Managing Vascular Pedicle Exposure in Free Tissue Transfer Using a Reprocessed Micronized Dermal Substitute in Lower Extremity Reconstructions
Abstract
:1. Introduction
2. Materials and Methods
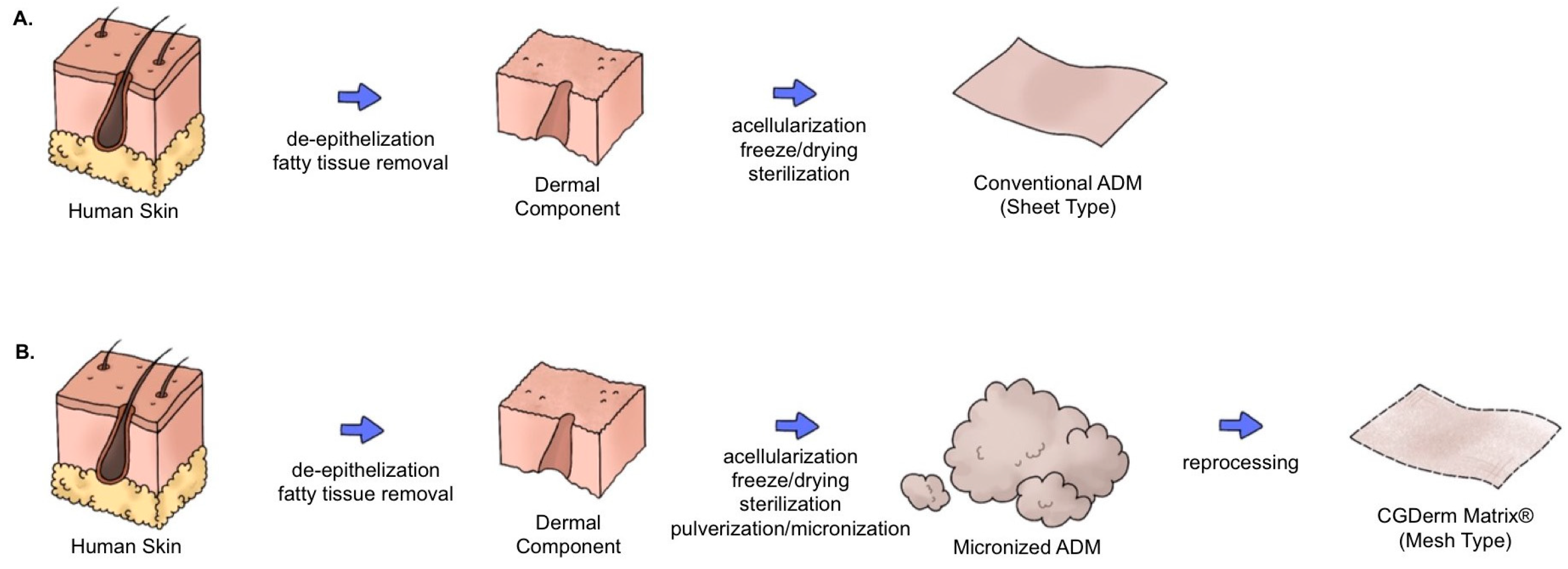
2.1. Materials
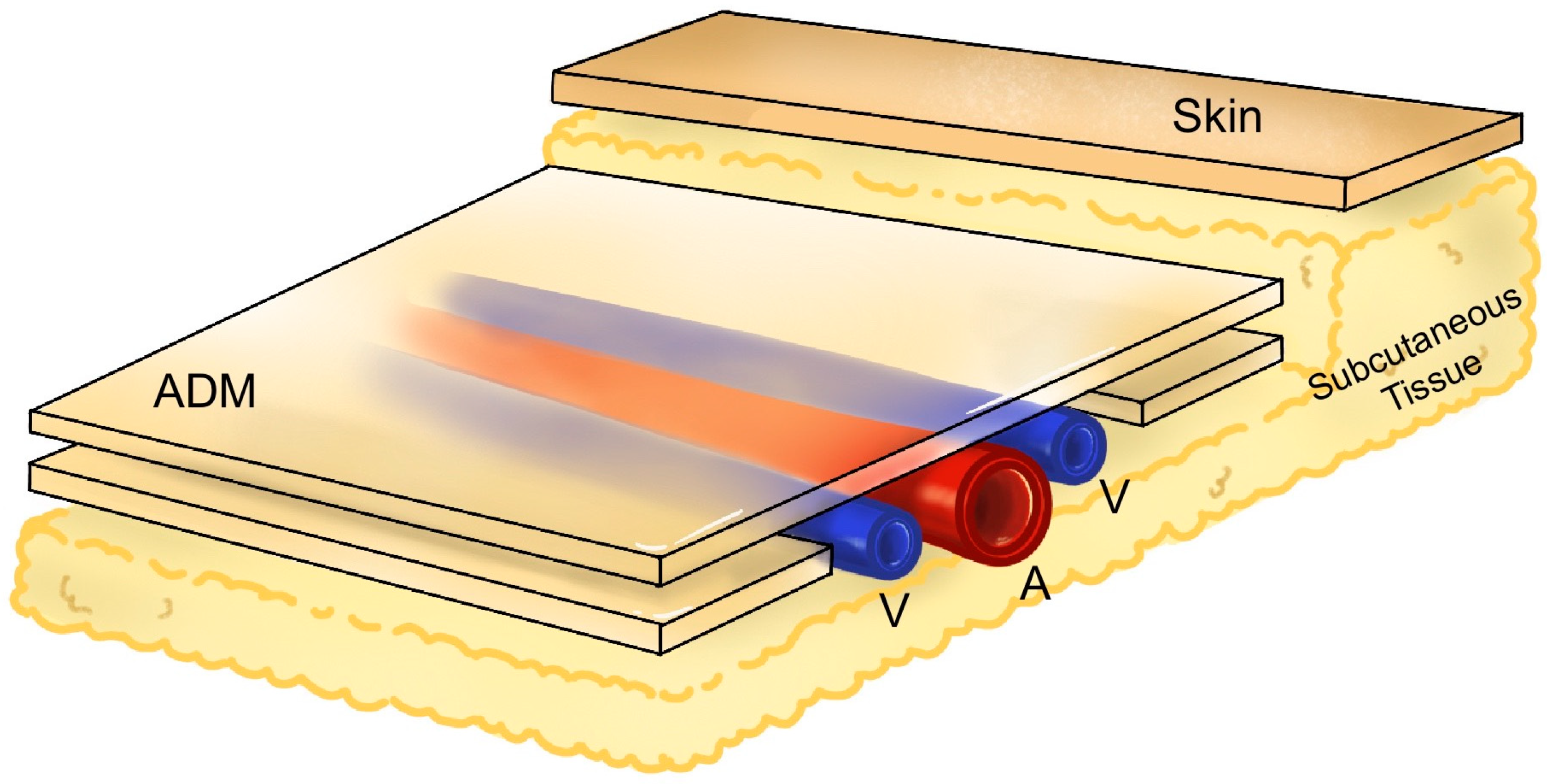
2.2. Surgical Techniques
2.3. Postoperative Care
2.4. Measured Parameters and Outcomes
3. Results
3.1. Patient Demographics
3.2. Reconstruction Characteristics
3.3. Treatment Outcomes
3.4. Case Reports
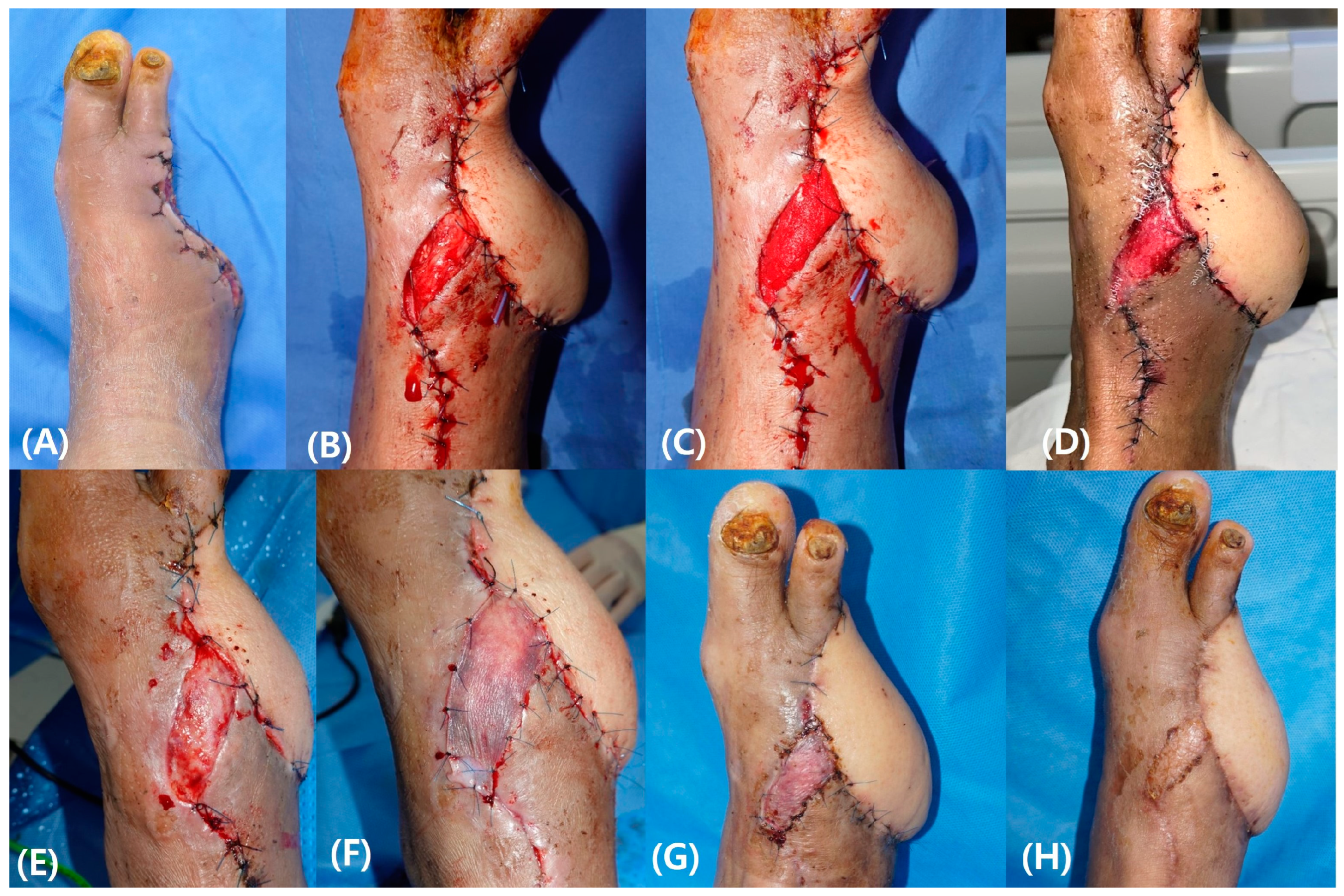
3.4.1. Case 1
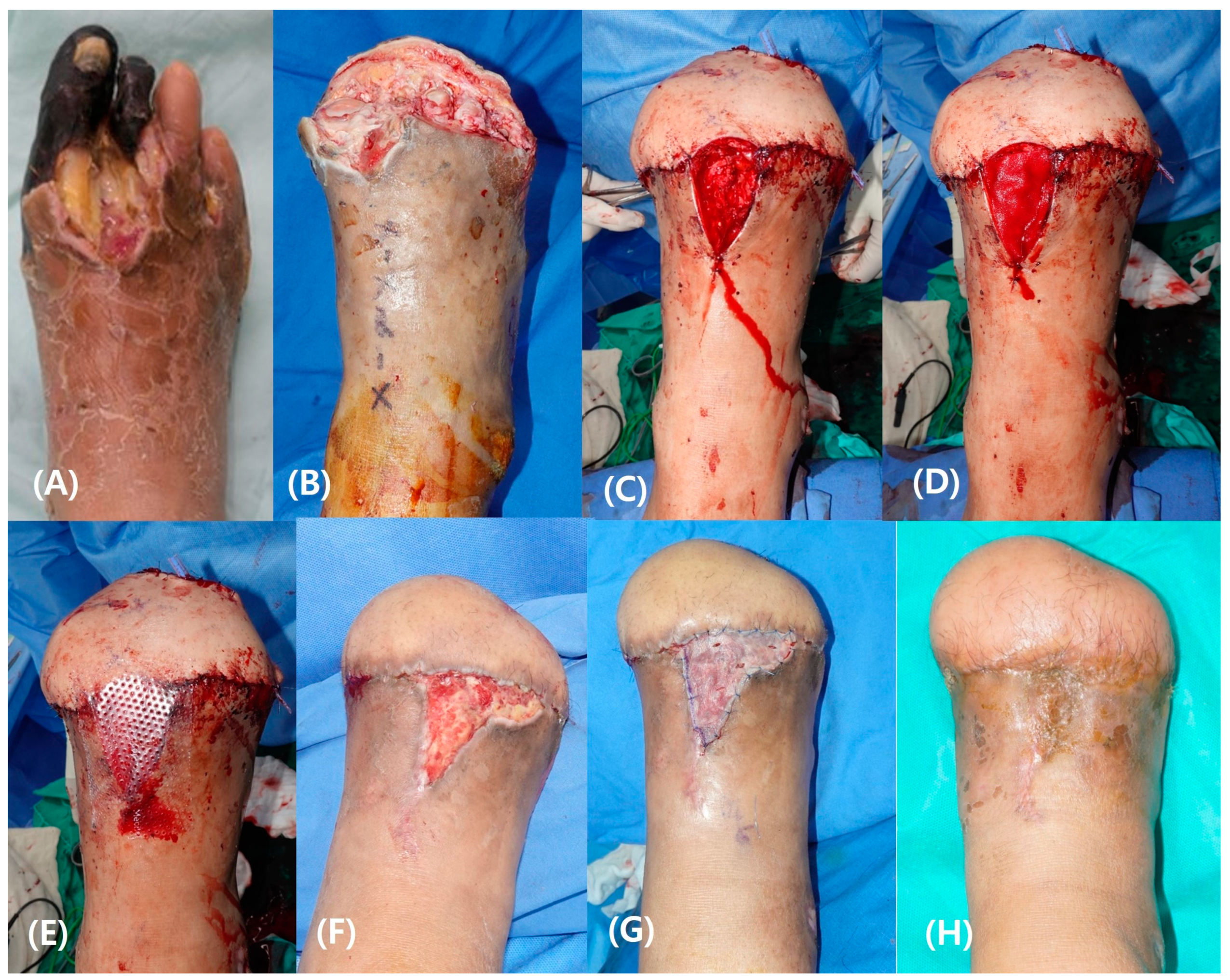
3.4.2. Case 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govshievich, A.; Bauder, A.; Kovach, S.J.; Levin, L.S. Aesthetic considerations in extremity salvage and reconstruction. Plast. Reconstr. Surg. 2023, 151, 679e–687e. [Google Scholar] [CrossRef]
- Kang, M.J.; Chung, C.H.; Chang, Y.J.; Kim, K.H. Reconstruction of the lower extremity using free flaps. Arch. Plast. Surg. 2013, 40, 575–583. [Google Scholar] [CrossRef]
- Xiong, L.; Gazyakan, E.; Kremer, T.; Hernekamp, F.J.; Harhaus, L.; Saint-Cyr, M.; Kneser, U.; Hirche, C. Free flaps for reconstruction of soft tissue defects in lower extremity: A meta-analysis on microsurgical outcome and safety. Microsurgery 2016, 36, 511–524. [Google Scholar] [CrossRef]
- Bigdeli, A.K.; Gazyakan, E.; Schmidt, V.J.; Bauer, C.; Germann, G.; Radu, C.A.; Kneser, U.; Hirche, C. Long-term outcome after successful lower extremity free flap salvage. J. Reconstr. Microsurg. 2019, 35, 263–269. [Google Scholar] [CrossRef]
