Primary Stability of Implants Inserted into Polyurethane Blocks: Micro-CT and Analysis In Vitro
Abstract
:1. Introduction
2. Material and Methods
- -
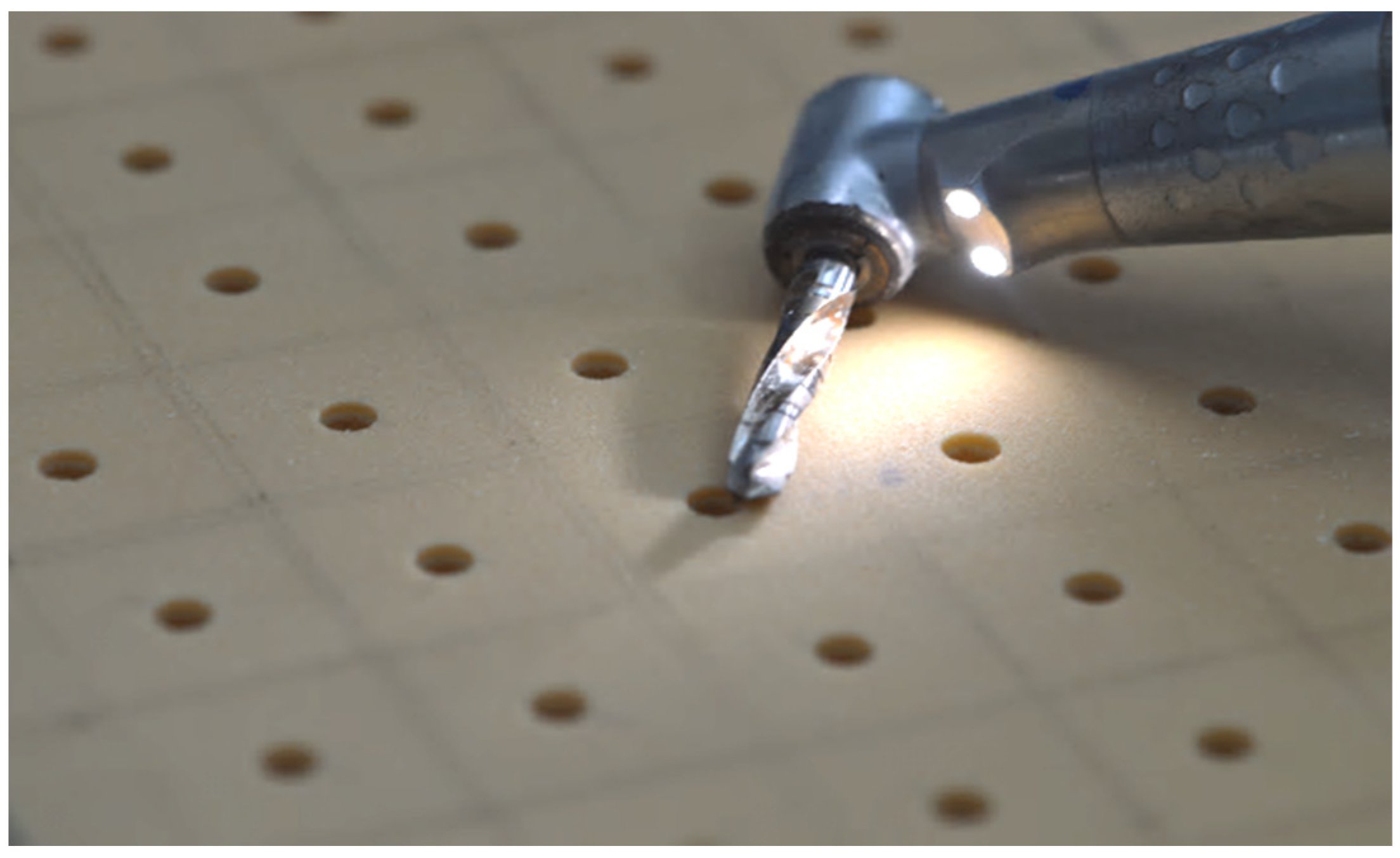
- TD: The drill preparation of the implant was performed with Winsix, Biosafin company (Ancona, Italy). The sequence began with a 2.0-mm-diameter precision pilot drill, followed by conical drills of progressively larger diameters (2.6 mm and 3.0 mm), moreover; the final cutter used for all of the blocks had a diameter of 3.4 mm. The sequence of drills was performed using an implant motor set to a speed of 800 revolutions per minute (rpm) and with the assistance of external cooling. The implants were mechanically screwed at the standard rate of 35 rpm.
- -
- PES: The piezoelectric preparation of the implant site was performed with the S.U.S. (surgery ultrasonic site, Esacrom, Imola, Italy). All S.U.S. have a uniform octagonal star section but vary in diameters and tapers. The sequence comprised 4 successive steps. It began with the insertion of a first guide (ES052XGT), followed by a series of conical inserts with gradually larger diameters of 2.8 (ES02.8T), 3.2 (ES03.2T), and 3.6 mm (ES03.6T), and the frequency of 22-35 kHz. According to the manufacturer’s recommendations, the inserts were used with abundant irrigation, and the operator performed a combination of vertical inward and outward motions together with rotational movements.
- -
- MM: Due to the geometry of the tips, which are conical at the end and then parallel with a diamond-like carbon (DLC) coating, they are able to penetrate bone much more easily than conventional osteotomes [31]. DLC is an innovative carbon-based coating that reduces abrasion, slippage, and chemical-aggression-related issues. This material is distinguished by its high hardness, resistance to wear and attrition, low coefficient of friction, resistance to scratching, and biocompatibility. The inserts had the sequences of 2.26 (BLK-R1), 2.60 (BLK-R2), 3.10 (BLK-R3), and 3.6 mm (BLK-R4).
