Nonthermal Atmospheric Pressure Plasma Treatment of Endosteal Implants for Osseointegration and Antimicrobial Efficacy: A Comprehensive Review
Abstract
:1. Introduction
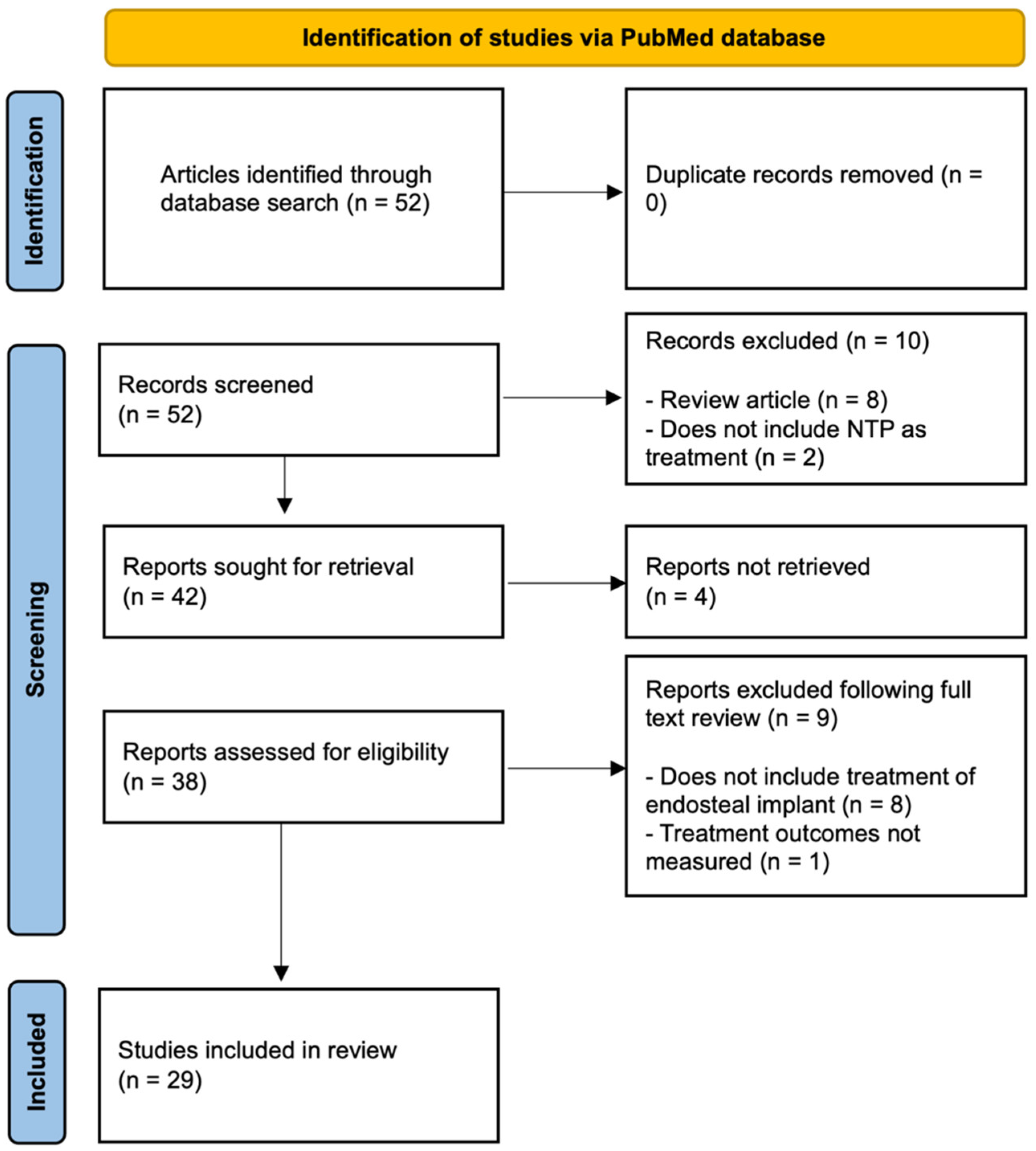
2. Methods
3. Atmospheric Pressure Plasma
3.1. Surface Modifications and Characterization
3.2. The Long-Term Effects of NTP Treatment
4. In Vitro Studies Using NTPs
4.1. NTP Effects on Cell Proliferation and Adhesion
4.2. NTP Effects on Disinfection
5. In Vivo Studies Using NTPs
5.1. Preclinical and Clinical Studies on Osseointegration and Disinfection
5.2. Preclinical and Clinical Studies on Wound Healing
6. Novel Applications of NTP
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Dental Implants Market Size, Share and Growth Report. Available online: https://www.grandviewresearch.com/industry-analysis/dental-implants-market (accessed on 10 February 2024).