- Kamolz, L.-P.; Kotzbeck, P.; Schintler, M.; Spendel, S. Skin regeneration, repair, and reconstruction: Present and future. Eur. Surg. 2022, 54, 163–169. [Google Scholar] [CrossRef]
- Jefferson, R.; Janis, J.; Banyard, D.; Bourgeois, J.; Widgerow, A.; Evans, G.; Tenenhaus, M.; Rennekampff, H.; Badylak, S.; Wan, D. Acellular Dermal Matrices: Applications in Plastic Surgery. In Seminars in Plastic Surgery; Thieme Medical Publishers: New York, NY, USA, 2019; pp. 173–184. [Google Scholar]
- Nahabedian, M.Y. The bioengineered prosthetic breast reconstruction: Advancements, evidence, and outcomes. Gland. Surg. 2019, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, A.M.; Kokini, K.; Vaughn, L.C.; Waisner, B.Z.; Voytik-Harbin, S.L. Extracellular matrix (ECM) microstructural composition regulates local cell-ECM biomechanics and fundamental fibroblast behavior: A multidimensional perspective. J. Appl. Physiol. 2005, 98, 1909–1921. [Google Scholar] [CrossRef]
- Seo, Y.K.; Song, K.Y.; Kim, Y.J.; Park, J.K. Wound healing effect of acellular artificial dermis containing extracellular matrix secreted by human skin fibroblasts. Artif. Organs 2007, 31, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Pianigiani, E.; Ierardi, F.; Lorenzini, G.; Casella, D.; Liso, F.G.; De Pascalis, A.; Cinotti, E.; Rubegni, P. The use of human acellular dermal matrices in advanced wound healing and surgical procedures: State of the art. Dermatol. Ther. 2021, 34, e14987. [Google Scholar] [CrossRef]
