Unlocking the Potential of Stem Cell Microenvironments In Vitro
Abstract
:1. Introduction
2. The Cellular Microenvironment
2.1. Soluble and Immobilized Signaling Factors
2.2. Cell–Extracellular Matrix Interactions
2.3. Direct Cell–Cell Interactions
2.4. Physicochemical Environment
2.5. Mechanical Forces
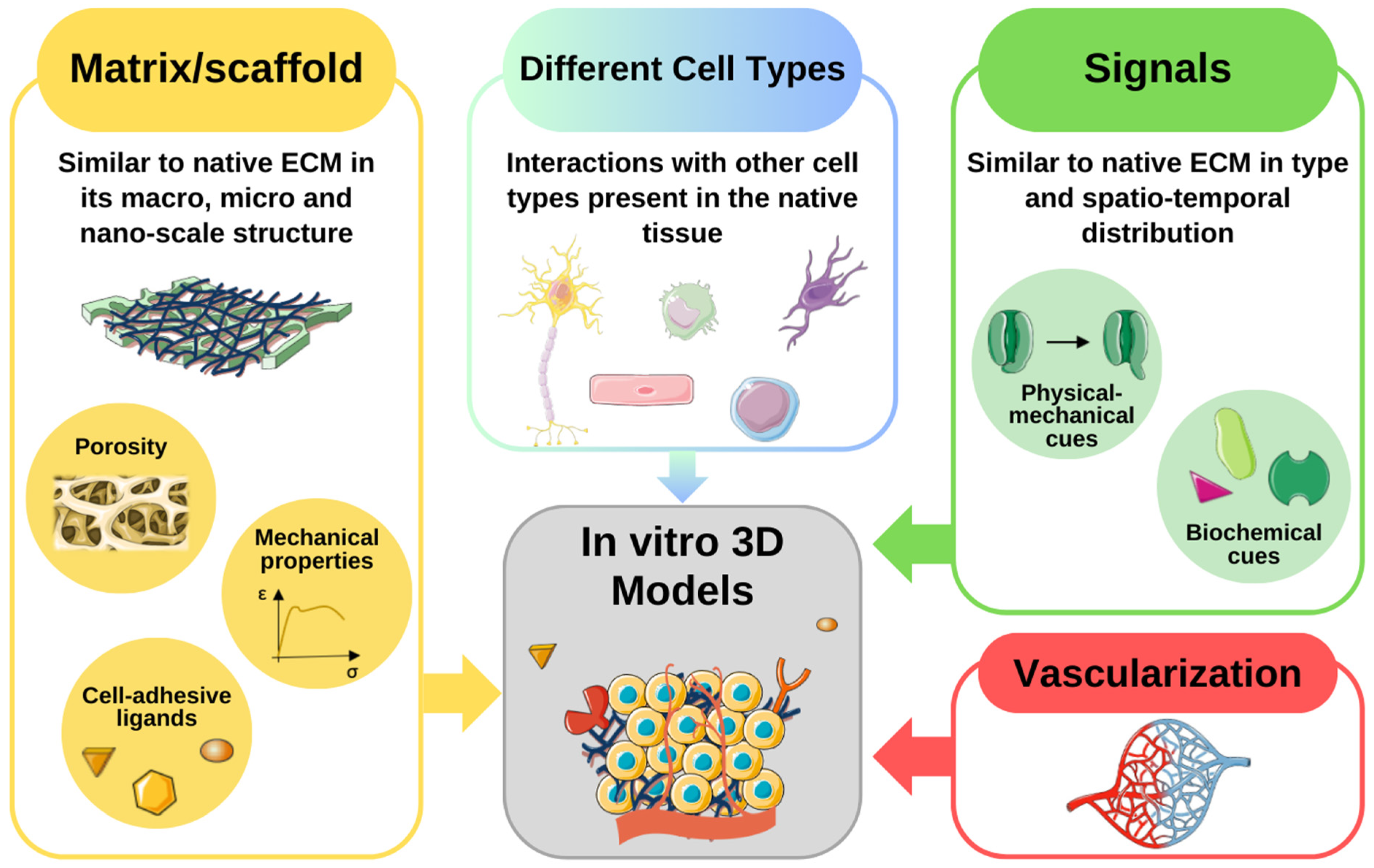
3. Recent Advances in In Vitro Microenvironment Modeling
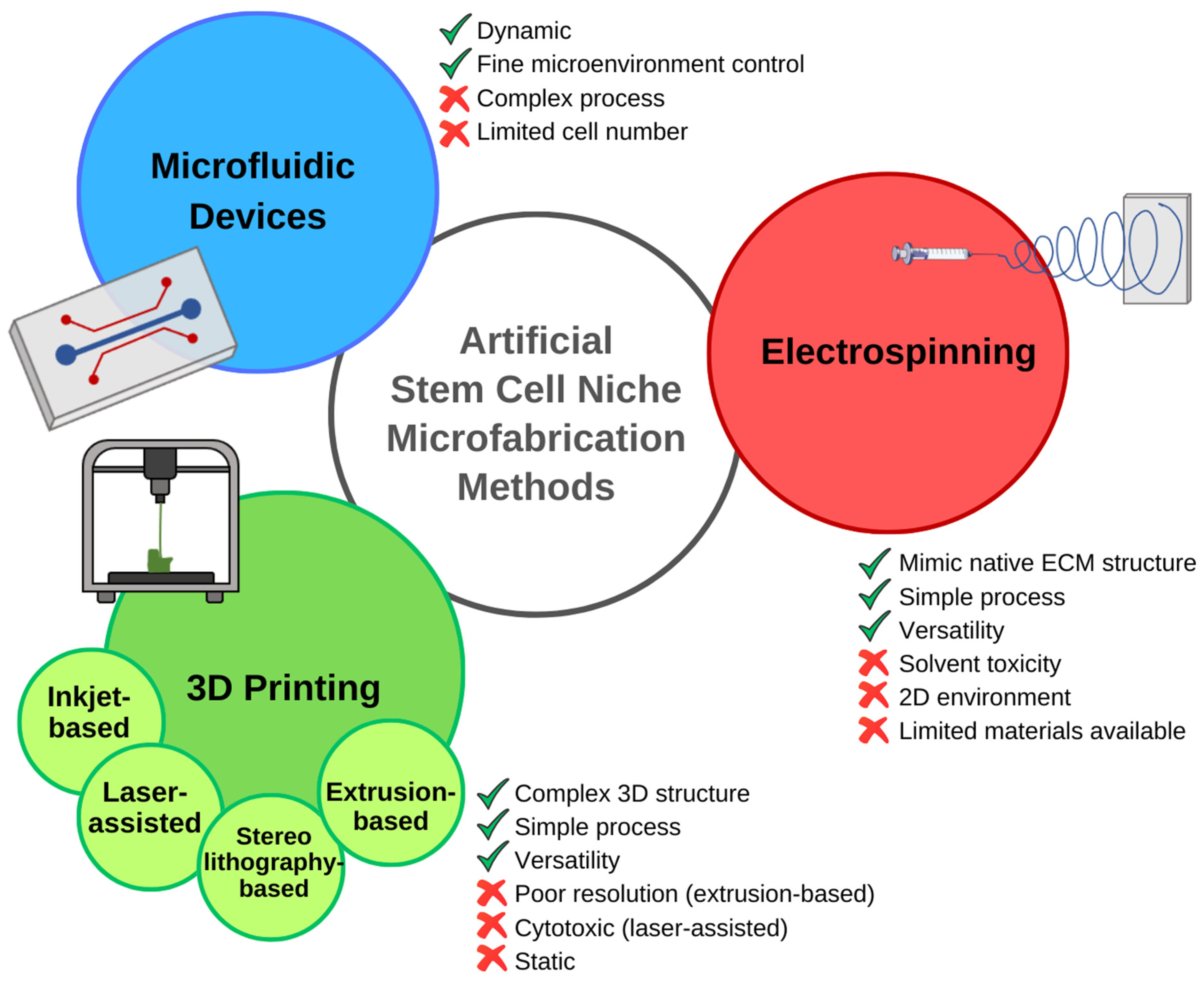
3.1. Emerging Technologies for Recreating the Cellular Niche

3.2. Examples and Case Studies
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Ortuño-Costela, M.D.C.; Cerrada, V.; García-López, M.; Gallardo, M.E. The Challenge of Bringing IPSCs to the Patient. Int. J. Mol. Sci. 2019, 20, 6305. [Google Scholar] [CrossRef] [PubMed]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and Their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ahmad, A.; Azmi, A.S.; Ali, S.; Sarkar, F.H. Overview of Cancer Stem Cells (CSCs) and Mechanisms of Their Regulation: Implications for Cancer Therapy. Curr. Protoc. Pharmacol. 2013, 61, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling Self-Renewal and Differentiation of Stem Cells via Mechanical Cues. J. Biomed. Biotechnol. 2012, 2012, 797410. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Blau, H.M. Artificial Stem Cell Niches. Adv. Mater. 2009, 21, 3255–3268. [Google Scholar] [CrossRef] [PubMed]
- Batada, N.N.; Shepp, L.A.; Siegmund, D.O.; Levitt, M. Spatial Regulation and the Rate of Signal Transduction Activation. PLoS Comput. Biol. 2006, 2, e44. [Google Scholar] [CrossRef]
- Putnam, A.J. The Instructive Role of the Vasculature in Stem Cell Niches. Biomater. Sci. 2014, 2, 1562–1573. [Google Scholar] [CrossRef]
- Pinho, S.; Frenette, P.S. Haematopoietic Stem Cell Activity and Interactions with the Niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem Cells and the Impact of ROS Signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef] [PubMed]
- Scadden, D.T. The Stem-Cell Niche as an Entity of Action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, S.; Lerner, R.G.; Petritsch, C. Asymmetric Cell Division of Stem and Progenitor Cells during Homeostasis and Cancer. Cell. Mol. Life Sci. 2014, 71, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem Cell Paracrine Actions and Tissue Regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Schliermann, A.; Nickel, J. Unraveling the Connection between Fibroblast Growth Factor and Bone Morphogenetic Protein Signaling. Int. J. Mol. Sci. 2018, 19, 3220. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.A. Signaling in Cell Differentiation and Morphogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008151. [Google Scholar] [CrossRef]
- Tata, P.R.; Rajagopal, J. Regulatory Circuits and Bi-Directional Signaling between Stem Cells and Their Progeny. Cell Stem Cell 2016, 19, 686–689. [Google Scholar] [CrossRef]
- Bendall, S.C.; Stewart, M.H.; Menendez, P.; George, D.; Vijayaragavan, K.; Werbowetski-Ogilvie, T.; Ramos-Mejia, V.; Rouleau, A.; Yang, J.; Bossé, M.; et al. IGF and FGF Cooperatively Establish the Regulatory Stem Cell Niche of Pluripotent Human Cells In Vitro. Nature 2007, 448, 1015–1021. [Google Scholar] [CrossRef]