2.1. Osstell® IDx Analysis
2.2. X-ray Microcomputed Tomography Analysis
3. Results
- -
- Screw to site preparation occupation ratio: Table 2 demonstrates the occupation rate for all six protocols. Evidently, there were notable variations between the 30 PCF and the 15 PCF bone density measurements, with 30 PCF exhibiting superior average outcomes.
- -
- Screw site preparation micro-gap: The results demonstrated that there were no significant differences in the maximum distance between the 3D-printed screw and the wall of the preparation site when comparing the same preparation techniques in different bone densities, specifically 15 and 30 PCF. Nevertheless, notable disparities emerged when comparing the outcomes of the various approaches. This suggests that the technique employed for the preparation has an important impact on the micro-gap and, consequently, the primary stability of dental implants. The preparations undertook with a bone density of 15 PCF had marginally greater mean values compared to those of 30 PCF. The TD technique demonstrated the smallest micro-gap compared to the other two procedures. When comparing the PES and MM, there were moderate variations observed between these two distinct bone preparation techniques, wherein the PES produced higher outcomes, regardless of whether the bone density was 15 or 30 PCF.
- -
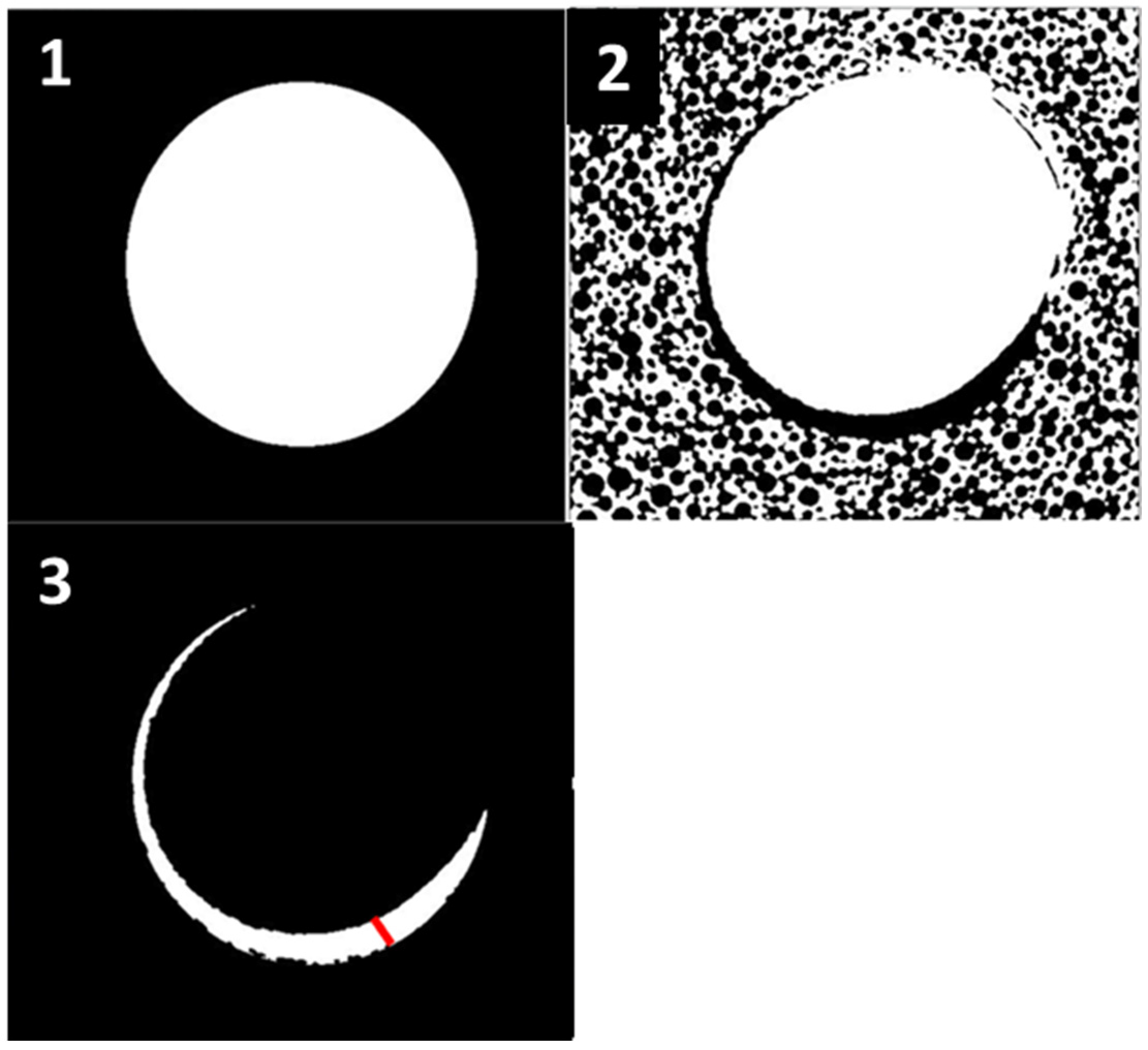
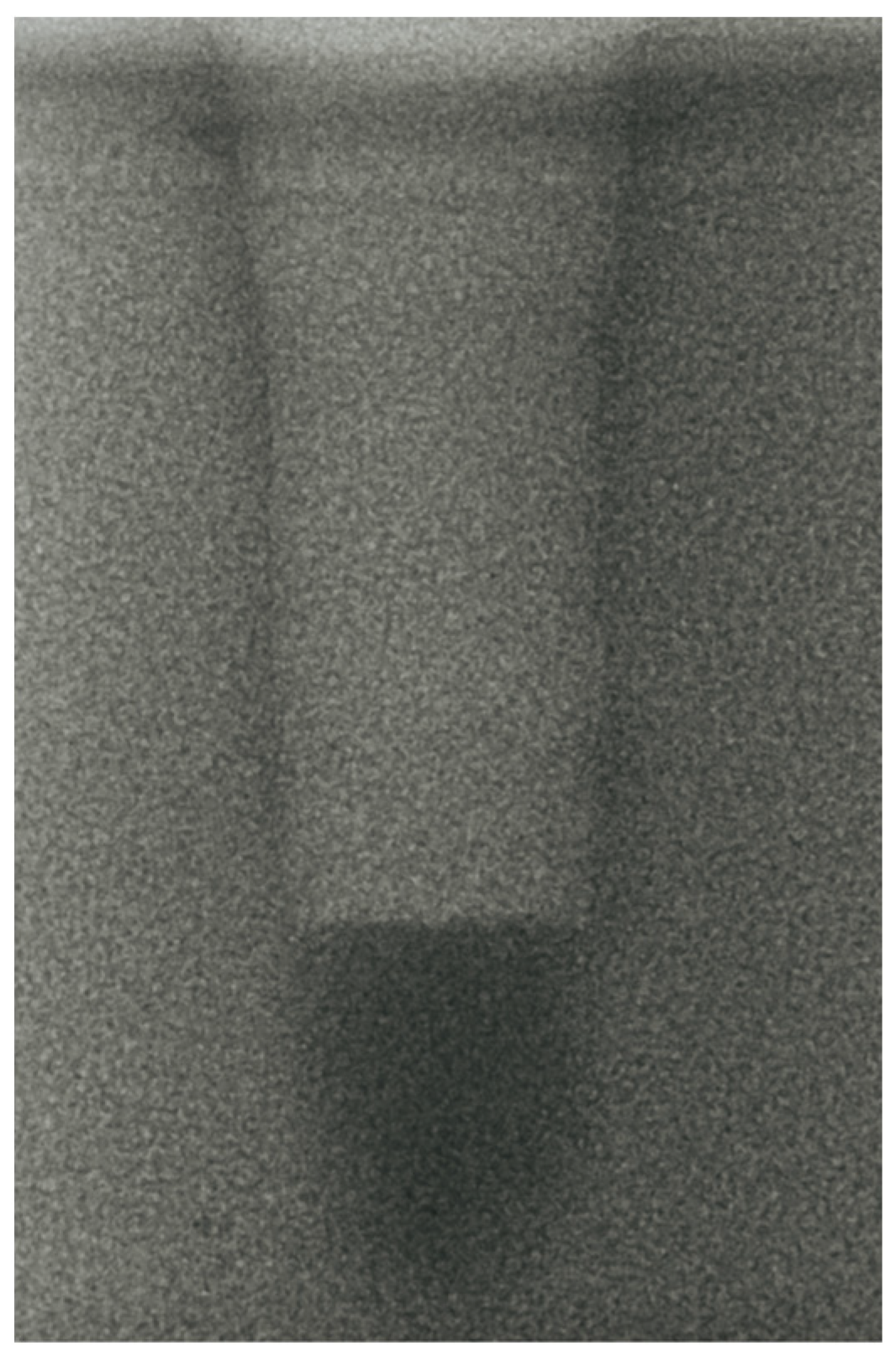
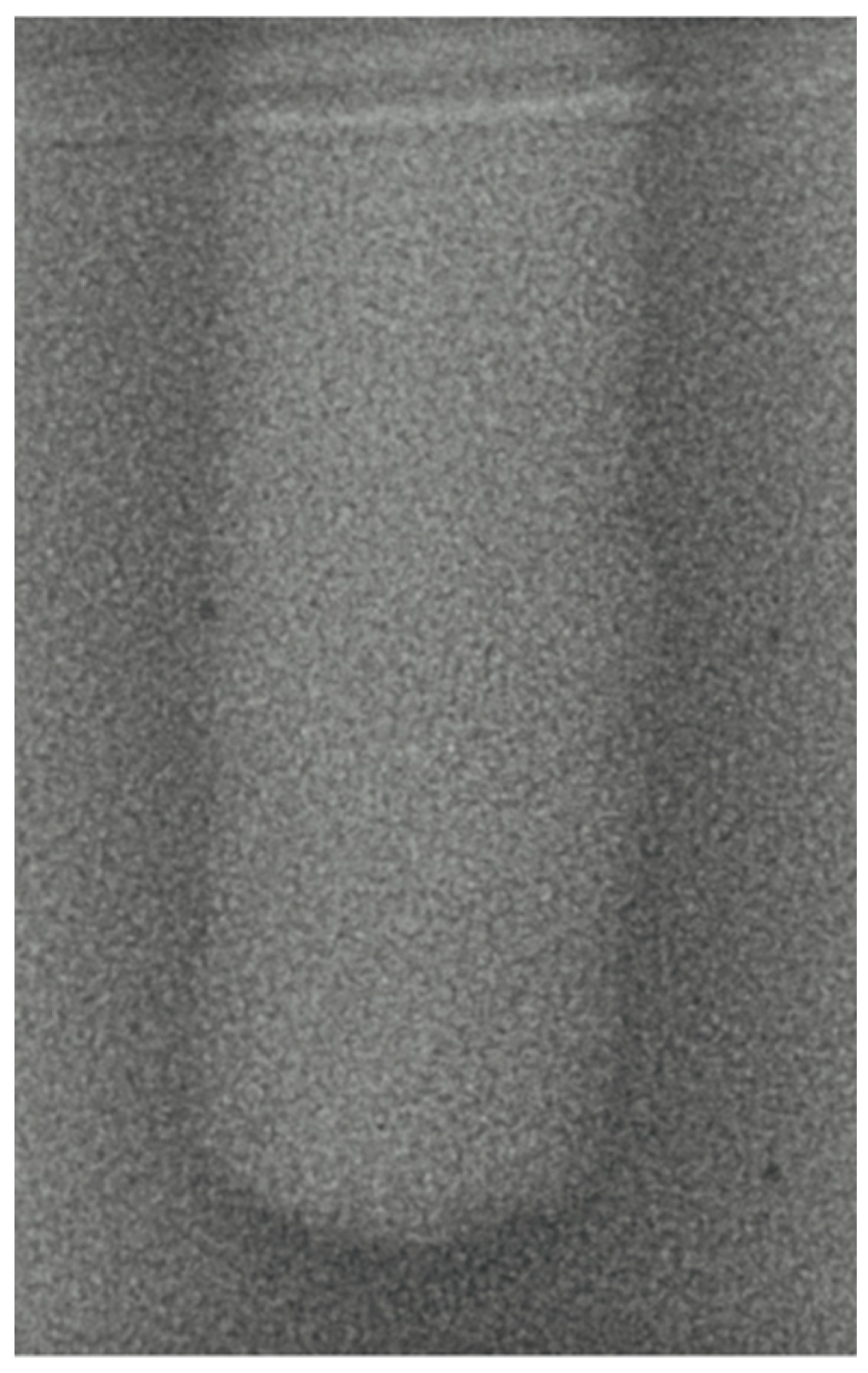
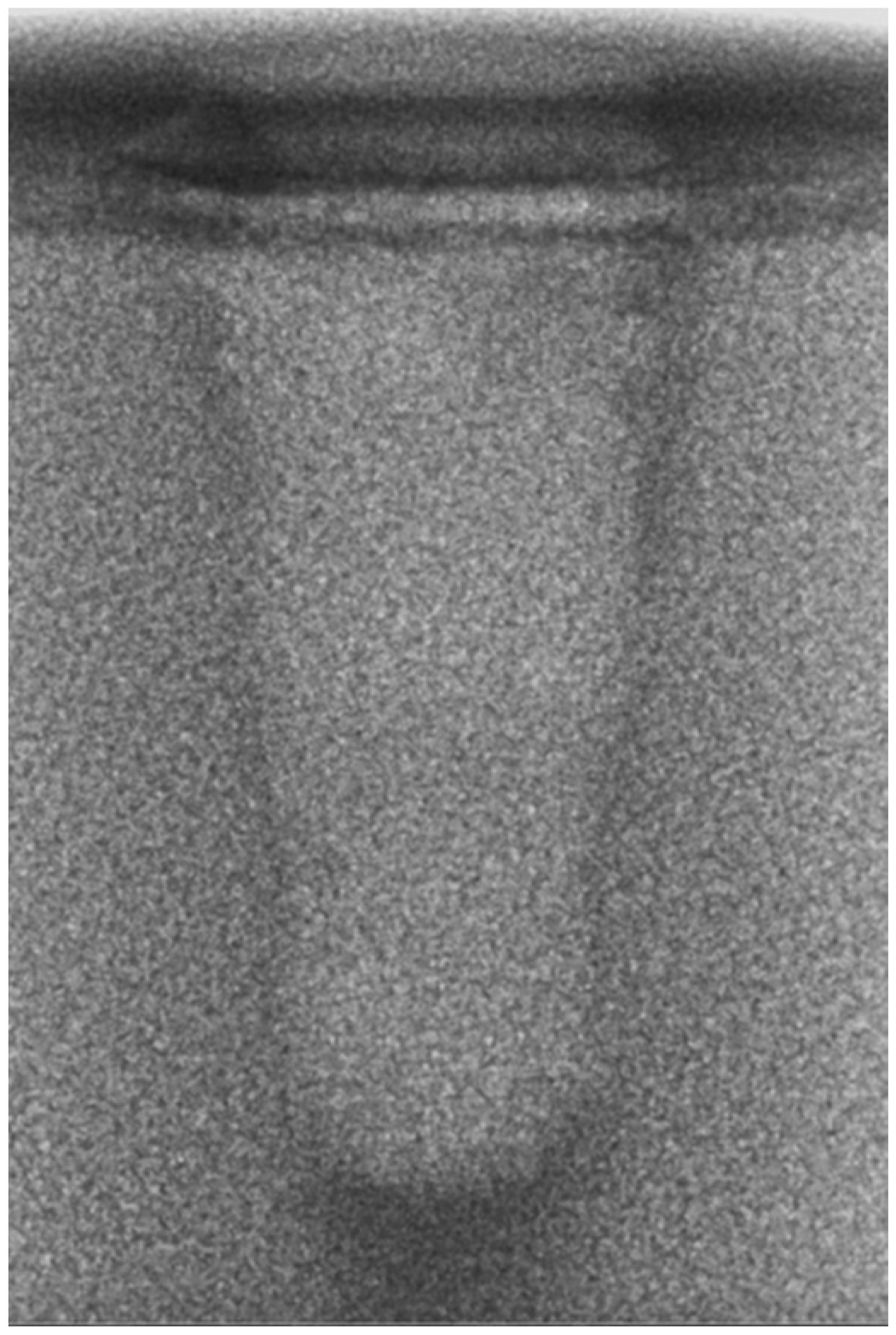
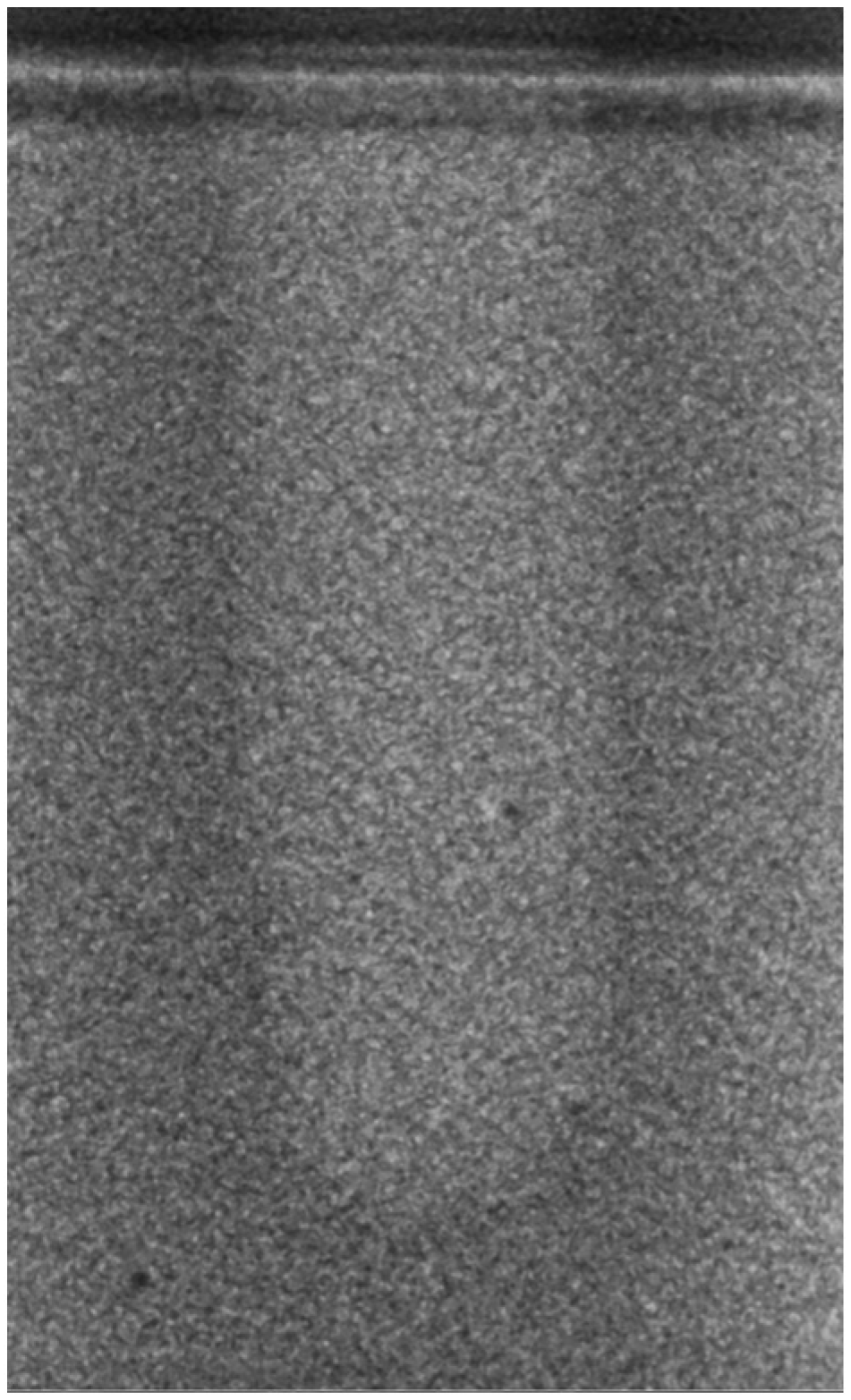