- Hahn, H.M.; Jeong, Y.S.; Lee, I.J.; Kim, M.J.; Lim, H. Efficacy of split-thickness skin graft combined with novel sheet-type reprocessed micronized acellular dermal matrix. BMC Surg. 2022, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Thione, A.; Cavadas, P.; Landin, L.; Ibañez, J. Microvascular pedicle coverage with split thickness skin graft: Indications and surgical tips. Indian J. Plast. Surg. 2011, 44, 528–529. [Google Scholar] [CrossRef]
- Persson, N.H.; Takolander, R.; Bergovist, D. Edema after lower limb arterial reconstruction. Influence of background factors, surgical technique and potentially prophylactic methods. VASA 1991, 20, 57–62. [Google Scholar]
- Szczesny, G.; Olszewski, W.L. The pathomechanism of posttraumatic edema of lower limbs: I. The effect of extravasated blood, bone marrow cells, and bacterial colonization on tissues, lymphatics, and lymph nodes. J. Trauma. Acute Care Surg. 2002, 52, 315–322. [Google Scholar] [CrossRef]
- Szczesny, G.; Olszewski, W.L. The pathomechanism of posttraumatic edema of the lower limbs: II—Changes in the lymphatic system. J. Trauma. Acute Care Surg. 2003, 55, 350–354. [Google Scholar] [CrossRef]
- Han, H.H.; Min, K.H. Is split-thickness skin graft safe for coverage of the vascular pedicle in free tissue transfer? J. Plast. Surg. Hand Surg. 2019, 53, 138–142. [Google Scholar] [CrossRef]
- Kovar, A.; Diamond, S.; Iorio, M.L. Skin grafting the vascular pedicle: A useful technique to avoid microvascular collapse in free tissue transfer for limb salvage. PAR 2019, 6, 10. [Google Scholar] [CrossRef]