- Bavaro, T.; Tengattini, S.; Rezwan, R.; Chiesa, E.; Temporini, C.; Dorati, R.; Massolini, G.; Conti, B.; Ubiali, D.; Terreni, M. Design of Epidermal Growth Factor Immobilization on 3D Biocompatible Scaffolds to Promote Tissue Repair and Regeneration. Sci. Rep. 2021, 11, 2629. [Google Scholar] [CrossRef]
- Jha, A.K.; Mathur, A.; Svedlund, F.L.; Ye, J.; Yeghiazarians, Y.; Healy, K.E. Molecular Weight and Concentration of Heparin in Hyaluronic Acid-Based Matrices Modulates Growth Factor Retention Kinetics and Stem Cell Fate. J. Control Release 2015, 209, 308–316. [Google Scholar] [CrossRef]
- Zhu, J.; Clark, R.A.F. Fibronectin at Select Sites Binds Multiple Growth Factors and Enhances Their Activity: Expansion of the Collaborative ECM-GF Paradigm. J. Investig. Dermatol. 2014, 134, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; McMahon, A.P. Cholesterol Modification of Hedgehog Family Proteins. J. Clin. Investig. 2002, 110, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Schaffer, D.V. Engineering Microenvironments to Control Stem Cell Fate and Function. StemBook 2008, 510, 67–72. [Google Scholar] [CrossRef]
- Mercier, F.; Arikawa-Hirasawa, E. Heparan Sulfate Niche for Cell Proliferation in the Adult Brain. Neurosci. Lett. 2012, 510, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Ng, H.-H. The Metabolic Programming of Stem Cells. Genes Dev. 2017, 31, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Votteler, M.; Kluger, P.J.; Walles, H.; Schenke-Layland, K. Stem Cell Microenvironments-Unveiling the Secret of How Stem Cell Fate Is Defined. Macromol. Biosci. 2010, 10, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular Matrix Composition of Connective Tissues: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef]
- Streuli, C.H. Integrins and Cell-Fate Determination. J. Cell Sci. 2009, 122, 171–177. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The Extracellular Matrix: Structure, Composition, Age-Related Differences, Tools for Analysis and Applications for Tissue Engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Choudhary, M.; Zhang, X.; Stojković, P.; Hyslop, L.; Anyfantis, G.; Herbert, M.; Murdoch, A.P.; Stojković, M.; Lako, M. Putative Role of Hyaluronan and Its Related Genes, HAS2 and RHAMM, in Human Early Preimplantation Embryogenesis and Embryonic Stem Cell Characterization. Stem Cells 2007, 25, 3045–3057. [Google Scholar] [CrossRef]
- Peters, A.; Sherman, L.S. Diverse Roles for Hyaluronan and Hyaluronan Receptors in the Developing and Adult Nervous System. Int. J. Mol. Sci. 2020, 21, 5988. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.K.; Haylock, D.N.; Johnston, H.M.; Occhiodoro, T.; Brown, T.J.; Simmons, P.J. Hyaluronan Is Synthesized by Primitive Hemopoietic Cells, Participates in Their Lodgment at the Endosteum Following Transplantation, and Is Involved in the Regulation of Their Proliferation and Differentiation In Vitro. Blood 2003, 101, 856–862. [Google Scholar] [CrossRef]