- Vertical effect of magnetic mallet: The forces produced by the MM are transmitted to the tip of the osteotome in order to achieve the plastic deformation of the bone. These forces can affect the bone in three dimensions: horizontally, vertically, and sagittally. However, the vertical effect of the MM goes beyond the area that is in direct contact with the instrument’s tip. By employing a Micro-CT scan, we accurately measured the extent of condensed bone located apically to the preparation site. The results indicate a direct correlation between the size of the condensed area and the density of the bone. Figure 3 shows an implant prepared site employing the MM technique in a bone density of 30 PCF. The length of our preparation measured 11 mm, while the condensed bone resulting from osteotomy extended 3.7 ± 0.14 mm apically to the implant site preparation. By comparing the MM with the other preparation techniques in the same bone density, we observed that, in the sites prepared using the PES approach, the vertical effect of the preparation was noticeably less than that of the MM. It extended apically to the implant site preparation at 0.36 ± 0.08 mm, as shown in Figure 4. When employing the TD technique, we noticed that the vertical effect of this preparation method was minimal compared to the MM and PES, measuring 0.15 ± 0.04 mm, as shown in Figure 5.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takanashi, Y.; Penrod, J.R.; Lund, J.P.; Feine, J.S. A cost comparison of mandibular two-implant overdenture and conventional denture treatment. Int. J. Prosthodont. 2004, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.J.; Lee, K.W.; Han, D.H. The effects of local factors on the survival rate of dental implants: A 19-year retrospective study. J. Korean Acad. Prosthodont. 2010, 48, 28–40. [Google Scholar] [CrossRef]
