.; Bakin, A.V.; et al. XBP1-KLF9 Axis Acts as a Molecular Rheostat to Control the Transition from Adaptive to Cytotoxic Unfolded Protein Response. Cell Rep. 2018, 25, 212–223.e4. [Google Scholar] [CrossRef]
- Formas-Oliveira, A.S.; Basílio, J.S.; Rodrigues, A.F.; Coroadinha, A.S. Overexpression of ER Protein Processing and Apoptosis Regulator Genes in Human Embryonic Kidney 293 Cells Improves Gene Therapy Vectors Production. Biotechnol. J. 2020, 15, e1900562. [Google Scholar] [CrossRef]
- Ku, S.C.Y.; Toh, P.C.; Lee, Y.Y.; Chusainow, J.; Yap, M.G.S.; Chao, S. Regulation of XBP-1 signaling during transient and stable recombinant protein production in CHO cells. Biotechnol. Prog. 2010, 26, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.L.; Biaggio, R.T.; Swiech, K. Strategies to suspension serum-free adaptation of mammalian cell lines for recombinant glycoprotein production. Methods Mol. Biol. 2018, 1674, 75–85. [Google Scholar] [PubMed]
- Zagari, F.; Jordan, M.; Stettler, M.; Broly, H.; Wurm, F.M. Lactate metabolism shift in CHO cell culture: The role of mitochondrial oxidative activity. New Biotechnol. 2013, 30, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Fendt, S.-M.; Frezza, C.; Erez, A. Targeting Metabolic Plasticity and Flexibility Dynamics for Cancer Therapy. Cancer Discov. 2020, 10, 1797–1807. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 2022, 13, 4981. [Google Scholar] [CrossRef]
- Chintalacharuvu, K.R.; Gurbaxani, B.; Morrison, S.L. Incomplete assembly of IgA2m(2) in Chinese hamster ovary cells. Mol. Immunol. 2007, 44, 3445–3452. [Google Scholar] [CrossRef]
- Sauer, P.W.; Burky, J.E.; Wesson, M.C.; Sternard, H.D.; Qu, L. A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol. Bioeng. 2000, 67, 585–597. [Google Scholar] [CrossRef]
- Bueno-Soler, A.; Palacios-Oliva, J.; Dorvignit-Pedroso, D.; Quintana-Cantillo, A.; Ramirez-Roque, Y.; Tomas-Pompa, J.S.; Solazabal-Armstrong, J.A.; Ruiz-Ramirez, I.; Acosta, C.M.-D.; Boggiano-Ayo, T.; et al. Production of an anti-TNFα antibody in murine myeloma cells by perfusion culture. Appl. Microbiol. Biotechnol. 2022, 106, 5007–5021. [Google Scholar] [CrossRef]
- Rossi, D.L.; Rossi, E.A.; Goldenberg, D.M.; Chang, C.-H. A new mammalian host cell with enhanced survival enables completely serum-free development of high-level protein production cell lines. Biotechnol. Prog. 2011, 27, 766–775. [Google Scholar] [CrossRef]
- Grammer, M.J. Mammalian cell cultures for biologics manufacturing. In Advances in Biochemical Engineering/Biotechnology; Kantardjieff, A., Zhou, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 139, pp. 1–9. [Google Scholar]
- Cain, K.; Peters, S.; Hailu, H.; Sweeney, B.; Stephens, P.; Heads, J.; Sarkar, K.; Ventom, A.; Page, C.; Dickson, A. A CHO cell line engineered to express XBP1 and ERO1-Lα has increased levels of transient protein expression. Biotechnol. Prog. 2013, 29, 697–706. [Google Scholar] [CrossRef]
- Codamo, J.; Hou, J.J.C.; Hughes, B.S.; Gray, P.P.; Munro, T.P. Efficient mAb production in CHO cells incorporating PEI-mediated transfection, mild hypothermia and the co-expression of XBP-1. J. Chem. Technol. Biotechnol. 2011, 86, 923–934. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Liu, S.; Liu, G.; Yao, K.; Yin, Y. l-Glutamine Attenuates Apoptosis Induced by Endoplasmic Reticulum Stress by Activating the IRE1α-XBP1 Axis in IPEC-J2: A Novel Mechanism of l-Glutamine in Promoting Intestinal Health. Int. J. Mol. Sci. 2017, 18, 2617. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Ni, M.; Gill, P.; Lee, A.S. Cell Surface Relocalization of the Endoplasmic Reticulum Chaperone and Unfolded Protein Response Regulator GRP78/BiP. J. Biol. Chem. 2010, 285, 15065–15075. