Nerve Reconstruction Using ActiGraft Blood Clot in Rabbit Acute Peripheral Injury Model: Preliminary Study
Abstract
:1. Introduction
1.1. Strategies for Treating Complete Peripheral Nerve Injuries
1.2. Peripheral Nerve Grafts
1.2.1. Autologous Nerve Grafts
1.2.2. Allografts
1.2.3. Nerve Tubes
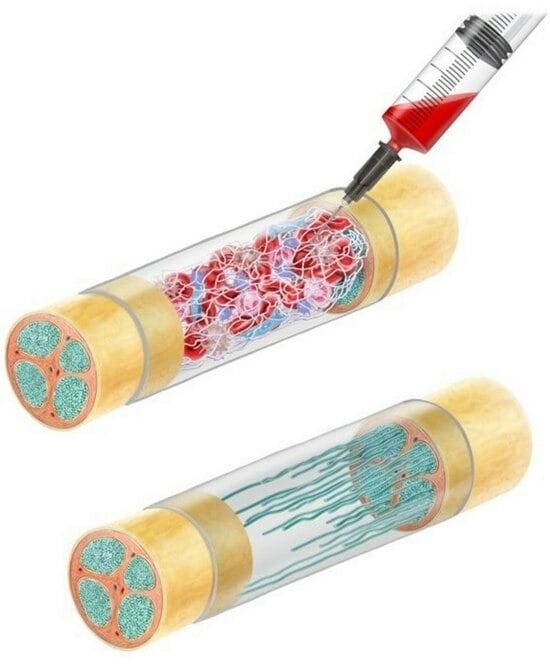
1.2.4. Autologous Blood Clots
1.2.5. Fibrin as a Filler for the Nerve Tube
2. Materials and Methods
2.1. Animals and Surgical Procedure
2.2. Rabbit PNI Model
2.3. Induction and Repair of Peripheral Nerve Injury (PNI)
Method of Blood Sampling Used to Prepare the ActiGraft Blood Clot
2.4. Organ/Tissue Collection and Fixation
2.4.1. Slide Preparation
2.4.2. Light Microscopy Photography
2.4.3. Histopathological Evaluation
- Grade 0—the tissue appears normal.
- Grade 1—minimal pathological findings.
- Grade 2—mild pathological findings.
- Grade 3—moderate pathological findings.
- Grade 4—severe pathological findings.
2.4.4. IHC of MBP for Myelin Staining (Per a ×20 Field)
- Grade 0 = no positive staining reaction.
- Grade 1 = only a few cells are immune positive (<5 cells).
- Grade 2 = very mild immune reaction (5–15 cells).
- Grade 3 = mild immune reaction (15–25 cells).
- Grade 4 = moderate immune reaction (25–50 cells).
- Grade 5 = significant immune reaction (>50 cells).
2.4.5. Electromyography (EMG) Examinations
- Amplitude = the peak of the action potential of the electrical signal, measured in microvolts (μV);
- Latency = the time for the action potential to take place, measured in milliseconds (ms).