- Su, W.; Matsumoto, S.; Sorg, B.; Sherman, L.S. Distinct Roles for Hyaluronan in Neural Stem Cell Niches and Perineuronal Nets. Matrix Biol. 2019, 78–79, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lewallen, M.; Xie, T. Adhesion in the Stem Cell Niche: Biological Roles and Regulation. Development 2013, 140, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Kale, V. Physiological Cues Involved in the Regulation of Adhesion Mechanisms in Hematopoietic Stem Cell Fate Decision. Front. Cell Dev. Biol. 2020, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef]
- Dong, J.; Pan, Y.-B.; Wu, X.-R.; He, L.-N.; Liu, X.-D.; Feng, D.-F.; Xu, T.-L.; Sun, S.; Xu, N.-J. A Neuronal Molecular Switch through Cell-Cell Contact That Regulates Quiescent Neural Stem Cells. Sci. Adv. 2019, 5, eaav4416. [Google Scholar] [CrossRef]
- Goldberg, J.S.; Hirschi, K.K. Diverse Roles of the Vasculature within the Neural Stem Cell Niche. Regen. Med. 2009, 4, 879–897. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef]
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, C.; Vaegler, M.; Gieseke, F.; Pfister, S.M.; Handgretinger, R.; Kerst, G.; Müller, I. Low Physiologic Oxygen Tensions Reduce Proliferation and Differentiation of Human Multipotent Mesenchymal Stromal Cells. BMC Cell Biol. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.G.; Diogo, M.M.; Fernandes-Platzgummer, A.; da Silva, C.L.; Cabral, J.M.S. Different Stages of Pluripotency Determine Distinct Patterns of Proliferation, Metabolism, and Lineage Commitment of Embryonic Stem Cells under Hypoxia. Stem Cell Res. 2010, 5, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Inglés, M.; Gimeno-Mallench, L.; El Alami, M.; Viña-Almunia, J.; Gambini, J.; Viña, J.; Borrás, C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1195. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.S.C.; Fernandes, T.G.; Dias, T.P.; Diogo, M.M.; Cabral, J.M.S. New Insights into the Mechanisms of Embryonic Stem Cell Self-Renewal under Hypoxia: A Multifactorial Analysis Approach. PLoS ONE 2012, 7, e38963. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.G.; Park, C.V.; Kenneth, N.S. Translating the Hypoxic Response—The Role of HIF Protein Translation in the Cellular Response to Low Oxygen. Cells 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Yfantis, A.; Mylonis, I.; Chachami, G.; Nikolaidis, M.; Amoutzias, G.D.; Paraskeva, E.; Simos, G. Transcriptional Response to Hypoxia: The Role of HIF-1-Associated Co-Regulators. Cells 2023, 12, 798. [Google Scholar] [CrossRef]
- Keung, A.J.; Healy, K.E.; Kumar, S.; Schaffer, D.V. Biophysics and Dynamics of Natural and Engineered Stem Cell Microenvironments. WIREs Syst. Biol. Med. 2010, 2, 49–64. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An Overview of Substrate Stiffness Guided Cellular Response and Its Applications in Tissue Regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Bongiorno, T.; Kazlow, J.; Mezencev, R.; Griffiths, S.; Olivares-Navarrete, R.; McDonald, J.F.; Schwartz, Z.; Boyan, B.D.; McDevitt, T.C.; Sulchek, T. Mechanical Stiffness as an Improved Single-Cell Indicator of Osteoblastic Human Mesenchymal Stem Cell Differentiation. J. Biomech. 2014, 47, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways That Control Cell Morphology. Annu. Rev. Physiol. 