- Eriksson, A.; Albrektsson, T.; Grane, B.; McQueen, D. Thermal injury to bone: A vital microscopic description of heat effects. Int. J. Oral Surg. 1982, 11, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.; Kaiser, A.; Prochnow, N.; Schmitz, I.; Hoffmann, E.; Haurer, P. Heat Production during different Ultrasonic and conventional osteotomy preparation for dental Implants. Clin. Oral Implant. Res. 2011, 22, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Vercellotti, T.; Paoli, S.D.; Nevins, M. The Piezoelectric Bony Window Osteotomy and Sinus Membrane Elevation: Introduction of a New Technique for Simplification of the Sinus Augmentation Procedure. Int. J. Perio. Rest. Dent. 2001, 21, 561–567. [Google Scholar]
- Labanca, M.; Azzola, F.; Vinci, R.; Rodella, L.F. Piezoelectric surgery: Twenty years of use. Br. J. Oral Maxillofac. Surg. 2008, 46, 265–269. [Google Scholar] [CrossRef]
- Visale, K.; Manimala, V.; Vidhyasankari, N.; Shanmugapriya, S.V. Magnetic mallets—A stroke of luck in implantology: A review. J. Acad. Dent. Educ. 2021, 7, 6–9. [Google Scholar] [CrossRef]
- Gencer, R.C.; Ozel, A.; Uckan, S.I. Bilateral sagittal split ramus osteotomy using a conventional osteotome-hammer and a magnetic mallet device: An in vitro comparison. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 58–65. [Google Scholar]
- Branemark, P.; Hansson, B.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw—Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. 1977, 16, 1–132. [Google Scholar]
- Manz, M.C.; Morris, H.F.; Ochi, S. An evaluation of the Periotest system. Part I: Examiner reliability and repeatability of readings. Implant Dent. 1992, 1, 142–146. [Google Scholar] [CrossRef]
- Manz, M.C.; Morris, H.F.; Ochi, S. An evaluation of the Periotest system. Part II: Reliability and repeatability of instruments. Implant Dent. 1992, 1, 221–226. [Google Scholar] [CrossRef]