2.4.6. Statistics
3. Results
3.1. Mortality Incidents
3.2. Electromyography (EMG) Examinations
3.3. Histopathology
3.3.1. H&E Staining
3.3.2. Myelin-Based Protein (MBP) Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2000, 23, 863–873. [Google Scholar] [CrossRef]
- Hammi, C.; Yeung, B. Neuropathy. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Saperstein, D.S.; Katz, J.S.; Amato, A.A.; Barohn, R.J. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve 2001, 24, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Sindrup, S.H.; Jensen, T.S. Pharmacologic treatment of pain in polyneuropathy. Neurology 2000, 55, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Ahlawat, S.; Belzberg, A.; Andreseik, G. Peripheral nerve injury grading simplified on MR neurography: As referenced to Seddon and Sunderland classifications. Indian J. Radiol. Imaging 2014, 24, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.J.; Lavernia, C.J.; Owens, P.W. Anatomy and physiology of peripheral nerve injury and repair. Am. J. Orthop. 2000, 29, 167–173. [Google Scholar]
- Oberlin, S.; Rantissi, M. Gunshot injuries to the nerves. Chir. Main 2011, 30, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Myckatyn, T.M.; Mackinnon, S.E. A review of research endeavors to optimize peripheral nerve reconstruction. Neurol. Res. 2004, 26, 124–138. [Google Scholar] [CrossRef]
- Beris, A.; Gkiatas, I.; Gelalis, I.; Papadopoulos, D.; Kostas-Agnantis, I. Current concepts in peripheral nerve surgery. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 263–269. [Google Scholar] [CrossRef]
- Bhandari, P. Management of peripheral nerve injury. J. Clin. Orthop. Trauma 2019, 10, 862–866. [Google Scholar] [CrossRef]
- Panagopoulos, G.N.; Megaloikonomos, P.D.; Mavrogenis, A.F. The present and future for peripheral nerve regeneration. Orthopedics 2017, 40, 141–156. [Google Scholar] [CrossRef]
- Spearman, B.S.; Desai, V.H.; Mobini, S.; McDermott, M.D.; Graham, J.B.; Otto, K.J.; Judy, J.W.; Schmidt, C.E. Tissue-engineered peripheral nerve interfaces. Adv. Funct. Mater. 2018, 28, 1701713. [Google Scholar] [CrossRef]
- Millesi, H. Techniques for nerve grafting. Hand Clin. 2000, 16, 73–91. [Google Scholar] [CrossRef]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.; Joosten, E.A.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobıol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Siemionow, M.; Brzezicki, G. Chapter 8: Current, techniques and concepts in peripheral nerve repair. In International Review of Neurobiology; Stefano, G., Pierluigi, T., Bruno, B., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 141–172. [Google Scholar]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Gluck, T. Ueber Transplantation, Regeneration und entzündliche Neubildung. Klin. Wochenschr. 1881, 18, 554–557. [Google Scholar]
- Snyder, R.J.; Schultz, G.; Wachuku, C.; Arij, M.; Rashid, A.M.; Ead, J.K. Proposed Mechanism of Action of Topically Applied Autologous Blood Clot Tissue. A Quintessential Cellular and Tissue-Based Therapy. J. Am. Podiatr. Med. Assoc. 2023, 113. [Google Scholar] [CrossRef]
- Kushnir, I.; Kushnir, A.; Serena, T.E.; Garfinkel, D. Efficacy and safety of a novel autologous wound matrix in the management of complicated, chronic wounds: A pilot study. Wounds 2016, 28, 317–327. [Google Scholar]
- Galla, T.; Vedecnik, S.; Halbgewachs, J.; Steinmann, S.; Friedrich, C.; Stark, G. Fibrin/Schwann cell matrix in poly-epsilon-caprolactone conduits enhances guided nerve regeneration. Int. J. Artif. Organs 2004, 27, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Han, S.-G.; Kim, S.-H.; Zhu, S.-J.; Huh, J.-Y.; Jung, J.-H.; Lee, S.-H.; Kim, B.-Y. Autologous fibrin glue in peripheral nerve regeneration in vivo. Microsurgery 2005, 25, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Hosseinpour, S.; Nazeman, P.; Dehghan, M. The effect of a platelet-rich fibrin conduit on neurosensory recovery following inferior alveolar nerve lateralization: A preliminary clinical study. Int. J. Oral Maxillofac. Surg. 2016, 45, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.; Fernandes, M.; Valente, S.G.; Santos, J.B.; Furukawa, R.B.; Fernandes, C.H.; Leite, V.M.; Faloppa, F. Platelet-rich fibrin conduits as an alternative to nerve autografts for peripheralnerve repair. J. Reconstr. Microsurg. 2017, 33, 549–556. [Google Scholar]
