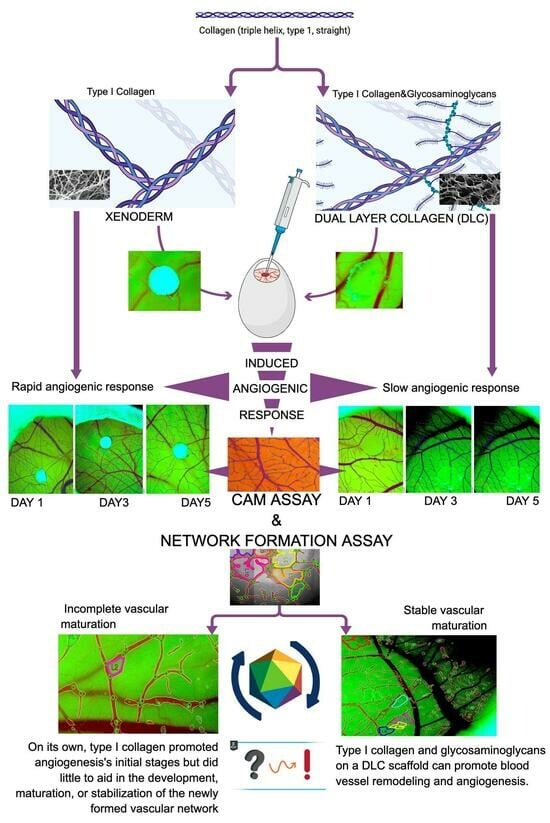
Glycosaminoglycans Modulate the Angiogenic Ability of Type I Collagen-Based Scaffolds by Acting on Vascular Network Remodeling and Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Selection and Material Grafting into the Chick Embryo CAM
2.2. IKOSA CAM Assay and Network Formation Assay
3. Results
3.1. CAM Assay
3.1.1. Vessel Total Area/ROI
3.1.2. Vessels_Num_Branching_Points/ROI
3.1.3. Vessels_Number_Branching_Points/Vessels_Total_Area
3.1.4. Total_Tube_Length [Px]/Covered_Area
3.1.5. Number_Tubes/Total_Tube_Length [Px]
3.1.6. Vascular_Mean_Thickness
3.2. Vascular Loops Analysis by Network Formation Assay (NFA)
3.3. Comparative Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. OnLine 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffold Design and Fabrication Technologies for Engineering Tissues--State of the Art and Future Perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical Applications of Collagen and Collagen-Based Materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent Progress of Collagen, Chitosan, Alginate and Other Hydrogels in Skin Repair and Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, D.; Van Gulik, T.M. Evolution of Fibrinogen-coated Collagen Patch for Use as a Topical Hemostatic Agent. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85B, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.N.; Mousavinasab, S.; Rahmanpour, H.; Fallahnezhad, M. A Biological Dressing versus “conventional” Treatment in Patients with Massive Burns: A Clinical Trial. Ulus. Travma Ve Acil Cerrahi Derg. Turk. J. Trauma Emerg. Surg. TJTES 2009, 15, 135–140. [Google Scholar]
- Matouskova, E.; Mestak, O. The Effect of Different Biologic and Biosynthetic Wound Covers on Keratinocyte Growth, Stratification and Differentiation in Vitro. J. Tissue Eng. 2014, 5, 2041731414554966. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, L.; Wang, Y.; Li, M.; Xu, S.; Zhang, C. A Collagen-Based Bi-Layered Composite Dressing for Accelerated Wound Healing. J. Tissue Viability 2022, 31, 180–189. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Extracellular Matrix-Based Scaffolding Technologies for Periodontal and Peri-Implant Soft Tissue Regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Peng, Y.Y.; Glattauer, V.; Werkmeister, J.A. Collagens as Biomaterials. J. Mater. Sci. Mater. Med. 2009, 20, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Vijayalekha, A.; Anandasadagopan, S.K.; Pandurangan, A. An Overview of Collagen-Based Composite Scaffold for Bone Tissue Engineering. Appl. Biochem. Biotechnol. 2023, 195, 4617–4636. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The Effect of Dehydrothermal Treatment on the Mechanical and Structural Properties of collagen-GAG Scaffolds. J. Biomed. Mater. Res. A 2009, 89A, 363–369. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F. Influence of Freezing Rate on Pore Structure in Freeze-Dried Collagen-GAG Scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Ellermann, E.; Meyer, N.; Cameron, R.E.; Best, S.M. In Vitro Angiogenesis in Response to Biomaterial Properties for Bone Tissue Engineering: A Review of the State of the Art. Regen. Biomater. 2023, 10, rbad027. [Google Scholar] [CrossRef]
