Applying Self-Supervised Learning to Image Quality Assessment in Chest CT Imaging
Abstract
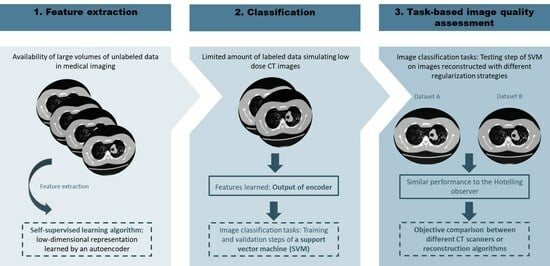
:1. Introduction
2. Materials and Methods
2.1. Data
2.1.1. Signal Known Exactly (SKE)
2.1.2. Signal Known Statistically (SKS)
2.2. Hotelling Observer
2.3. Supervised Learning-Based Model Observer
2.4. SSL-Based Model Observer
2.4.1. AE
2.4.2. SVM
3. Results
3.1. Optimal Structure of the CNN
3.2. Optimal Parameters for the SSL-Based Model Observer
3.3. Detectability Comparison between Observers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solomon, J.; Lyu, P.; Marin, D.; Samei, E. Noise and spatial resolution properties of a commercially available deep learning-based CT reconstruction algorithm. Med. Phys. 2020, 47, 3961–3971. [Google Scholar] [CrossRef] [PubMed]
- Funama, Y.; Nakaura, T.; Hasegawa, A.; Sakabe, D.; Oda, S.; Kidoh, M.; Nagayama, Y.; Hirai, T. Noise power spectrum properties of deep learning-based reconstruction and iterative reconstruction algorithms: Phantom and clinical study. Eur. J. Radiol. 2023, 165, 110914. [Google Scholar] [CrossRef]
- Kim, G.; Han, M.; Shim, H.; Baek, J. A convolutional neural network-based model observer for breast CT images. Med. Phys. 2020, 47, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leng, S.; Yu, L.; Carter, R.E.; McCollough, C.H. Correlation between human and model observer performance for discrimination task in CT. Phys. Med. Biol. 2014, 59, 3389–3404. [Google Scholar] [CrossRef] [PubMed]
- Granstedt, J.L.; Zhou, W.; Anastasio, M.A. Approximating the Hotelling Observer with Autoencoder-Learned Efficient Channels for Binary Signal Detection Tasks. arXiv 2020, arXiv:2003.02321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, H.; Anastasio, M.A. Approximating the Ideal Observer and Hotelling Observer for Binary Signal Detection Tasks by Use of Supervised Learning Methods. IEEE Trans. Med. Imaging 2019, 38, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Gallas, B.D.; Barrett, H.H. Validating the use of channels to estimate the ideal linear observer. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2003, 20, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.H.; Denny, J.L.; Wagner, R.F.; Myers, K.J. Objective assessment of image quality. II. Fisher information, Fourier crosstalk, and figures of merit for task performance. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1995, 12, 834–852. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.K.; Catalano, M.; Pfeiffer, D.; Fingerle, A.A.; Rummeny, E.J.; Noël, P.B. CNN as model observer in a liver lesion detection task for x-ray computed tomography: A phantom study. Med. Phys. 2018, 45, 4439–4447. [Google Scholar] [CrossRef]
- Zhou, W.; Li, H.; Anastasio, M.A. Learning the Hotelling observer for SKE detection tasks by use of supervised learning methods. In Medical Imaging 2019: Image Perception, Observer Performance, and Technology Assessment; SPIE: San Diego, CA, USA, 2019; Volume 10952, pp. 41–46. [Google Scholar] [CrossRef]
- Zhou, W.; Anastasio, M.A. Learning the ideal observer for SKE detection tasks by use of convolutional neural networks (Cum Laude Poster Award). In Medical Imaging 2018: Image Perception, Observer Performance, and Technology Assessment; SPIE: San Diego, CA, USA, 2018; Volume 10577, pp. 287–292. [Google Scholar] [CrossRef]