- Wijaya, S.K.; Sumikawa, T.; Saratani, K.; Kawazoe, T.; Oka, H. Development of implant movement checker for determining dental implant stability. Med. Eng. Phys. 2004, 26, 513–522. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Souza, J.P.V.; Neto, C.L.M.M.; Piacenza, L.T. Relation between Insertion Torque and Implant Stability Quotient: A Clinical Study. Eur. J. Dent. 2021, 15, 618–623. [Google Scholar]
- Sarfaraz, H.; Shefali, J.; Sucheta, P.; Rao, S. Study to assess the relationship between insertion torque value and implant stability quotient and its influence on timing of functional implant loading. J. Indian Prosthodont. Soc. 2018, 18, 139–146. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Santos-Garcia, R.; Jaramillo-Santos, R. Assessment of Osstell ISQ’s reliability for implant stability measurement: A cross-sectional clinical study. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, 877–882. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Ting, C.C.; Du, J.K.; Chen, C.M.; Wu, J.H.; Chen, H.S. Insertion torque, resonance frequency, and removal torque analysis of microimplants. Kaohsiung J. Med. Sci. 2016, 32, 469–474. [Google Scholar] [CrossRef]
- Park, I.P.; Kim, S.K.; Lee, S.J. The relationship between initial implant stability quotient values and bone-to-implant contact ratio in the rabbit tibia. J. Adv. Prosthodont. 2011, 3, 76–80. [Google Scholar] [CrossRef]