- Hallock, G.G. Methods for providing vascularized tissue protection of microanastomoses. Ann. Plast. Surg. 1991, 27, 305–311. [Google Scholar] [CrossRef]
- Durand, P.D.; Couto, R.A.; Isakov, R.; Gurunluoglu, R.; Bernard, S. Adipofascial flap in the recipient incision site for coverage of vascular pedicle in free tissue transfer. Plast. Reconstr. Surg. 2016, 137, 1039–1041. [Google Scholar] [CrossRef]
- Osada, A.; Matsumine, H. Coverage of free flap vascular pedicles by basic fibroblast growth factor-impregnated collagen-gelatin sponge without skin graft. Int. J. Surg. Wound Care 2021, 2, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Kim, Y.H.; Kim, S.W. Effect of fibrin sealant in positioning and stabilizing microvascular pedicle: A comparative study. Microsurgery 2017, 37, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.; Schildhauer, T.A.; Dudda, M.; Sauber, J.; Spindler, N. Fibrin glue as a protective tool for microanastomoses in limb reconstructive surgery. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2015, 4, Doc14. [Google Scholar] [CrossRef]
- Livesey, S.A.; Herndon, D.N.; Hollyoak, M.A.; Atkinson, Y.H.; Nag, A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 1995, 60, 1–9. [Google Scholar] [CrossRef]
- Janis, J.E.; Kwon, R.K.; Attinger, C.E. The new reconstructive ladder: Modifications to the traditional model. Plast. Reconstr. Surg. 2011, 127, 205S–212S. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Sharma, V.; Kang, N.V.; García-Gareta, E. A universal classification system of skin substitutes inspired by factorial design. Tissue Eng. Part. B Rev. 2018, 24, 279–288. [Google Scholar] [CrossRef]