- Lee, S.K.; Ji, M.K.; Jo, Y.J.; Park, C.; Cho, H.; Lim, H.P. Effect of Non-Thermal Plasma Treatment of Contaminated Zirconia Surface on Porphyromonas gingivalis Adhesion and Osteoblast Viability. Materials 2022, 15, 5348. [Google Scholar] [CrossRef]
- Maillet, C.; Klein, F.M.; Le Bras, F.; Velard, F.; Guillaume, C.; Gangloff, S.C.; Gelle, M.P. Cytocompatibility of titanium and poly(etheretherketone) surfaces after O2 non-thermal plasma sterilization. PLoS ONE 2023, 18, e0290820. [Google Scholar] [CrossRef]
- Ochsenbein, A.; Chai, F.; Winter, S.; Traisnel, M.; Breme, J.; Hildebrand, H.F. Osteoblast responses to different oxide coatings produced by the sol-gel process on titanium substrates. Acta Biomater. 2008, 4, 1506–1517. [Google Scholar] [CrossRef]
- Cheng, F.; Shi, P.; Man, H.C. Anatase coating on NiTi via a low-temperature sol–gel route for improving corrosion resistance. Scr. Mater. 2004, 51, 1041–1045. [Google Scholar] [CrossRef]
- Echeverry-Rendón, M.; Galvis, O.; Quintero Giraldo, D.; Pavón, J.; López-Lacomba, J.L.; Jiménez-Piqué, E.; Anglada, M.; Robledo, S.M.; Castaño, J.G.; Echeverría, F. Osseointegration improvement by plasma electrolytic oxidation of modified titanium alloys surfaces. J. Mater. Sci. Mater. Med. 2015, 26, 72. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.Y.; Jurng, J.; Park, Y.-K. The synthesis and coating process of TiO2 nanoparticles using CVD process. Powder Technol. 2011, 214, 64–68. [Google Scholar] [CrossRef]
- Yoshida, R.; Suzuki, Y.; Yoshikawa, S. Syntheses of TiO2(B) nanowires and TiO2 anatase nanowires by hydrothermal and post-heat treatments. J. Solid. State Chem. 2005, 178, 2179–2185. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Pedeferri, M.P. Effect of anodic oxidation parameters on the titanium oxides formation. Corros. Sci. 2007, 49, 939–948. [Google Scholar] [CrossRef]
- Fadl-allah, S.; El-sherif, R.; Badawy, W. Electrochemical formation and characterization of porous titania (TiO2) films on Ti. J. Appl. Electrochem. 2008, 38, 1459–1466. [Google Scholar] [CrossRef]
- Santos Junior, E.; Kuromoto, N.; Soares, G. Mechanical properties of titania films used as biomaterials. Mater. Chem. Phys. 2007, 102, 92–97. [Google Scholar] [CrossRef]
- Feng, F.; Wu, Y.; Xin, H.; Chen, X.; Guo, Y.; Qin, D.; An, B.; Diao, X.; Luo, H. Surface Characteristics and Biocompatibility of Ultrafine-Grain Ti after Sandblasting and Acid Etching for Dental Implants. ACS Biomater. Sci. Eng. 2019, 5, 5107–5115. [Google Scholar] [CrossRef]
- De Angelis, E.; Ravanetti, F.; Cacchioli, A.; Corradi, A.; Giordano, C.; Candiani, G.; Chiesa, R.; Gabbi, C.; Borghetti, P. Attachment, proliferation and osteogenic response of osteoblast-like cells cultured on titanium treated by a novel multiphase anodic spark deposition process. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 280–289. [Google Scholar] [CrossRef]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 635–645. [Google Scholar] [CrossRef]
- Takebe, J.; Ito, S.; Miura, S.; Miyata, K.; Ishibashi, K. Physicochemical state of the nanotopographic surface of commercially pure titanium following anodization-hydrothermal treatment reveals significantly improved hydrophilicity and surface energy profiles. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 55–60. [Google Scholar] [CrossRef]
- Sharma, A.; McQuillan, A.J.; Sharma, L.A.; Waddell, J.N.; Shibata, Y.; Duncan, W.J. Spark anodization of titanium-zirconium alloy: Surface characterization and bioactivity assessment. J. Mater. Sci. Mater. Med. 2015, 26, 221. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. 2018, 27, 1055–1059. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Rościszewska, M.; Majkowska-Marzec, B.; Jażdżewska, M.; Bartmański, M.; Zieliński, A.; Tybuszewska, N.; Samsel, P. Influence of Surface Modification of Titanium and Its Alloys for Medical Implants on Their Corrosion Behavior. Materials 2022, 15, 7556. [Google Scholar] [CrossRef] [PubMed]
- ISO 14937:2009; Sterilization of Health Care Products—General Requirements for Characterization of a Sterilizing Agent and the Development, Validation and Routine Control of a Sterilization Process for Medical Devices. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/44954.html (accessed on 19 February 2024).