- Minor, A.J.; Coulombe, K.L.K. Engineering a Collagen Matrix for Cell-Instructive Regenerative Angiogenesis. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Two New Applications in the Study of Angiogenesis the CAM Assay: Acellular Scaffolds and Organoids. Microvasc. Res. 2021, 140, 104304. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Tamma, R. The Use of the Chick Embryo CAM Assay in the Study of Angiogenic Activiy of Biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Ribatti, D. The CAM Assay as an Alternative In Vivo Model for Drug Testing. In Organotypic Models in Drug Development; Schäfer-Korting, M., Stuchi Maria-Engler, S., Landsiedel, R., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2020; Volume 265, pp. 303–323. ISBN 978-3-030-70062-1. [Google Scholar]
- Muthukumar, T.; Sreekumar, G.; Sastry, T.P.; Chamundeeswari, M. Collagen as a Potential Biomaterial in Biomedical Applications. Rev. Adv. Mater. Sci. 2018, 53, 29–39. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Binti Haji Idrus, R. Collagen Type I: A Versatile Biomaterial. Adv. Exp. Med. Biol. 2018, 1077, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-Based Formulations for Wound Healing: A Literature Review. Life Sci. 2022, 290, 120096. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.M.; Llopis-Hernández, V.; Salmerón-Sánchez, M.; Altankov, G. Dynamic Reorganization and Enzymatic Remodeling of Type IV Collagen at Cell–Biomaterial Interface. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 105, pp. 81–104. ISBN 978-0-12-804825-2. [Google Scholar]
- Hosseini, S.N.; Mousavinasab, S.N.; Fallahnezhat, M. Xenoderm Dressing in the Treatment of Second Degree Burns. Burns 2007, 33, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, J.M.; Costantino, P.D.; Wolpoe, M.E.; Bederson, J.B.; Griffey, E.S.; Zhang, W.X. Use of an Acellular Dermal Allograft for Dural Replacement: An Experimental Study. Neurosurgery 1999, 45, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of Collagen and Mesenchymal Stem Cells in Regenerative Dentistry. Curr. Stem Cell Res. Ther. 2022, 17, 606–620. [Google Scholar] [CrossRef]
- Zegarra-Caceres, L.; Orellano-Merluzzi, A.; Muniz, F.W.M.G.; de Souza, S.L.S.; Faveri, M.; Meza-Mauricio, J. Xenogeneic Collagen Matrix vs. Connective Tissue Graft for the Treatment of Multiple Gingival Recession: A Systematic Review and Meta-Analysis. Odontology 2024, 112, 317–340. [Google Scholar] [CrossRef]
- Nocini, R.; Abdulraheem, M.; Galzignato, P.-F.; Manzini, J.; Bernardi, P.; Conti, G.; Sbarbati, A.; Chirumbolo, S.; Bertossi, D. Histology and Long-Term Clinical Outcome of Crushed Cartilage with Double-Layer Gelatin Sponge Membrane for Dorsum Refinement in Primary Rhinoplasty. Facial Plast. Surg. 2023, 39, 679–685. [Google Scholar] [CrossRef]
- Hoogenkamp, H.R.; Koens, M.J.W.; Geutjes, P.J.; Ainoedhofer, H.; Wanten, G.; Tiemessen, D.M.; Hilborn, J.; Gupta, B.; Feitz, W.F.J.; Daamen, W.F.; et al. Seamless Vascularized Large-Diameter Tubular Collagen Scaffolds Reinforced with Polymer Knittings for Esophageal Regenerative Medicine. Tissue Eng. Part C Methods 2014, 20, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef] [PubMed]
- Salleh, A.; Mustafa, N.; Teow, Y.H.; Fatimah, M.N.; Khairudin, F.A.; Ahmad, I.; Fauzi, M.B. Dual-Layered Approach of Ovine Collagen-Gelatin/Cellulose Hybrid Biomatrix Containing Graphene Oxide-Silver Nanoparticles for Cutaneous Wound Healing: Fabrication, Physicochemical, Cytotoxicity and Antibacterial Characterisation. Biomedicines 2022, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chi, L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers 2022, 14, 5014. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Angello, J.C.; Wight, T.N. Formation of Hyaluronan- and Versican-Rich Pericellular Matrix Is Required for Proliferation and Migration of Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Marini, L.; Gagliano, N.; Canciani, E.; Dellavia, C.; Cornaghi, L.B.; Costa, E.; Rojas, M.A. Clinical, Histological, Immunohistochemical, and Biomolecular Analysis of Hyaluronic Acid in Early Wound Healing of Human Gingival Tissues: A Randomized, Split-Mouth Trial. J. Periodontol. 