2019, 81, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Friedman, M.H. Adaptive Response of Vascular Endothelial Cells to an Acute Increase in Shear Stress Magnitude. Am. J. Physiol. Circ. Physiol. 2012, 302, H983–H991. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Yasu, T.; Ueba, H.; Sata, M.; Hashimoto, S.; Kuroki, M.; Saito, M.; Kawakami, M. Mechanical Stress Promotes the Expression of Smooth Muscle-like Properties in Marrow Stromal Cells. Exp. Hematol. 2004, 32, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gu, Y.; Li, C.; Wang, C.; Feng, Z.; Qiu, R.; Chen, B.; Li, J.; Zhang, S.; Wang, Z.; et al. Response of Mesenchymal Stem Cells to Shear Stress in Tissue-Engineered Vascular Grafts. Acta Pharmacol. Sin. 2009, 30, 530–536. [Google Scholar] [CrossRef]
- Yan, J.; Wang, W.-B.; Fan, Y.-J.; Bao, H.; Li, N.; Yao, Q.-P.; Huo, Y.-L.; Jiang, Z.-L.; Qi, Y.-X.; Han, Y. Cyclic Stretch Induces Vascular Smooth Muscle Cells to Secrete Connective Tissue Growth Factor and Promote Endothelial Progenitor Cell Differentiation and Angiogenesis. Front. Cell Dev. Biol. 2020, 8, 606989. [Google Scholar] [CrossRef] [PubMed]
- Sumigray, K.D.; Terwilliger, M.; Lechler, T. Morphogenesis and Compartmentalization of the Intestinal Crypt. Dev. Cell 2018, 45, 183–197.e5. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Taverna, E. Neural Progenitor Cell Polarity and Cortical Development. Front. Cell. Neurosci. 2017, 11, 384. [Google Scholar] [CrossRef]
- Doetsch, F.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cellular Composition and Three-Dimensional Organization of the Subventricular Germinal Zone in the Adult Mammalian Brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering Microscale Topographies to Control the Cell–Substrate Interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef]
- Daly, A.C.; Prendergast, M.E.; Hughes, A.J.; Burdick, J.A. Bioprinting for the Biologist. Cell 2021, 184, 18–32. [Google Scholar] [CrossRef]
- Anthon, S.G.; Valente, K.P. Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting. Int. J. Mol. Sci. 2022, 23, 14582. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, D.H.; MacNeil, S.; Claeyssens, F.; Asencio, I.O. The Use of Microfabrication Techniques for the Design and Manufacture of Artificial Stem Cell Microenvironments for Tissue Regeneration. Bioengineering 2021, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Heilshorn, S.C. Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche. Annu. Rev. Biomed. Eng. 2018, 20, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, D.H.; MacNeil, S.; Claeyssens, F.; Ortega Asencio, I. Delivery of Bioactive Compounds to Improve Skin Cell Responses on Microfabricated Electrospun Microenvironments. Bioengineering 2021, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- Gruene, M.; Pflaum, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Wilhelmi, M.; Haverich, A.; Chichkov, B.N. Adipogenic Differentiation of Laser-Printed 3D Tissue Grafts Consisting of Human Adipose-Derived Stem Cells. Biofabrication 2011, 3, 015005. [Google Scholar] [CrossRef]
- Phillippi, J.A.; Miller, E.; Weiss, L.; Huard, J.; Waggoner, A.; Campbell, P. Microenvironments Engineered by Inkjet Bioprinting Spatially Direct Adult Stem Cells Toward Muscle- and Bone-Like Subpopulations. Stem Cells 2008, 26, 127–134. [Google Scholar] [CrossRef]