- Montgomery, A.; Bolle-Reddat, R.; Formica, S.; Lundahl, B.; McDonnell, G. Regulatory Approach for Transitioning from Gamma Ray to X-ray Radiation Sterilization. Biomed. Instrum. Technol. 2021, 55, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H. Ethylene Oxide Gas Sterilization of Medical Devices. Biocontrol Sci. 2017, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile; U.S. Department of Health and Human Service, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research: Silver Spring, MD, USA, 2024; pp. 1–11.
- Török, G.; Gombocz, P.; Bognár, E.; Nagy, P.; Dinya, E.; Kispélyi, B.; Hermann, P. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy—Pilot study. BMC Oral Health 2020, 20, 19. [Google Scholar] [CrossRef]
- Morsy, M.S.M.; Hassan, A.A.A.; Alshawkani, H.A.; Mattoo, K.A.; Mathur, A.; Fiorillo, L. Effect of Repeated Moist Heat Sterilization on Titanium Implant-Abutment Interface-An In Vitro Study. Eur. J. Dent. 2024. [Google Scholar] [CrossRef]
- Morrison, R.J.; Kashlan, K.N.; Flanangan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.J.; Weatherwax, K.J. Regulatory Considerations in the Design and Manufacturing of Implantable 3D-Printed Medical Devices. Clin. Transl. Sci. 2015, 8, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Time-dependent effects of ultraviolet and nonthermal atmospheric pressure plasma on the biological activity of titanium. Sci. Rep. 2016, 6, 33421. [Google Scholar] [CrossRef] [PubMed]
- Cogollo de Cádiz, M.; López Arrabal, A.; Díaz Lantada, A.; Aguirre, M.V. Materials degradation in non-thermal plasma generators by corona discharge. Sci. Rep. 2021, 11, 24175. [Google Scholar] [CrossRef] [PubMed]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Elaissi, S.; Alsaif, N.A.M. Modelling of Nonthermal Dielectric Barrier Discharge Plasma at Atmospheric Pressure and Role of Produced Reactive Species in Surface Polymer Microbial Purification. Polymers 2023, 15, 1235. [Google Scholar] [CrossRef]
- Morelli, A.; Hawker, M.J. Utilizing Radio Frequency Plasma Treatment to Modify Polymeric Materials for Biomedical Applications. ACS Biomater. Sci. Eng. 2023, 9, 3760–3777. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.H.; Lin, J.C.Y.; Chen, M.K.; Salamanca, E.; Choy, C.S.; Tsai, P.Y.; Leu, S.J.; Yang, K.C.; Huang, H.M.; Yao, W.L.; et al. Glow Discharge Plasma Treatment on Zirconia Surface to Enhance Osteoblastic-Like Cell Differentiation and Antimicrobial Effects. Materials 2020, 13, 3771. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Rolauffs, B.; Adolfsson, E.; Altmann, B. Human osteoblast and fibroblast response to oral implant biomaterials functionalized with non-thermal oxygen plasma. Sci. Rep. 2021, 11, 17302. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Tendero, C.; Dublanche-Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric Pressure Plasmas: A Review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Jang, M.H.; Park, Y.B.; Kwon, J.S.; Kim, Y.J.; Lee, J.H. Osseointegration of Plasma Jet Treated Titanium Implant Surface in an Animal Model. Materials 2021, 14, 1942. [Google Scholar] [CrossRef]
- Liu, T.; Wu, L.; Babu, J.P.; Hottel, T.L.; Garcia-Godoy, F.; Hong, L. Effects of atmospheric non-thermal argon/oxygen plasma on biofilm viability and hydrophobicity of oral bacteria. Am. J. Dent. 2017, 30, 52–56. [Google Scholar] [PubMed]