- Ahn, E.; Kumar, A.; Fulham, M.; Feng, D.D.F.; Kim, J. Unsupervised Domain Adaptation to Classify Medical Images Using Zero-Bias Convolutional Auto-Encoders and Context-Based Feature Augmentation. IEEE Trans. Med. Imaging 2020, 39, 2385–2394. [Google Scholar] [CrossRef]
- Ahn, E.; Kumar, A.; Feng, D.; Fulham, M.; Kim, J. Unsupervised Deep Transfer Feature Learning for Medical Image Classification. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 1915–1918. [Google Scholar] [CrossRef]
- Ahn, E.; Kim, J.; Kumar, A.; Fulham, M.; Feng, D. Convolutional Sparse Kernel Network for Unsupervised Medical Image Analysis. Med. Image Anal. 2019, 56, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Rosenthal, J.; Waring, J.; Umeton, R. Applying Self-Supervised Learning to Medicine: Review of the State of the Art and Medical Implementations. Informatics 2021, 8, 59. [Google Scholar] [CrossRef]
- Kwak, M.G.; Su, Y.; Chen, K.; Weidman, D.; Wu, T.; Lure, F.; Li, J. Self-Supervised Contrastive Learning to Predict Alzheimer’s Disease Progression with 3D Amyloid-PET. medRxiv 2023. [Google Scholar] [CrossRef]
- Xing, X.; Liang, G.; Wang, C.; Jacobs, N.; Lin, A.L. Self-Supervised Learning Application on COVID-19 Chest X-ray Image Classification Using Masked AutoEncoder. Bioengineering 2023, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wan, Y. A novel efficient method for training sparse auto-encoders. In Proceedings of the 2013 6th International Congress on Image and Signal Processing (CISP), Hangzhou, China, 16–18 December 2013; Volume 2, pp. 1019–1023. [Google Scholar] [CrossRef]
- Vincent, P.; Larochelle, H.; Bengio, Y.; Manzagol, P.A. Extracting and composing robust features with denoising autoencoders. In Proceedings of the 25th International Conference on Machine Learning, ICML ’08. Association for Computing Machinery, Montreal, QC, Canada, 11–15 April 2016; pp. 1096–1103. [Google Scholar] [CrossRef]
- Vincent, P.; Larochelle, H.; Lajoie, I.; Bengio, Y.; Manzagol, P.A. Stacked Denoising Autoencoders: Learning Useful Representations in a Deep Network with a Local Denoising Criterion. J. Mach. Learn. Res. 2010, 11, 3371–3408. [Google Scholar]
- Al-Qatf, M.; Lasheng, Y.; Al-Habib, M.; Al-Sabahi, K. Deep Learning Approach Combining Sparse Autoencoder With SVM for Network Intrusion Detection. IEEE Access 2018, 6, 52843–52856. [Google Scholar] [CrossRef]
- Binbusayyis, A.; Vaiyapuri, T. Unsupervised deep learning approach for network intrusion detection combining convolutional autoencoder and one-class SVM. Appl Intell. 2021, 51, 7094–7108. [Google Scholar] [CrossRef]
- Meng, Q.; Catchpoole, D.; Skillicom, D.; Kennedy, P.J. Relational autoencoder for feature extraction. In Proceedings of the 2017 International Joint Conference on Neural Networks (IJCNN), Anchorage, AK, USA, 14–19 May 2017; pp. 364–371. [Google Scholar] [CrossRef]
- Meng, L.; Ding, S.; Xue, Y. Research on denoising sparse autoencoder. Int. J. Mach. Learn. Cyber. 2017, 8, 1719–1729. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Wu, H.; Han, W.; Li, C.; Chen, H. Joint optimization of autoencoder and Self-Supervised Classifier: Anomaly detection of strawberries using hyperspectral imaging. Comput. Electron. Agric. 2022, 198, 107007. [Google Scholar] [CrossRef]
- Riquelme, D.; Akhloufi, M.A. Deep Learning for Lung Cancer Nodules Detection and Classification in CT Scans. AI 2020, 1, 28–67. [Google Scholar] [CrossRef]
- Multipurpose Chest Phantom N1 “LUNGMAN”|KYOTO KAGAKU. Available online: https://www.kyotokagaku.com/en/products_data/ph-1_01/ (accessed on 2 August 2021).