- Braham, M.V.J.; Ahlfeld, T.; Akkineni, A.R.; Minnema, M.C.; Dhert, W.J.A.; Öner, F.C.; Robin, C.; Lode, A.; Gelinsky, M.; Alblas, J. Endosteal and Perivascular Subniches in a 3D Bone Marrow Model for Multiple Myeloma. Tissue Eng. Part C Methods 2018, 24, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Creff, J.; Courson, R.; Mangeat, T.; Foncy, J.; Souleille, S.; Thibault, C.; Besson, A.; Malaquin, L. Fabrication of 3D Scaffolds Reproducing Intestinal Epithelium Topography by High-Resolution 3D Stereolithography. Biomaterials 2019, 221, 119404. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef] [PubMed]
- Somers, S.M.; Gilbert-Honick, J.; Choi, I.Y.; Lo, K.W.E.; Lim, H.; Dias, S.; Wagner, K.R.; Mao, H.-Q.; Cahan, P.; Lee, G.; et al. Engineering Skeletal Muscle Grafts with PAX7::GFP-Sorted Human Pluripotent Stem Cell-Derived Myogenic Progenitors on Fibrin Microfiber Bundles for Tissue Regeneration. Bioengineering 2022, 9, 693. [Google Scholar] [CrossRef]
- Gottwald, E.; Giselbrecht, S.; Augspurger, C.; Lahni, B.; Dambrowsky, N.; Truckenmüller, R.; Piotter, V.; Gietzelt, T.; Wendt, O.; Pfleging, W.; et al. A Chip-Based Platform for the in Vitro Generation of Tissues in Three-Dimensional Organization. Lab Chip 2007, 7, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Eichholz, K.; Chariyev-Prinz, F.; Pitacco, P.; Aydin, H.M.; Kelly, D.J.; Vargel, İ. Biofabrication of Poly(Glycerol Sebacate) Scaffolds Functionalized with a Decellularized Bone Extracellular Matrix for Bone Tissue Engineering. Bioengineering 2022, 10, 30. [Google Scholar] [CrossRef]
- Baumgartner, W.; Wolint, P.; Hofmann, S.; Nüesch, C.; Calcagni, M.; Brunelli, M.; Buschmann, J. Impact of Electrospun Piezoelectric Core–Shell PVDFhfp/PDMS Mesh on Tenogenic and Inflammatory Gene Expression in Human Adipose-Derived Stem Cells: Comparison of Static Cultivation with Uniaxial Cyclic Tensile Stretching. Bioengineering 2022, 9, 21. [Google Scholar] [CrossRef]
- Koyanagi, A.; Onishi, I.; Muraoka, K.; Sato, I.; Sato, S.; Kimura, T.; Kishida, A.; Yamamoto, K.; Kitagawa, M.; Kurata, M. Identification of the Factor That Leads Human Mesenchymal Stem Cell Lines into Decellularized Bone. Bioengineering 2022, 9, 490. [Google Scholar] [CrossRef]
- Rodrigues, G.M.C.; Rodrigues, C.A.V.; Fernandes, T.G.; Diogo, M.M.; Cabral, J.M.S. Clinical-Scale Purification of Pluripotent Stem Cell Derivatives for Cell-Based Therapies. Biotechnol. J. 2015, 10, 1103–1114. [Google Scholar] [CrossRef]
- Badenes, S.M.; Fernandes, T.G.; Rodrigues, C.A.V.; Diogo, M.M.; Cabral, J.M.S. Scalable Expansion of Human-Induced Pluripotent Stem Cells in Xeno-Free Microcarriers. Methods Mol. Biol. 2015, 1283, 23–29. [Google Scholar] [CrossRef]