- Pandiyaraj, K.N.; Kumar, A.A.; Ramkumar, M.C.; Sachdev, A.; Gopinath, P.; Cools, P.; De Geyter, N.; Morent, R.; Deshmukh, R.R.; Hegde, P.; et al. Influence of non-thermal TiCl4/Ar + O2 plasma-assisted TiOx based coatings on the surface of polypropylene (PP) films for the tailoring of surface properties and cytocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 908–918. [Google Scholar] [CrossRef]
- Nayak, V.V.; Mirsky, N.A.; Slavin, B.V.; Witek, L.; Coelho, P.G.; Tovar, N. Non-Thermal Plasma Treatment of Poly(tetrafluoroethylene) Dental Membranes and Its Effects on Cellular Adhesion. Materials 2023, 16, 6633. [Google Scholar] [CrossRef]
- Silva, N.R.F.A.; Coelho, P.G.; Valverde, G.B.; Becker, K.; Ihrke, R.; Quade, A.; Thompson, V.P. Surface characterization of Ti and Y-TZP following non-thermal plasma exposure. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99B, 199–206. [Google Scholar] [CrossRef]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. A 2013, 101, 98–103. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, K.H.; Park, S.Y.; Yoon, S.Y.; Kim, G.H.; Lee, Y.M.; Rhyu, I.C.; Seol, Y.J. The bactericidal effect of an atmospheric-pressure plasma jet on Porphyromonas gingivalis biofilms on sandblasted and acid-etched titanium discs. J. Periodontal Implant. Sci. 2019, 49, 319–329. [Google Scholar] [CrossRef]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet treatment overcomes time-related degrading bioactivity of titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Lee, K.J.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Overcoming the biological aging of titanium using a wet storage method after ultraviolet treatment. Sci. Rep. 2017, 7, 3833. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Heiland, M.; Cacaci, C.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. 1), 4–7. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, W.S.; Seo, S.J.; Kim, H.W.; Kim, K.N.; Choi, E.H.; Kim, K.M. Non-thermal atmospheric pressure plasma functionalized dental implant for enhancement of bacterial resistance and osseointegration. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, 257–270. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Huang, A.; Ding, C.; Chu, P.K. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials 2005, 26, 6129–6135. [Google Scholar] [CrossRef]
- Akçay, H.; Ercan, U.K.; Bahçeci, S.; Ulu, M.; Ibiş, F.; Enhoş, Ş. The Effect of Atmospheric Pressure Cold Plasma Application on Titanium Barriers: A Vertical Bone Augmentation. J. Craniofac. Surg. 2020, 31, 2054–2058. [Google Scholar] [CrossRef]
- Henningsen, A.; Precht, C.; Karnatz, N.; Bibiza, E.; Yan, M.; Guo, L.; Gosau, M.; Smeets, R. Osseointegration of titanium implants after surface treatment with ultraviolet light or cold atmospheric plasma in vivo. Int. J. Oral Implantol. 2023, 16, 197–208. [Google Scholar]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. A 2012, 100, 1901–1906. [Google Scholar] [CrossRef]
- Evert, K.; Kocher, T.; Schindler, A.; Müller, M.; Müller, K.; Pink, C.; Holtfreter, B.; Schmidt, A.; Dombrowski, F.; Schubert, A.; et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci. Rep. 2021, 11, 20672. [Google Scholar] [CrossRef]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef]
- Danna, N.R.; Beutel, B.G.; Tovar, N.; Witek, L.; Marin, C.; Bonfante, E.A.; Granato, R.; Suzuki, M.; Coelho, P.G. Assessment of Atmospheric Pressure Plasma Treatment for Implant Osseointegration. BioMed Res. Int. 2015, 2015, 761718. [Google Scholar] [CrossRef]
- Beutel, B.G.; Danna, N.R.; Gangolli, R.; Granato, R.; Manne, L.; Tovar, N.; Coelho, P.G. Evaluation of bone response to synthetic bone grafting material treated with argon-based atmospheric pressure plasma. Mater. Sci. Eng. C 2014, 45, 484–490. [Google Scholar] [CrossRef]