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Ryno, L.M.; Genereux, J.C.; Moresco, J.J.; Tu, P.G.; Wu, C.; Yates, J.R., III; Su, A.I.; Kelly, J.W.; Wiseman, R.L. Stress-Independent Activation of XBP1s and/or ATF6 Reveals Three Functionally Diverse ER Proteostasis Environments. Cell Rep. 2013, 3, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Kitagaki, M.; Itagaki, H.; Aiba, S. Quantitative measurement of spliced XBP1 mRNA as an indicator of endoplasmic reticulum stress. J. Toxicol. Sci. 2006, 31, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, M.; Nasimian, A.; Rahmati, M.; Kawalec, P.; Machaj, F.; Rosik, J.; Bhushan, B.; Bathaie, S.Z.; Azarpira, N.; Los, M.J.; et al. The Role of BiP and the IRE1α–XBP1 Axis in Rhabdomyosarcoma Pathology. Cancers 2021, 13, 4927. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; San-Miguel, B.; Prause, C.; Marroni, N.; Cuevas, M.J.; González-Gallego, J.; Tuñón, M.J. Glutamine Treatment Attenuates Endoplasmic Reticulum Stress and Apoptosis in TNBS-Induced Colitis. PLoS ONE 2012, 7, e50407. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- English, B.C.; Savage, H.P.; Mahan, S.P.; Diaz-Ochoa, V.E.; Young, B.M.; Abuaita, B.H.; Sule, G.; Knight, J.S.; O’riordan, M.X.; Bäumler, A.J.; et al. The IRE1α-XBP1 Signaling Axis Promotes Glycolytic Reprogramming in Response to Inflammatory Stimuli. mBio 2023, 14, e0306822. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yu, C.; Yin, F.; Xu, W.; Pan, Y.; Yang, B.; Huang, T.; Chen, S.; Luo, W.; Su, T.; et al. IRE1α-XBP1 regulates PDK1-dependent induction of epithelial-mesenchymal transition in non-small cell lung cancer cells. Exp. Cell Res. 2022, 421, 113376. [Google Scholar] [CrossRef]
- Iovino, M.; Colonval, M.; Wilkin, C.; L’homme, L.; Lassence, C.; Campas, M.; Peulen, O.; de Tullio, P.; Piette, J.; Legrand-Poels, S. Novel XBP1s-independent function of IRE1 RNase in HIF-1α-mediated glycolysis upregulation in human macrophages upon stimulation with LPS or saturated fatty acid. Front. Immunol. 2023, 14, 1204126. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, X.; Liu, M.; Yang, Z.; Bi, Y.; Zou, H.; Wu, J.; Che, H.; Li, C.; Wang, X.; et al. XBP1 silencing decreases glioma cell viability and glycolysis possibly by inhibiting HK2 expression. J. Neuro-Oncol. 2016, 126, 455–462. [Google Scholar] [CrossRef]
- Piperi, C.; Adamopoulos, C.; Papavassiliou, A.G. XBP1: A Pivotal Transcriptional Regulator of Glucose and Lipid Metabolism. Trends Endocrinol. Metab. 2016, 27, 119–122. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, S.; Reno, C.M.; Sun, C. Regulation of Glucose Homeostasis Through a XBP-1-FoxO1 Unteraction. Nat. Med. 2011, 17, 356–365. [Google Scholar] [CrossRef]
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-Box Binding Protein 1 Couples the Unfolded Protein Response to Hexosamine Biosynthetic pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bhattacharya, A.; Qi, L. Endoplasmic Reticulum Quality Control in Cancer: Friend or Foe. Semin. Cancer Biol. 2015, 33, 25–33. [Google Scholar] [CrossRef]
- Bloedjes, T.A.; de Wilde, G.; Guikema, J.E.J. Metabolic Effects of Recurrent Genetic Aberrations in Multiple Myeloma. Cancers 2021, 13, 396. [Google Scholar] [CrossRef]
- Jahandar, H.; Vaziri, B.; Nematollahi, L.; Afsharirad, T.; Mirabzadeh, E.; Torkashvand, F.; Khalaj, V. Effect of Cysteamine on Cell Growth and IgG4 Production in Recombinant Sp2.0 Cells. Iran. J. Pharm. Res. 2015, 14, 177–187. [Google Scholar] [PubMed]