- Schuh, C.M.; Day, A.G.; Redl, H.; Phillips, J. An optimized collagen-fibrin blend engineered neural tissue promotes peripheral nerve repair. Tissue Eng. Part A 2018, 24, 1332–1340. [Google Scholar] [CrossRef]
- Carriel, V.; Garrido-Gómez, J.; Hernández-Cortés, P.; Garzón, I.; García-García, S.; Sáez-Moreno, J.A.; del Carmen Sanchez-Quevedo, M.; Campos, A.; Alaminos, M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J. Neural. Eng. 2013, 10, 026022. [Google Scholar] [CrossRef]
- Chato-Astrain, J.; Campos, F.; Roda, O.; Miralles, E.; Durand-Herrera, D.; Sáez-Moreno, J.A.; García-García, S.; Alaminos, M.; Campos, A.; Carriel, V. In vivo evaluation of nanostructured fibrin-agarose hydrogels with mesenchymal stem cells for peripheral nerve repair. Front. Cell. Neurosci. 2018, 12, 501. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Yao, S.; Mao, H.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y.; Xiao, B.; Wang, Y.; et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017, 55, 296–309. [Google Scholar] [CrossRef]
- Sorkin, J.A.; Rechany, Z.; Almog, M.; Dietzmeyer, N.; Shapira, Y.; Haastert-Talini, K.; Rochkind, S. A Rabbit Model for Peripheral Nerve Reconstruction Studies Avoiding Automutilation Behavior. J. Brachial. Plex. Peripher. Nerve Inj. 2022, 17, e22–e29. [Google Scholar] [CrossRef] [PubMed]
- Schafer, K.A.; Eighmy, J.; Fikes, J.D.; Halpern, W.G.; Hukkanen, R.R.; Long, G.G.; Meseck, E.K.; Patrick, D.J.; Thibodeau, M.S.; Wood, C.E.; et al. Use of Severity Grades to characterize Histopathologic Changes. Toxicol. Pathol. 2018, 46, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, A.; Borschel, G.H.; Luciano, J.P.; Whitlock, E.L.; Hayashi, A.; Hunter, D.A.; Mackinnon, S.E. The impact of motor and sensory nerve architecture on nerve regeneration. Exp. Neurol. 2008, 212, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Bellamkonda, R.V. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials 2006, 27, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- Battiston, B.; Raimondo, S.; Tos, P.; Gaidano, V.; Audisio, C.; Scevola, A. Chapter 11: Tissue engineering of peripheral nerves. Int. Rev. Neurobiol. 2009, 87, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Dodla, M.C.; Bellamkonda, R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008, 29, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, F.; Chen, M.B.; Lineaweaver, W.C. Chapter 10: Conduit luminal additives for peripheral nerve repair. Int. Rev. Neurobiol. 2009, 87, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Letourneau, P.C.; Tranquillo, R.T. Guided neurite elongation and Schwann cell invasion into magnetically aligned collagen in simulated peripheral nerve regeneration. Exp. Neurol. 1999, 158, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.I.; Siegel, R.A.; Grosberg, A.; Tranquillo, R.T. Rational design of contact guiding, neurotrophic matrices for peripheral nerve regeneration. Ann. Biomed. Eng. 2003, 31, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M. The past the present and the future of face transplantation. Curr. Opin. Organ Transplant. 2020, 25, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Leach, J.B. Neural tissue engineering: Strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Astachov, L.; El-Ani, D.; Hayon, T.; Graif, M.; Barsky, L.; Alon, M.; Odvak, I.; Nevo, Z.; Shahar, A. Further development of reconstructive and cell tissue-engineering technology for treatment of complete peripheral nerve injury in rats. Neurol. Res. 2004, 26, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Nevo, Z. Recovery of Peripheral Nerve with Massive Loss Defect by Tissue Engineered Guiding Regenerative Gel. BioMed Res. Int. 2014, 2014, 327578. [Google Scholar] [CrossRef]