- Hayes, J.S.; Seidenglanz, U.; Pearce, A.I.; Pearce, S.G.; Archer, C.W.; Richards, R.G. Surface polishing positively influences ease of plate and screw removal. Eur. Cell Mater. 2010, 19, 117–126. [Google Scholar] [CrossRef]
- Hayes, J.S.; Welton, J.L.; Wieling, R.; Richards, R.G. In vivo evaluation of defined polished titanium surfaces to prevent soft tissue adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 611–617. [Google Scholar] [CrossRef]
- Meredith, D.O.; Eschbach, L.; Riehle, M.O.; Curtis, A.S.; Richards, R.G. Microtopography of metal surfaces influence fibroblast growth by modifying cell shape, cytoskeleton, and adhesion. J. Orthop. Res. 2007, 25, 1523–1533. [Google Scholar] [CrossRef]
- Tsujita, H.; Nishizaki, H.; Miyake, A.; Takao, S.; Komasa, S. Effect of Plasma Treatment on Titanium Surface on the Tissue Surrounding Implant Material. Int. J. Mol. Sci. 2021, 22, 6931. [Google Scholar] [CrossRef]
- Patelli, A.; Mussano, F.; Brun, P.; Genova, T.; Ambrosi, E.; Michieli, N.; Mattei, G.; Scopece, P.; Moroni, L. Nanoroughness, Surface Chemistry, and Drug Delivery Control by Atmospheric Plasma Jet on Implantable Devices. ACS Appl. Mater. Interfaces 2018, 10, 39512–39523. [Google Scholar] [CrossRef]
- Matthes, R.; Jablonowski, L.; Pitchika, V.; Holtfreter, B.; Eberhard, C.; Seifert, L.; Gerling, T.; Vilardell Scholten, L.; Schlüter, R.; Kocher, T. Efficiency of biofilm removal by combination of water jet and cold plasma: An in-vitro study. BMC Oral Health 2022, 22, 157. [Google Scholar] [CrossRef]
- Wagner, G.; Eggers, B.; Duddeck, D.; Kramer, F.J.; Bourauel, C.; Jepsen, S.; Deschner, J.; Nokhbehsaim, M. Influence of cold atmospheric plasma on dental implant materials—An in vitro analysis. Clin. Oral Investig. 2022, 26, 2949–2963. [Google Scholar] [CrossRef]
- Kamionka, J.; Matthes, R.; Holtfreter, B.; Pink, C.; Schlüter, R.; von Woedtke, T.; Kocher, T.; Jablonowski, L. Efficiency of cold atmospheric plasma, cleaning powders and their combination for biofilm removal on two different titanium implant surfaces. Clin. Oral Investig. 2022, 26, 3179–3187. [Google Scholar] [CrossRef]
- Flörke, C.; Janning, J.; Hinrichs, C.; Behrens, E.; Liedtke, K.R.; Sen, S.; Christofzik, D.; Wiltfang, J.; Gülses, A. In-vitro assessment of the efficiency of cold atmospheric plasma on decontamination of titanium dental implants. Int. J. Implant. Dent. 2022, 8, 12. [Google Scholar] [CrossRef]
- Ji, M.K.; Lee, S.K.; Kim, H.S.; Oh, G.J.; Cho, H.; Lim, H.P. Assessment of Inhibition of Biofilm Formation on Non-Thermal Plasma-Treated TiO2 Nanotubes. Int. J. Mol. Sci. 2023, 24, 3335. [Google Scholar] [CrossRef]
- Preissner, S.; Wirtz, H.C.; Tietz, A.K.; Abu-Sirhan, S.; Herbst, S.R.; Hartwig, S.; Pierdzioch, P.; Schmidt-Westhausen, A.M.; Dommisch, H.; Hertel, M. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: An in-vitro-study. J. Biophotonics 2016, 9, 637–644. [Google Scholar] [CrossRef]
- Gallingani, T.; Resca, E.; Dominici, M.; Gavioli, G.; Laurita, R.; Liguori, A.; Mari, G.; Ortolani, L.; Pericolini, E.; Sala, A.; et al. A new strategy to prevent biofilm and clot formation in medical devices: The use of atmospheric non-thermal plasma assisted deposition of silver-based nanostructured coatings. PLoS ONE 2023, 18, e0282059. [Google Scholar] [CrossRef]