- Thanuthanakhun, N.; Kim, M.-H.; Kino-oka, M. Cell Behavioral Dynamics as a Cue in Optimizing Culture Stabilization in the Bioprocessing of Pluripotent Stem Cells. Bioengineering 2022, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.C.; Gomes, M.R.; Moço, M.; Cabral, J.M.S.; Ferreira, F.C.; Sanjuan-Alberte, P. A Concise Review on Electrospun Scaffolds for Kidney Tissue Engineering. Bioengineering 2022, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Al, M.; Kyeremeh, G.K.; Saeinasab, M.; Heidari Keshel, S.; Sefat, F. Stem Cell Niche Microenvironment: Review. Bioengineering 2021, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Raees, S.; Ullah, F.; Javed, F.; Akil, H.M.; Jadoon Khan, M.; Safdar, M.; Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Bakht, M.A.; et al. Classification, Processing, and Applications of Bioink and 3D Bioprinting: A Detailed Review. Int. J. Biol. Macromol. 2023, 232, 123476. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Hu, Q.; Shen, Z.; Rana, D.; Ramalingam, M. Designing Vascular Supportive Albumen-Rich Composite Bioink for Organ 3D Printing. J. Mech. Behav. Biomed. Mater. 2020, 104, 103642. [Google Scholar] [CrossRef] [PubMed]
- Jongpaiboonkit, L.; King, W.J.; Lyons, G.E.; Paguirigan, A.L.; Warrick, J.W.; Beebe, D.J.; Murphy, W.L. An Adaptable Hydrogel Array Format for 3-Dimensional Cell Culture and Analysis. Biomaterials 2008, 29, 3346–3356. [Google Scholar] [CrossRef]
- Liu, H.; Roy, K. Biomimetic Three-Dimensional Cultures Significantly Increase Hematopoietic Differentiation Efficacy of Embryonic Stem Cells. Tissue Eng. 2005, 11, 319–330. [Google Scholar] [CrossRef]
- Jones, C.N.; Lee, J.Y.; Zhu, J.; Stybayeva, G.; Ramanculov, E.; Zern, M.A.; Revzin, A. Multifunctional Protein Microarrays for Cultivation of Cells and Immunodetection of Secreted Cellular Products. Anal. Chem. 2008, 80, 6351–6357. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, D.Y.; Kang, J.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Oh, D.H.; Kim, J.-M.; Rhim, S.-J.; Kim, G.-D.; et al. Alternative Experimental Approaches to Reduce Animal Use in Biomedical Studies. J. Drug Deliv. Sci. Technol. 2022, 68, 103131. [Google Scholar] [CrossRef]
- Tenreiro, M.F.; Branco, M.A.; Cotovio, J.P.; Cabral, J.M.S.; Fernandes, T.G.; Diogo, M.M. Advancing Organoid Design through Co-Emergence, Assembly, and Bioengineering. Trends Biotechnol. 2023, 41, 923–938. [Google Scholar] [CrossRef]
- Fernandes, T.G. Design and Fabrication of Artificial Stem Cell Niches. Bioengineering 2022, 9, 813. [Google Scholar] [CrossRef]
- Fernandes, T.G. Organoids as Complex (Bio)Systems. Front. Cell Dev. Biol. 2023, 11, 1268540. [Google Scholar] [CrossRef]

| Technical Terms and Abbreviations | |
|---|---|
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| Biocompatibility | The ability to interact with a living system without producing an adverse effect |
| Bioink | A specialized material used in 3D bioprinting, which serves as the medium through which cells are deposited layer by layer to build complex tissue structures |
| Biomaterial | A natural or synthetic substance that interact with biological systems |
| Bioprinting | An advanced technology that enables the fabrication of 3D biological structures using living cells, biomaterials, and bioactive molecules. The process involves layer-by-layer deposition of materials capable of incorporating living components to create tissues, organs, and other biological constructs. Specific methods include inkjet, extrusion, and light-assisted bioprinting |