- Mohebichamkhorami, F.; Alizadeh, A. Skin substitutes; an updated review of products from year 1980 to 2017. J. Appl. Biotechnol. Rep. 2017, 4, 615–623. [Google Scholar]
- Bush, K.; Gertzman, A.A. Process development and manufacturing of human and animal acellular dermal matrices. In Skin Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–108. [Google Scholar]
- Leclère, F.M.; Desnouveaux, E.; Choughri, H.; Casoli, V. Acellular dermal matrix: New applications for free flap pedicle coverage–A prospective study in 10 patients. J. Cosmet. Laser Ther. 2018, 20, 200–204. [Google Scholar] [CrossRef]
- Anderson, J.J.; Wallin, K.J.; Spencer, L. Split thickness skin grafts for the treatment of non-healing foot and leg ulcers in patients with diabetes: A retrospective review. Diabet. Foot Ankle 2012, 3, 10204. [Google Scholar] [CrossRef]
- Mahmoud, S.; Mohamed, A.; Mahdi, S.; Ahmed, M. Split-skin graft in the management of diabetic foot ulcers. J. Wound Care 2008, 17, 303–306. [Google Scholar] [CrossRef]
- Veves, A.; Falanga, V.; Armstrong, D.G.; Sabolinski, M.L.; Study, A.D.F.U. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: A prospective randomized multicenter clinical trial. Diabetes Care 2001, 24, 290–295. [Google Scholar] [CrossRef]
- Jewell, L.; Guerrero, R.; Quesada, A.R.; Chan, L.S.; Garner, W.L. Rate of healing in skin-grafted burn wounds. Plast. Reconstr. Surg. 2007, 120, 451–456. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The treatment of impaired wound healing in diabetes: Looking among old drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total Patients (n = 10) |