- Heinemann, F.; Hasan, I.; Bourauel, C.; Biffar, R.; Mundt, T. Bone stability around dental implants: Treatment related factors. Ann. Anat. 2015, 199, 3–8. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Albertini, M.; Rios-Santos, J.V.; Lázaro-Calvo, P.; Fernández-Palacín, A.; Bullon, P. Resonance frequency analysis-reliability in third generation instruments: Osstell mentor®. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, 801–806. [Google Scholar] [CrossRef]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography). 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef]
- Waarsing, J.; Day, J.; van der Linden, J.; Ederveen, A.; Spanjers, C.; De Clerck, N.; Sasov, A.; Verhaar, J.A.; Weinans, H. Detecting and tracking local changes in the tibiae of individual rats: A novel method to analyse longitudinal in vivo micro-CT data. Bone 2004, 34, 163–169. [Google Scholar] [CrossRef]
- Schulze, R.K.; Berndt, D.; D’hoedt, B. On cone-beam computed tomography artifacts induced by titanium implants. Clin. Oral Implants Res. 2010, 21, 100–107. [Google Scholar] [CrossRef]
- Remeysen, K.; Swennen, R. Beam hardening artifact reduction in microfocus computed tomography for improved quantitative coal characterization. Int. J. Coal Geol. 2006, 67, 101–111. [Google Scholar] [CrossRef]
- Lombardo, G.; Pighi, J.; Marincola, M.; Corrocher, G.; Simancas-Pallares, M.; Nocini, P.F. Cumulative Success Rate of Short and Ultrashort Implants Supporting Single Crowns in the Posterior Maxilla: A 3-Year Retrospective Study. Int. J. Dent. 2017, 2017, 8434281. [Google Scholar] [CrossRef]
- Beverte, I.; Cabulis, U.; Andersons, J.; Kirpluks, M.; Skruls, V.; Cabulis, P. Characteristics of Components and Density of Rigid Nanoclay-Filled Medium-Density Polyurethane Foams Produced in a Sealed Mould. Polymers 2023, 15, 3228. [Google Scholar] [CrossRef]
- ASTM F1839-08; Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. ASTM Internalional: West Conshohocken, PA, USA, 2012.