| BMPs | Bone morphogenetic proteins, a group of signaling molecules part of the transforming growth factor-beta (TGF-β) superfamily |
| Cell niche | Refers to the specialized microenvironment in which cells reside within tissues or organs. It encompasses the physical, chemical, and biological factors that regulate the behavior, maintenance, and fate of cells |
| CRISPR-Cas9 | Clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9, a gene-editing technology that allows to make precise changes to an organism’s DNA |
| Cytotoxicity | The capacity of an agent to cause damage or death to cells |
| Differentiation | The process by which an immature cell becomes specialized, acquiring specific structures and functions that enable it to perform particular tasks within an organism |
| ECM | Extracellular matrix, a complex 3D network of proteins, glycoproteins, proteoglycans, and polysaccharides that surrounds and supports cells within tissues and organs in multicellular organisms |
| Electrospinning | A technique capable to produce ultrafine fibers. The process involves the use of an electric field to draw charged polymer solutions into thin fibers that are collected on a grounded substrate |
| FGFs | Fibroblast growth factors, a family of signaling proteins that bind to specific cell surface receptors and are involved in various biological processes, including cell growth, proliferation, differentiation, and tissue repair |
| GAGs | Glycosaminoglycans, a family of polysaccharides that are major components of the ECM. Examples are hyaluronic acid (HA), chondroitin sulfate, and heparan sulfate |
| Hedgehog | A family of secreted signaling proteins that play essential roles in embryonic development, tissue homeostasis, and stem cell regulation across various species, including humans |
| hESCs | Human embryonic stem cells, pluripotent stem cells derived from the inner cell mass of the blastocyst, an early stage of embryonic development |
| HIFs | Hypoxia-inducible factors, a family of transcription factors that regulate the cellular response to changes in oxygen levels |
| High-throughput | The capability of performing many analyses in parallel, typically using automation, miniaturization, and advanced technologies |
| HSCs | Hematopoietic stem cells, multipotent stem cells that give rise to all types of blood cells in the body |
| Hydrogel | A 3D network of hydrophilic polymer chains that are capable of absorbing and retaining large amounts of water |
| Hypoxia | Low oxygen levels |
| IGF | Insulin-like growth factor, peptide hormone with structural similarities to insulin. Plays essential roles in regulating growth, development, metabolism, and cellular function in various tissues throughout the body |
| iPSCs | Induced pluripotent stem cells, a type of pluripotent stem cell that can be generated from somatic cells through a process of cellular reprogramming |
| Microfluidic devices | Miniaturized platforms that manipulate small volumes of fluids at the microscale level |
| Microwell arrays | Microscale platforms composed of arrays of small wells or compartments arranged in a regular pattern on a substrate. Commonly fabricated using microfabrication techniques such as soft lithography or replica molding |