- Park, C.; Park, S.W.; Yun, K.D.; Ji, M.K.; Kim, S.; Yang, Y.P.; Lim, H.P. Effect of Plasma Treatment and Its Post Process Duration on Shear Bonding Strength and Antibacterial Effect of Dental Zirconia. Materials 2018, 11, 2233. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, M.; Jia, Y.N.; Li, J.; Li, H.P.; Tan, J.G. Time-dependent reactive oxygen species inhibit Streptococcus mutans growth on zirconia after a helium cold atmospheric plasma treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111633. [Google Scholar] [CrossRef]
- Park, L.; Kim, H.S.; Jang, W.; Ji, M.K.; Ryu, J.H.; Cho, H.; Lim, H.P. Antibacterial Evaluation of Zirconia Coated with Plasma-Based Graphene Oxide with Photothermal Properties. Int. J. Mol. Sci. 2023, 24, 8888. [Google Scholar] [CrossRef]
- Ulu, M.; Pekbagriyanik, T.; Ibis, F.; Enhos, S.; Ercan, U.K. Antibiofilm efficacies of cold plasma and er: YAG laser on Staphylococcus aureus biofilm on titanium for nonsurgical treatment of peri-implantitis. Niger. J. Clin. Pract. 2018, 21, 758–765. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, M.; Yang, Y.; Li, J.; Su, Y.F.; Li, H.P.; Tan, J.G. Inhibition of bacterial growth on zirconia abutment with a helium cold atmospheric plasma jet treatment. Clin. Oral Investig. 2020, 24, 1465–1477. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Jiang, H.B.; Choi, E.H.; Park, G.; Kim, K.M. The antibacterial effect of non-thermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Sci. Rep. 2019, 9, 1938. [Google Scholar] [CrossRef]
- Panariello, B.H.; Mody, D.P.; Eckert, G.J.; Witek, L.; Coelho, P.G.; Duarte, S.J.B.R.I. Low-temperature plasma short exposure to decontaminate peri-implantitis-related multispecies biofilms on titanium surfaces in vitro. BioMed Res. Int. 2022, 2022, 1549774. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, D.; Liang, D.; Zhang, W.; Shi, Q.; Cao, Y. Evaluation of modified cold-atmospheric pressure plasma (MCAP) for the treatment of peri-implantitis in beagles. Oral Dis. 2022, 28, 495–502. [Google Scholar] [CrossRef]
- Küçük, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Sağlam, M.; Köseoğlu, S. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef]
- Nevins, M.; Chen, C.Y.; Parma-Benfenati, S.; Kim, D.M. Gas Plasma Treatment Improves Titanium Dental Implant Osseointegration-A Preclinical In Vivo Experimental Study. Bioengineering 2023, 10, 1181. [Google Scholar] [CrossRef]
- Hong, Q.; Dong, X.; Chen, M.; Sun, H.; Hong, L.; Wang, Y.; Li, H.; Yu, Q. An in vitro and in vivo study of plasma treatment effects on oral biofilms. J. Oral Microbiol. 2019, 11, 1603524. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef]
- Game, F.L.; Apelqvist, J.; Attinger, C.; Hartemann, A.; Hinchliffe, R.J.; Löndahl, M.; Price, P.E.; Jeffcoate, W.J.; on behalf of the International Working Group on the Diabetic Foot (IWGDF). Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 154–168. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Li, Y.F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S. aureus and E. coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS ONE 2012, 7, e34610. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Murphy, A.B.; McLean, K.M.; Kong, M.G.; Ostrikov, K.K. Atmospheric pressure plasmas: Infection control and bacterial responses. Int. J. Antimicrob. Agents 2014, 43, 508–517. [Google Scholar] [CrossRef]
- Lunov, O.; Churpita, O.; Zablotskii, V.; Deyneka, I.G.; Meshkovskii, I.K.; Jäger, A.; Syková, E.; Kubinová, Š.; Dejneka, A. Non-thermal plasma mills bacteria: Scanning electron microscopy observations. Appl. Phys. Lett. 2015, 106, 053703. [Google Scholar] [CrossRef]