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The elastic moduli of human subchondral, trabecular and cortical bone tissue and the size dependency of cortical bone modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef]
- Misch, C.E. Bone Density: A Key Determinant for Treatment Planning. In Contemporary Implant Dentistry, 3rd ed.; Mosby: St Louis, MO, USA, 2007; pp. 130–146. [Google Scholar]
- Crespi, R.; Cappare, P.; Gherlone, E. A Comparison of Manual and Electrical Mallet in Maxillary Bone Condensing for Immediately Loaded Implants: A Randomized Study. Clin. Implant Dent. Relat. Res. 2014, 16, 374–382. [Google Scholar] [CrossRef]
- Turkyilmaz, I. Implant Dentistry—A Rapidly Evolving Practice; Intech: Rijeka, Croatia, 2011; pp. 111–126. [Google Scholar]
- Cheng, S.; Timonen, J.; Suominen, H. Elastic wave propagation in bone in vivo: Methodology. J. Biomech. 1995, 28, 471–478. [Google Scholar] [CrossRef]
- Rues, S.; Schmitter, M.; Kappel, S.; Sonntag, R.; Kretzer, J.P.; Nadorf, J. Effect of bone quality and quantity on the primary stability of dental implants in a simulated bicortical placement. Clin. Oral Investig. 2021, 25, 1769–1775. [Google Scholar] [CrossRef]
- Rozé, J.; Babu, S.; Saffarzadeh, A.; Gayet-Delacroix, M.; Hoornaert, A.; Layrolle, P. Correlating implant stability to bone structure. Clin. Oral Implants Res. 2009, 20, 1140–1145. [Google Scholar] [CrossRef]
- Vianna dos Santos, M.; Elias, C.N.; Cavalcanti Lima, J.H. The effects of superficial roughness and design on the primary stability of dental implants. Clin. Implant Dent. Relat. Res. 2011, 13, 215–223. [Google Scholar] [CrossRef]
- Olmedo-Gaya, M.V.; Romero-Olid, M.N.; Ocaña-Peinado, F.M.; Vallecillo-Rivas, M.; Vallecillo, C.; Reyes-Botella, C. Influence of different surgical techniques on primary implant stability in the posterior maxilla: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 3499–3508. [Google Scholar] [CrossRef]
- Yu, X.; Chang, C.; Guo, W.; Wu, Y.; Zhou, W.; Yu, D. Primary implant stability based on alternative site preparation techniques: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 580–590. [Google Scholar] [CrossRef]
- Schierano, G.; Baldi, D.; Peirone, B.; Mauthe von Degerfeld, M.; Navone, R.; Bragoni, A.; Colombo, J.; Autelli, R.; Muzio, G. Biomolecular, Histological, Clinical, and Radiological Analyses of Dental Implant Bone Sites Prepared Using Magnetic Mallet Technology: A Pilot Study in Animals. Materials 2021, 14, 6945. [Google Scholar] [CrossRef]
- Antonelli, A.; Barone, S.; Attanasio, F.; Salviati, M.; Cerra, M.G.; Calabria, E.; Bennardo, F.; Giudice, A. Effect of Implant Macro-Design and Magnetodynamic Surgical Preparation on Primary Implant Stability: An In Vitro Investigation. Dent. J. 2023, 11, 227. [Google Scholar] [CrossRef]
- Caccianiga, G.; Ferri, L.; Baldoni, M.; Alla, B.A.; Caccianiga, P. Magnetic Mallet and Laser for a Minimally Invasive Implantology: A Full Arch Case Report. Appl. Sci. 2022, 12, 1995. [Google Scholar] [CrossRef]
- Arakji, H.; Osman, E.; Aboelsaad, N.; Shokry, M. Evaluation of implant site preparation with piezosurgery versus conventional drills in terms of operation time, implant stability and bone density: A randomized controlled clinical trial-split mouth design. BMC Oral Health 2022, 22, 567. [Google Scholar] [CrossRef]
- Bennardo, F.; Barone, S.; Vocaturo, C. Usefulness of Magnetic Mallet in Oral Surgery and Implantology: A Systematic Review. J. Pers. Med. 2022, 12, 108. [Google Scholar] [CrossRef]
- Crespi, R.; Bruschi, G.B.; Gastaldi, G.; Capparé, P.; Gherlone, E.F. Immediate Loaded Implants in Split-Crest Procedure. Clin. Implant. Dent. Relat. Res. 2015, 17, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Cehreli, M.; Kökat, C.; Comert, A.M.; Akkocaoğlu, A.; Tekdemir, M.; Akça, I. Implant stability and bone density: Assessment of correlation in fresh cadavers using conventional and osteotome implant sockets. Clin. Oral Implants Res. 2009, 20, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Juodzbalys, G.; Wang, H.L.; Sabalys, G. Injury of the Inferior Alveolar Nerve during Implant Placement: A Literature Review. J. Oral Maxillofac. Res. 2011, 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Sennerby, L.; Meredith, N.; Lekholm, U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. Int. J. Oral Maxillofac. Surg. 1999, 28, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Büchter, A.; Kleinheinz, J.; Wiesmann, H.P.; Kersken, J.; Nienkemper, M.; Weyhrother, H.; Joos, U.; Meyer, U. Biological and biomechanical