- Pouget, E.; Dedieu, V. Comparison of supervised-learning approaches for designing a channelized observer for image quality assessment in CT. Med. Phys. 2023, 50, 4282–4295. [Google Scholar] [CrossRef]
- Brankov, J.G.; Yang, Y.; Wei, L.; El Naqa, I.; Wernick, M.N. Learning a Channelized Observer for Image Quality Assessment. IEEE Trans. Med. Imaging 2009, 28, 991–999. [Google Scholar] [CrossRef]
- Burgess, A.E.; Li, X.; Abbey, C.K. Nodule detection in two-component noise: Toward patient structure. In Medical Imaging 1997: Image Perception; SPIE: Newport Beach, CA, USA, 1997; Volume 3036, pp. 2–13. [Google Scholar] [CrossRef]
- Abbey, C.K.; Barrett, H.H. Human- and model-observer performance in ramp-spectrum noise: Effects of regularization and object variability. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2001, 18, 473–488. [Google Scholar] [CrossRef]
- Mathieu, K.B.; Ai, H.; Fox, P.S.; Godoy, M.C.B.; Munden, R.F.; de Groot, P.M.; Pan, T. Radiation dose reduction for CT lung cancer screening using ASIR and MBIR: A phantom study. J. Appl. Clin. Med. Phys. 2014, 15, 4515. [Google Scholar] [CrossRef]
- Park, S.; Witten, J.M.; Myers, K.J. Singular Vectors of a Linear Imaging System as Efficient Channels for the Bayesian Ideal Observer. IEEE Trans. Med. Imaging 2009, 28, 657–668. [Google Scholar] [CrossRef]
- Kupinski, M.A.; Edwards, D.C.; Giger, M.L.; Metz, C.E. Ideal observer approximation using Bayesian classification neural networks. IEEE Trans. Med. Imaging 2001, 20, 886–899. [Google Scholar] [CrossRef]
- Massanes, F.; Brankov, J.G. Evaluation of CNN as anthropomorphic model observer. Med. Imaging 2017, 10136, 101360Q. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. arXiv 2017. [Google Scholar] [CrossRef]
- Abadi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Devin, M.; Ghemawat, S.; Irving, G.; Isard, M.; et al. {TensorFlow}: A System for {Large-Scale} Machine Learning. In Proceedings of the 12th USENIX conference on Operating Systems Design and Implementation, Savannah, GA, USA, 2–4 November 2016; pp. 265–283. Available online: https://www.usenix.org/conference/osdi16/technical-sessions/presentation/abadi (accessed on 21 December 2023).
- Nishio, M.; Nagashima, C.; Hirabayashi, S.; Ohnishi, A.; Sasaki, K.; Sagawa, T.; Hamada, M.; Yamashita, T. Convolutional auto-encoder for image denoising of ultra-low-dose CT. Heliyon 2017, 3, e00393. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, C.; Alonso-Betanzos, A. An Unsupervised Deep Feature Learning Model Based on Parallel Convolutional Autoencoder for Intelligent Fault Diagnosis of Main Reducer. Intell. Neurosci. 2021, 2021, 8922656. [Google Scholar] [CrossRef] [PubMed]
- Laref, R.; Losson, E.; Sava, A.; Siadat, M. On the optimization of the support vector machine regression hyperparameters setting for gas sensors array applications. Chemom. Intell. Lab. Syst. 2019, 184, 22–27. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.Y.; Zhang, H.; Xiong, L.D.; Lei, H.; Deng, S.H. Hyperparameter optimization for machine learning models based on Bayesian optimization. J. Electron. Sci. Technol. 2019, 17, 26–40. [Google Scholar] [CrossRef]
- Nair, A. Grid Search VS Random Search VS Bayesian Optimization. Medium. Published 2 May 2022. Available online: https://towardsdatascience.com/grid-search-vs-random-search-vs-bayesian-optimization-2e68f57c3c46 (accessed on 21 June 2022).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Favazza, C.P.; Duan, X.; Zhang, Y.; Yu, L.; Leng, S.; Kofler, J.M.; Bruesewitz, M.R.; McCollough, C.H. A cross-platform survey of CT image quality and dose from routine abdomen protocols and a method to systematically standardize image quality. Phys. Med. Biol. 2015, 60, 8381–8397. [Google Scholar] [CrossRef]
- Chan, K.H.; Im, S.K.; Ke, W. A Multiple Classifier Approach for Concatenate-Designed Neural Networks. Neural Comput Applic. 2022, 34, 1359–1372. [Google Scholar] [CrossRef]




| CDAE | PCA | ||||
|---|---|---|---|---|---|
| λ = 0.00001 | λ = 0.0001 | λ = 0.001 | λ = 0.01 | ||
| C(SKE/BKS, SKS/BKS) | 104, 103 | 103, 102 | 101, 102 | 107, 106 | 103, 102 |
| σ(SKE/BKS, SKS/BKS) | 100, 100 | 10, 10 | 1, 1 | 0.01, 0.1 | 0.1, 0.1 |
| AUC (SKE/BKS, SKS/BKS) | 0.76, 0.74 | 0.77, 0.70 | 0.88, 0.91 | 0.63, 0.65 | 0.78, 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouget, E.; Dedieu, V. Applying Self-Supervised Learning to Image Quality Assessment in Chest CT Imaging. Bioengineering 2024, 11, 335. https://doi.org/10.3390/bioengineering11040335
Pouget E, Dedieu V. Applying Self-Supervised Learning to Image Quality Assessment in Chest CT Imaging. Bioengineering. 2024; 11(4):335. https://doi.org/10.3390/bioengineering11040335
Chicago/Turabian StylePouget, Eléonore, and Véronique Dedieu. 2024. "Applying Self-Supervised Learning to Image Quality Assessment in Chest CT Imaging" Bioengineering 11, no. 4: 335. https://doi.org/10.3390/bioengineering11040335