- Julák, J.; Vaňková, E.; Válková, M.; Kašparová, P.; Masák, J.; Scholtz, V. Combination of non-thermal plasma and subsequent antibiotic treatment for biofilm re-development prevention. Folia Microbiol. 2020, 65, 863–869. [Google Scholar] [CrossRef]
- Paldrychová, M.; Vaňková, E.; Kašparová, P.; Sembolová, E.; Maťátková, O.; Masák, J.; Scholtz, V.; Julák, J. Use of non-thermal plasma pre-treatment to enhance antibiotic action against mature Pseudomonas aeruginosa biofilms. World J. Microbiol. Biotechnol. 2020, 36, 108. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef]
- De Souza, A.M.T.; Braz, J.K.F.; Martins, G.M.; Vitoriano, J.D.O.; Neto, A.G.; Nery, D.M.; Sabino, V.G.; Lucena, E.E.D.S.; Rocha, H.A.D.O.; Barboza, C.A.G.; et al. Comparative analysis of the biocompatibility of endothelial cells on surfaces treated by thermal plasma and cold atmospheric plasma. An. Acad. Bras. Ciências 2023, 95, e20220865. [Google Scholar] [CrossRef]
- Pekbağrıyanık, T.; Dadas, F.K.; Enhoş, Ş. Effects of non-thermal atmospheric pressure plasma on palatal wound healing of free gingival grafts: A randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 6269–6278. [Google Scholar] [CrossRef]
- Kisch, T.; Schleusser, S.; Helmke, A.; Mauss, K.L.; Wenzel, E.T.; Hasemann, B.; Mailaender, P.; Kraemer, R. The repetitive use of non-thermal dielectric barrier discharge plasma boosts cutaneous microcirculatory effects. Microvasc. Res. 2016, 106, 8–13. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, S.Y.; Kim, K.H.; Yoon, S.Y.; Kim, G.H.; Lee, Y.M.; Seol, Y.J. Safety evaluation of atmospheric pressure plasma jets in in vitro and in vivo experiments. J. Periodontal Implant. Sci. 2021, 51, 213–223. [Google Scholar] [CrossRef]
- Hadian, K.; Babossalam, S.; Mahdikia, H.; Aghighi, M.; Talebi, A.; Abdollahimajd, F.; Shokri, B. Efficacy and safety of non-thermal nitrogen plasma versus long-pulsed Nd:YAG laser for hand rejuvenation. Lasers Med. Sci. 2022, 37, 181–191. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.M.; Ostrikov, K.K. Effect of atmospheric gas plasmas on cancer cell signaling. Int. J. Cancer 2014, 134, 1517–1528. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Nedrelow, D.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Doberschütz, P.; Finkelstein, S.; Von Hoff, D.; et al. Head and neck Cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Mohamed, H.; Esposito, R.A.; Kutzler, M.A.; Wigdahl, B.; Krebs, F.C.; Miller, V. Nonthermal plasma as part of a novel strategy for vaccination. Plasma Process Polym. 2020, 17, 2000051. [Google Scholar] [CrossRef]
- Han, I.; Mumtaz, S.; Ashokkumar, S.; Yadav, D.K.; Choi, E.H. Review of Developments in Combating COVID-19 by Vaccines, Inhibitors, Radiations, and Nonthermal Plasma. Curr. Issues Mol. Biol. 2022, 44, 5666–5690. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Marar, T.; Patil, S. Non-thermal plasma: An advanced technology for food industry. Food Sci. Technol. Int. 2020, 26, 727–740. [Google Scholar] [CrossRef] [PubMed]

| Cell Proliferation | Material +/− Additional Surface Treatment | Type of Application/Treatment | Control | n/Condition | Type of Cells | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Titanium discs, roughed | NTP (10 s) | Untreated | 5 | rBMM- SC | Cell adhesion and viability | Lee 2017 [51] | |
| Titanium discs, SA | NTP | Untreated | N/A | MC3T3 | Cell adhesion and viability, proliferation, protein absorption | Patelli 2018 [64] | |
| Titanium discs, SA | NTP (30 s; 70 s) | Untreated | 9 | MG-63 | Cell spreading | Matthes 2022 [65] | |