evaluation of bone remodelling and implant stability after using an osteotome technique. Clin. Oral Implants Res. 2005, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, H.N.; Choi, Y.C.; Heo, S.J.; Lee, C.W.; Choie, M.K. Effects of anodized oxidation or turned implants on bone healing after using conventional drilling or trabecular compaction technique: Histomorphometric analysis and RFA. Clin. Oral Implants Res. 2006, 17, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.H.; Hong, A.; Yang, L.Y.; Chang, W.Y.; Huang, Y.F.; Lin, Y.T. Implant stability quotients of osteotome bone expansion and conventional drilling technique for 4.1 mm diameter implant at posterior mandible. Clin. Implant Dent. Relat. Res. 2017, 19, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Khandelwal, N.; Petrov, S.; Drew, H.J.; Mupparapu, M. Resonance frequency analysis (RFA) and insertional torque (IT) stability comparisons of implants placed using osteotomes versus drilling techniques: A preliminary case study. Quintessence Int. 2015, 46, 789–798. [Google Scholar] [PubMed]
- Renapurkar, S.; Nagamalla, S. Piezosurgery in Oral and Maxillofacial Surgery. In Oral and Maxillofacial Surgery for the Clinician; Springer: Singapore, 2021. [Google Scholar]
- Stacchi, C.; Bassi, F.; Troiano, G.; Rapani, A.; Lombardi, T.; Jokstad, A.; Sennerby, L.; Schierano, G. Piezoelectric bone surgery for implant site preparation compared with conventional drilling techniques: A systematic review, meta-analysis and trial sequential analysis. Int. J. Oral Implantol. 2020, 13, 141–158. [Google Scholar]
- Baker, J.A.; Vora, S.; Bairam, L.; Kim, H.I.; Davis, E.L.; Andreana, S. Piezoelectric vs. conventional implant site preparation: Ex vivo implant primary stability. Clin. Oral Implants Res. 2012, 23, 433–437. [Google Scholar] [CrossRef]
- Blus, C.; Szmukler-Moncler, S. Atraumatic tooth extraction and immediate implant placement with Piezosurgery: Evaluation of 40 sites after at least 1 year of loading. Int. J. Periodontics Restor. Dent. 2010, 30, 355–363. [Google Scholar]
- Delgado-Ruiz, R.A.; Sacks, D.; Palermo, A. Temperature and time variations during osteotomies performed with different piezosurgical devices: An in vitro study. Clin. Oral Implants Res. 2016, 27, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Preti, G.; Martinasso, G.; Peirone, B.; Navone, R.; Manzella, C.; Muzio, G.; Russo, C.; Canuto, R.A.; Schierano, G. Cytokines and growth factors involved in the osseointegration of oral titanium implants positioned using piezoelectric bone surgery versus a drill technique: A pilot study in minipigs. J. Periodontol. 2007, 78, 716–722. [Google Scholar] [CrossRef] [PubMed]
- da Silva Neto, U.; Joly, J.C.; Gehrke, S.A. Clinical analysis of the stability of dental implants after preparation of the site by conventional drilling or Piezosurgery. Br. J. Oral Maxillofac. Surg. 2014, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]


| ISQ measurement | Bone Density | 15 PCF (Mean ± SD) | 30 PCF (Mean ± SD) | ||||
| Groups | TD | PES | MM | TD | PES | MM | |
| Outcomes | 60.6 ± 1.81 | 72.3 ± 1.63 | 62.4 ± 1.77 | 65.8 ± 1.5 | 75.6 ± 1.73 | 69.8 ± 1.91 | |
| Micro-CT measurement | Bone Density | 15 PCF (Mean ± SD) | 30 PCF (Mean ± SD) | ||||
| Groups | TD | PES | MM | TD | PES | MM | |
| Occupation ratio (Percent) | 90 ± 1.31 | 89.6 ± 1.22 | 88.4± 1.17 | 94 ± 1.88 | 96 ± 1.75 | 90.3 ± 2.11 | |
| Maximum Micro-gap (μm) | 238 ± 17 | 318 ± 21 | 301 ± 20 | 221 ± 16 | 299 ± 20 | 281 ± 19 | |
| Vertical Effect (mm) | 0.12 ± 0.03 | 0.27 ± 0.06 | 1.4 ± 0.11 | 0.15 ± 0.04 | 0.36 ± 0.08 | 3.7 ± 0.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dura Haddad, C.; Andreatti, L.; Zelezetsky, I.; Porrelli, D.; Turco, G.; Bevilacqua, L.; Maglione, M. Primary Stability of Implants Inserted into Polyurethane Blocks: Micro-CT and Analysis In Vitro. Bioengineering 2024, 11, 383. https://doi.org/10.3390/bioengineering11040383
Dura Haddad C, Andreatti L, Zelezetsky I, Porrelli D, Turco G, Bevilacqua L, Maglione M. Primary Stability of Implants Inserted into Polyurethane Blocks: Micro-CT and Analysis In Vitro. Bioengineering. 2024; 11(4):383. https://doi.org/10.3390/bioengineering11040383
Chicago/Turabian StyleDura Haddad, Chadi, Ludovica Andreatti, Igor Zelezetsky, Davide Porrelli, Gianluca Turco, Lorenzo Bevilacqua, and Michele Maglione. 2024. "Primary Stability of Implants Inserted into Polyurethane Blocks: Micro-CT and Analysis In Vitro" Bioengineering 11, no. 4: 383. https://doi.org/10.3390/bioengineering11040383