|---|---|
| Age (years) | 55.80 ± 20.70 (6–78) |
| Sex | |
| Male | 9 |
| Female | 1 |
| DM | 6 |
| HTN | 2 |
| Current smoker | 5 |
| Cause of defect | |
| Diabetic foot | 6 |
| Trauma | 3 |
| Tumor | 1 |
| N | Type of Surgery | Cause of Revision | Defect Location | Type of Flap | Flap Dimensions (cm2) | Recipient Artery and Vein |
|---|---|---|---|---|---|---|
| 1 | Primary | Great toe | MSAP | 9 × 5 | DPA & DPV | |
| 2 | Primary | Foot | ALT | 15 × 7 | DPA & DPV | |
| 3 | Revision | Venous thrombosis | Foot | SIEA | 20 × 8 | DPA & GSV |
| 4 | Revision | Arterial insufficiency | Great toe | RASP | 2.5 × 4 | FDMA & FDMV |
| 5 | Revision | Arterial insufficiency | Knee | SGAP | 11 × 6 | MSA & MSV |
| 6 | Primary | Ankle | SIEA | 20 × 12 | PTA & PTV | |
| 7 | Primary | Foot | MSAP | 15 × 5 | ATA & ATV | |
| 8 | Primary | Foot | ALT | 25 × 16 | PTA & GSV | |
| 9 | Primary | Foot | SIEA | 14 × 7 | PTA & PTV | |
| 10 | Primary | Foot | MSAP | 7 × 4 | DPA & GSV |
| N | ADM Size (cm2) | Method of Coverage | Interval between ADM and Skin Grafting (Days) | Time to Complete Epithelialization (Days) | Flap Complication |
|---|---|---|---|---|---|
| 1 | 2 × 4 | STSG | 9 | 46 | None |
| 2 | 2 × 5 | STSG | 21 | 44 | None |
| 3 | 5 × 6 | STSG | 42 | 71 | None |
| 4 | 1 × 2 | Healing by secondary intention | NA | 36 | None |
| 5 | 2 × 2 | STSG | 46 | 46 | Partial flap necrosis |