| Titanium discs Zirconia discs | NTP | Untreated | 6 | HGF-1 MG-63 | Cell adhesion, gene expression, | Wagner 2022 [66] | |
| Titanium discs PEEK discs | O2-NTP (120 min) Autoclave (20 min) Gamma-ray irradiation | Untreated | 108 | MG-63 | Cell viability, proliferation, cytotoxicity | Maillet 2023 [5] | |
| Disinfection | Material +/− Additional Surface Treatment | Type of application/treatment | Control | n/Condition | Bacterial stain | Outcome | Reference |
| Titanium discs, mirror-polished | NTP (2 min; 10 min) | Untreated | 3 | S. mutants, S. aureus, K. oxytoca, K. pneumonuae | CFU, biofilm formation, viability, SEM | Lee 2019 [46] | |
| Titanium discs, anodized, SA | NTP (9 min), APG + NTP(9 min) APE + NTP (9 min) | Untreated, APG, APE, | 2 | Ex vivo Human biofilm | Biofilm reduction | Kamionka 2022 [67] | |
| Zirconia discs | NTP (60 s; 300 s; 600 s) | Untreated | N/A | P. gingivalis | Bacterial adhesion SEM | Lee 2022 [4] | |
| Titanium dental implant | NTP (3 min) | Untreated Photodynamic therapy PDT phosphoric acid gel PAG | 15 | E. faecalis | CFU, fluorescent staining | Floerke 2022 [68] | |
| Titanium discs, anodized, heat treated (600 °C) | NTP (120 s) | Untreated | 3 | S. mutans P. gingivalis | Biofilm formation, fluorescent staining | Ji 2023 [69] |
| Osseointegration | Material +/− Additional Surface Treatment | Type of Application/Treatment | Control | n/Condition | In Vivo Model | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Titanium implant | NTP (60 s) | Untreated | n = 24 total | canine | Surface energy BIC BAFO | Coelho 2012 [55] | |
| Titanium implant, SA | NTP (10 min) | Untreated | 10 | canine | BV BIC | Jang 2021 [40] | |
| Titanium implant | NTP, MD | MD + 2% CHX irrigation | 10 | canine | SBI PD BH IL-1β, IL-6, IL-17 Peri-implant sulcular fluid | Zhou 2022 [79] | |
| Titanium implant | NTP | Untreated UV | 18 | porcine | BIC BAFO | Henningsen 2023 [54] | |
| Titanium implant | NTP | Untreated | 6 | canine | Radiographic SEM BIC | Nevins 2023 [81] | |
| Disinfection | Material +/− additional surface treatment | Type of application/treatment | Control | n/Condition | In vivo model | Outcome | Reference |
| Direct NTP treatment to infected tooth | NTP | Standard therapy | 25 (internal control | human | CAL PCR ELISA | Küçük 2019 [80] | |
| Direct NTP to tooth | NTP | Untreated | 60 (internal control) | rat | Caries index | Hong et al. 2019 [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schafer, S.; Swain, T.; Parra, M.; Slavin, B.V.; Mirsky, N.A.; Nayak, V.V.; Witek, L.; Coelho, P.G. Nonthermal Atmospheric Pressure Plasma Treatment of Endosteal Implants for Osseointegration and Antimicrobial Efficacy: A Comprehensive Review. Bioengineering 2024, 11, 320. https://doi.org/10.3390/bioengineering11040320
Schafer S, Swain T, Parra M, Slavin BV, Mirsky NA, Nayak VV, Witek L, Coelho PG. Nonthermal Atmospheric Pressure Plasma Treatment of Endosteal Implants for Osseointegration and Antimicrobial Efficacy: A Comprehensive Review. Bioengineering. 2024; 11(4):320. https://doi.org/10.3390/bioengineering11040320
Chicago/Turabian StyleSchafer, Sogand, Tina Swain, Marcelo Parra, Blaire V. Slavin, Nicholas A. Mirsky, Vasudev Vivekanand Nayak, Lukasz Witek, and Paulo G. Coelho. 2024. "Nonthermal Atmospheric Pressure Plasma Treatment of Endosteal Implants for Osseointegration and Antimicrobial Efficacy: A Comprehensive Review" Bioengineering 11, no. 4: 320. https://doi.org/10.3390/bioengineering11040320