- Rochkind, S.; Almog, M.; Meilin, S.; Nevo, Z. Reviving Matrix for Nerve Reconstruction in Rabbit model of chronic peripheral nerve injury with massive loss defect. Front. Surg. 2021, 7, 609638. [Google Scholar] [CrossRef]
- Fellin, C.R.; Steiner, R.C.; Buchen, J.T.; Anders, J.J.; Jariwala, S.H. Photobiomodulation therapy (PBMT) utilizes visible and near-infrared electromagnetic energy to induce therapeutic photochemical changes and improve clinical recovery outcomes. Photobiomodul. Photomed. Laser Surg. 2024, 42, 1–10. [Google Scholar]
- Rosso, M.P.d.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef]
- Anders, J.J.; Geuna, S.; Rochkind, S. Phototherapy promotes regeneration and functionalrecovery of injured peripheral nerve. Neurol. Res. 2004, 26, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Gigo-Benato, D.; Geuna, S.; Rochkind, S. Phototherapy for enhancing peripheral nerve repair: A review of the literature. Muscle Nerve 2005, 31, 694–701. [Google Scholar] [CrossRef]
- Rochkind, S.; Geuna, S.; Shainberg, A. Chapter 25 Phototherapy in Peripheral Nerve Injury: Effects on Muscle Preservation and Nerve Regeneration. Int. Rev. Neurobiol. 2009, 87, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Leider-Trejo, L.; Nissan, M.; Shamir, M.H.; Kharenko, O.; Alon, M. Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study). Photomed. Laser Surg. 2007, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Yang, Y.C.; Liu, B.S. Effects of large-area irradiated laser phototherapy on peripheral nerve regeneration across a large gap in a biomaterial conduit. J. Biomed. Mater. Res. A 2013, 101, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J. Treatment of nonhealing ulcers with allografts. Clin Dermato. 2005, 23, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Kasper, M.A.; Patel, K.; Carter, M.J.; Kushnir, I.; Kushnir, A.; Serena, T.E. Safety and Efficacy of an Autologous Blood Clot Product in the Management of Texas 1A or 2A Neuropathic Diabetic Foot Ulcers: A Prospective, Multicenter, Open Label Pilot Study. Wounds 2018, 30, 205–212. [Google Scholar]
- Gao, H.; You, Y.; Zhang, G.; Zhao, F.; Sha, Z.; Shen, Y. The use of fiber-reinforced scaffolds cocultured with Schwann cells and vascular endothelial cells to repair rabbit sciatic nerve defect with vascularization. Biomed. Res. Int. 2013, 2013, 362918. [Google Scholar] [CrossRef]
- Geuna, S.; Tos, P.; Battiston, B.; Giacobini-Robecchi, M.G. Bridging peripheral nerve defects with muscle-vein combined guides. Neurol. Res. 2004, 26, 139–144. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chan, S.H.; Chiang, C.M.; Chen, C.C.; Jiang, C.F. Peripheral nerve regeneration using a microporous polylactic acid asymmetric conduit in a rabbit long-gap sciatic nerve transection model. Biomaterials 2011, 32, 3764–3775. [Google Scholar] [CrossRef] [PubMed]





| Treatment | N |
|---|---|
| 1. NeuraGen® Nerve Guide | 3 |
| 2. NeuraGen® Nerve Guide filled with ActiGraft Blood Clot | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochkind, S.; Sirota, S.; Kushnir, A. Nerve Reconstruction Using ActiGraft Blood Clot in Rabbit Acute Peripheral Injury Model: Preliminary Study. Bioengineering 2024, 11, 298. https://doi.org/10.3390/bioengineering11040298
Rochkind S, Sirota S, Kushnir A. Nerve Reconstruction Using ActiGraft Blood Clot in Rabbit Acute Peripheral Injury Model: Preliminary Study. Bioengineering. 2024; 11(4):298. https://doi.org/10.3390/bioengineering11040298
Chicago/Turabian StyleRochkind, Shimon, Sharon Sirota, and Alon Kushnir. 2024. "Nerve Reconstruction Using ActiGraft Blood Clot in Rabbit Acute Peripheral Injury Model: Preliminary Study" Bioengineering 11, no. 4: 298. https://doi.org/10.3390/bioengineering11040298