| 6 | 3 × 5 | NA | NA | NA | Total flap necrosis due to bypass failure |
| 7 | 2 × 3 | STSG | 49 | 70 | None |
| 8 | 1 × 4 | Healing by secondary intention | NA | 56 | Partial flap necrosis |
| 9 | 1 × 4 | STSG | 33 | 59 | None |
| 10 | 1 × 4 | FTSG | 15 | 30 | None |
| 8.70 ± 8.41 | 30.71 ± 15.18 | 50.89 ± 14.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Lee, J.H.; Park, M.S.; Ahn, M.R.; Jun, D.; Lee, J.H. Managing Vascular Pedicle Exposure in Free Tissue Transfer Using a Reprocessed Micronized Dermal Substitute in Lower Extremity Reconstructions. Bioengineering 2024, 11, 241. https://doi.org/10.3390/bioengineering11030241
Kim D, Lee JH, Park MS, Ahn MR, Jun D, Lee JH. Managing Vascular Pedicle Exposure in Free Tissue Transfer Using a Reprocessed Micronized Dermal Substitute in Lower Extremity Reconstructions. Bioengineering. 2024; 11(3):241. https://doi.org/10.3390/bioengineering11030241
Chicago/Turabian StyleKim, Daheui, Jun Hyeok Lee, Min Suk Park, Ma Rhip Ahn, Daiwon Jun, and Jung Ho Lee. 2024. "Managing Vascular Pedicle Exposure in Free Tissue Transfer Using a Reprocessed Micronized Dermal Substitute in Lower Extremity Reconstructions" Bioengineering 11, no. 3: 241. https://doi.org/10.3390/bioengineering11030241
APA StyleKim, D., Lee, J. H., Park, M. S., Ahn, M. R., Jun, D., & Lee, J. H. (2024). Managing Vascular Pedicle Exposure in Free Tissue Transfer Using a Reprocessed Micronized Dermal Substitute in Lower Extremity Reconstructions. Bioengineering, 11(3), 241. https://doi.org/10.3390/bioengineering11030241