- Foster, L.; Catzel, D.; Atwa, S.; Zarka, M.; Mahler, S. Increase in synthesis of human monoclonal antibodies by transfected Sp2/0 myeloma mouse cell line under conditions of microgravity. Biotechnol. Lett. 2003, 25, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, V.; Marincola, F.M. Hypoxia and the phenomenon of immune exclusion. J. Transl. Med. 2021, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewska, S.; Collawn, J.F.; Bartoszewski, R. The Role of the Hypoxia-Related Unfolded Protein Response (UPR) in the Tumor Microenvironment. Cancers 2022, 14, 4870. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Adachi, M.; Zhao, S.; Hareyama, M.; Koong, A.C.; Luo, D.; Rando, T.A.; Imai, K.; Shinomura, Y. Preventing oxidative stress: A new role for XBP1. Cell Death Differ. 2009, 16, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Moisant, R.; Cowles, E.; Broutel, L.; Deparis, V.; Baud, A.; von Hagen, J.; Kolmar, H. Characterization of lipoprotein supplement and influence of its oxidized lipid content on cell culture performance and monoclonal antibody production by a SP2/0 hybridoma cell line Rémy. Biotechnol. Prog. 2023, 39, e3372. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nogimori, Y.; Imamura, H.; Ando, J. Shear stress activates mitochondrial oxidative phosphorylation by reducing plasma membrane cholesterol in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2020, 117, 33660–33667. [Google Scholar] [CrossRef]
- Yang, Z.; Huo, Y.; Zhou, S.; Guo, J.; Ma, X.; Li, T.; Fan, C.; Wang, L. Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 2022, 34, 2018–2035.e8. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xiao, J.X.; Li, S.; Liu, J.J.; Alugongo, G.M.; Cao, Z.J.; Yang, H.J.; Wang, S.X.; Swanson, K.C. Protein Metabolism and Signal Pathway Regulation in Rumen and Mammary Gland. Curr. Protein Pept. Sci. 2017, 18, 636–651. [Google Scholar] [CrossRef]
- Feichtinger, J.; Hernández, I.; Fischer, C.; Hanscho, M.; Auer, N.; Hackl, M.; Jadhav, V.; Baumann, M.; Krempl, P.M.; Schmidl, C.; et al. Comprehensive genome and epigenome characterization of CHO cells in response to evolutionary pressures and over time. Biotechnol. Bioeng. 2016, 113, 2241–2253. [Google Scholar] [CrossRef] [PubMed]
- Chintalacharuvu, K.R.; Chuang, P.D.; Dragoman, A.; Fernandez, C.Z.; Qiu, J.; Plaut, A.G.; Trinh, K.R.; Gala, F.A.; Morrison, S.L. Cleavage of the Human Immunoglobulin A1 (IgA1) Hinge Region by IgA1 Proteases Requires Structures in the Fc region of IgA. Infect. Immun. 2003, 71, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Rifai, A.; Fadden, K.; Morrison, S.L.; Chintalacharuvu, K.R. The N-Glycans Determine the Differential Blood Clearance and Hepatic Uptake of Human Immunoglobulin (Ig)a1 and Iga2 Isotypes. J. Exp. Med. 2000, 191, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Betts, Z.; Dickson, A.J. Assessment of UCOE on Recombinant EPO Production and Expression Stability in Amplified Chinese Hamster Ovary Cells. Mol. Biotechnol. 2015, 57, 846–858. [Google Scholar] [CrossRef]
- Torres, M.; Dickson, A.J. Combined gene and environmental engineering offers a synergetic strategy to enhance r-protein production in Chinese hamster ovary cells. Biotechnol. Bioeng. 2022, 119, 550–565. [Google Scholar] [CrossRef]
- Jiménez, A.B.; González, C.C. Estadistica Con Ejemplos Aplicados en R, 1st ed.; Editorial Académica Española: London, UK, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Pereira, P.; Trinh, R.; Vasuthasawat, A.; Bartsch-Jiménez, A.; Nuñez-Soto, C.; Altamirano, C. Enhancing Antibody-Specific Productivity: Unraveling the Impact of XBP1s Overexpression and Glutamine Availability in SP2/0 Cells. Bioengineering 2024, 11, 201. https://doi.org/10.3390/bioengineering11030201
González-Pereira P, Trinh R, Vasuthasawat A, Bartsch-Jiménez A, Nuñez-Soto C, Altamirano C. Enhancing Antibody-Specific Productivity: Unraveling the Impact of XBP1s Overexpression and Glutamine Availability in SP2/0 Cells. Bioengineering. 2024; 11(3):201. https://doi.org/10.3390/bioengineering11030201
Chicago/Turabian StyleGonzález-Pereira, Priscilla, Ryan Trinh, Alex Vasuthasawat, Angelo Bartsch-Jiménez, Constanza Nuñez-Soto, and Claudia Altamirano. 2024. "Enhancing Antibody-Specific Productivity: Unraveling the Impact of XBP1s Overexpression and Glutamine Availability in SP2/0 Cells" Bioengineering 11, no. 3: 201. https://doi.org/10.3390/bioengineering11030201