| MSCs | Mesenchymal stem/stromal cells, multipotent cells that can differentiate into a variety of cell types, including bone, cartilage, fat, and other connective tissue cells |
| NSCs | Neural stem cells, a type of stem cell found in the nervous system. Can differentiate into neurons, astrocytes, and oligodendrocytes |
| Organoids | 3D miniature organ-like structures that are derived from stem or progenitor cells and exhibit rudimentary organ function and organization |
| PGs | Proteoglycans, a type of glycoprotein found in the ECM of tissues. They consist of a protein core to which GAGs are attached |
| Printability | The feasibility of a given material to be use in a printing process |
| PSCs | Pluripotent stem cells, a type of stem cell that can differentiate into all cell types in the body. Include ESCs and iPSCs |
| Regenerative medicine | A multidisciplinary field that aims to restore, repair, or replace damaged tissue or organs in the body |
| ROS | Reactive oxygen species, are chemically reactive molecules containing oxygen |
| Scaffold | 3D structure or framework that provides mechanical support, guidance, and a conducive environment for cells to attach, grow, and differentiate |
| Shear stress | A mechanical force exerted parallel to the surface of an object or fluid layer |
| Soft lithography | A set of techniques used in microfabrication to pattern and fabricate structures on the micrometer scale using elastomeric materials as stamps or molds |
| Soluble factors | Molecules or compounds that are soluble in biological fluids, and play critical roles in cellular signaling, communication, and regulation of physiological processes |
| Stem cell | Undifferentiated cells with the capacity to self-renew and ability to differentiate into various specialized cell types |
| Stiffness | A property that refers to the resistance of a material to deformation in response to an applied force or load |
| WNTs | Wingless INTs, a family of highly conserved signaling molecules that play crucial roles in embryonic development, tissue homeostasis, and adult stem cell regulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scodellaro, C.; Pina, R.R.; Ferreira, F.C.; Sanjuan-Alberte, P.; Fernandes, T.G. Unlocking the Potential of Stem Cell Microenvironments In Vitro. Bioengineering 2024, 11, 289. https://doi.org/10.3390/bioengineering11030289
Scodellaro C, Pina RR, Ferreira FC, Sanjuan-Alberte P, Fernandes TG. Unlocking the Potential of Stem Cell Microenvironments In Vitro. Bioengineering. 2024; 11(3):289. https://doi.org/10.3390/bioengineering11030289
Chicago/Turabian StyleScodellaro, Chiara, Raquel R. Pina, Frederico Castelo Ferreira, Paola Sanjuan-Alberte, and Tiago G. Fernandes. 2024. "Unlocking the Potential of Stem Cell Microenvironments In Vitro" Bioengineering 11, no. 3: 289. https://doi.org/10.3390/bioengineering11030289
APA StyleScodellaro, C., Pina, R. R., Ferreira, F. C., Sanjuan-Alberte, P., & Fernandes, T. G. (2024). Unlocking the Potential of Stem Cell Microenvironments In Vitro. Bioengineering, 11(3), 289. https://doi.org/10.3390/bioengineering11030289