2023, 94, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Dimitrievska, S.; Niklason, L.E. Historical Perspective and Future Direction of Blood Vessel Developments. Cold Spring Harb. Perspect. Med. 2018, 8, a025742. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of New Blood-Vessel Formation and Proliferative Heterogeneity of Endothelial Cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef]
- Ausprunk, D.H. Distribution of Hyaluronic Acid and Sulfated Glycosaminoglycans during Blood-vessel Development in the Chick Chorioallantoic Membrane. Am. J. Anat. 1986, 177, 313–331. [Google Scholar] [CrossRef]
- Huang, K.-F.; Hsu, W.-C.; Hsiao, J.-K.; Chen, G.-S.; Wang, J.-Y. Collagen-Glycosaminoglycan Matrix Implantation Promotes Angiogenesis Following Surgical Brain Trauma. BioMed Res. Int. 2014, 2014, 672409. [Google Scholar] [CrossRef] [PubMed]




| DLC | Xenoderm | |
|---|---|---|
| Parameteres | CAM Assay | CAM Assay |
| Vessels_total_area [Px^2]/ROI | ||
| 1 | 16.53% | 18.29% |
| 3 | 22.72% | 32.10% |
| 5 | 18.86% | 16.30% |
| Vessels_num_branching_points/ROI | ||
| 1 | 0.00% | 0.01% |
| 2 | 0.01% | 0.02% |
| 3 | 0.02% | 0.02% |
| Vessels_num_branching_points/vessels_total_area | ||
| 0.02% | 0.07% | |
| 0.02% | 0.06% | |
| 0.00% | 0.13% | |
| NFA | NFA | |
| Total_tube_length [Px]/covered_area [Px^2] | ||
| 1 | 1.15% | 3.53% |
| 2 | 1.10% | 3.27% |
| 3 | 4.04% | 4.01% |
| Num_tubes/total_tube_length [Px] | ||
| 1 | 3.29% | 2.81% |
| 2 | 3.35% | 4.26% |
| 3 | 3.05% | 5.40% |
| Vessels_Mean_Thickness [Px] | DLC CAM Assay | Xenoderm CAM Assay |
|---|---|---|
| 25.12 | 11.98 | |
| 23.32 | 16.51 | |
| 120.18 | 10.10 |
| DLC_Vascular Mean Thickness | DLC_Branching Points | ||
|---|---|---|---|
| DLC_branching points | Pearson’s r | 1.000 ** | — |
| p-value | 0.005 | — | |
| Spearman’s rho | 0.866 | — | |
| p-value | 0.167 | — | |
| Kendall’s Tau B | 0.816 | — | |
| p-value | 0.110 | — | |
| DLC_total tube length/covered area | Pearson’s r | 1.000 *** | 1.000 ** |
| p-value | <0.001 | 0.005 | |
| Spearman’s rho | 1.000 | 0.866 | |
| p-value | 0.167 | 0.167 | |
| Kendall’s Tau B | 1.000 | 0.816 | |
| p-value | 0.167 | 0.110 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvante, E.R.G.; Popoiu, A.V.; Saxena, A.K.; Popoiu, T.A.; Boia, E.S.; Cimpean, A.M.; Rus, F.S.; Dorobantu, F.R.; Chis, M. Glycosaminoglycans Modulate the Angiogenic Ability of Type I Collagen-Based Scaffolds by Acting on Vascular Network Remodeling and Maturation. Bioengineering 2024, 11, 423. https://doi.org/10.3390/bioengineering11050423
Salvante ERG, Popoiu AV, Saxena AK, Popoiu TA, Boia ES, Cimpean AM, Rus FS, Dorobantu FR, Chis M. Glycosaminoglycans Modulate the Angiogenic Ability of Type I Collagen-Based Scaffolds by Acting on Vascular Network Remodeling and Maturation. Bioengineering. 2024; 11(5):423. https://doi.org/10.3390/bioengineering11050423
Chicago/Turabian StyleSalvante, Enrica Raffaella Grazia, Anca Voichita Popoiu, Amulya K. Saxena, Tudor Alexandru Popoiu, Eugen Sorin Boia, Anca Maria Cimpean, Florina Stefania Rus, Florica Ramona Dorobantu, and Monica Chis. 2024. "Glycosaminoglycans Modulate the Angiogenic Ability of Type I Collagen-Based Scaffolds by Acting on Vascular Network Remodeling and Maturation" Bioengineering 11, no. 5: 423. https://doi.org/10.3390/bioengineering11050423
APA StyleSalvante, E. R. G., Popoiu, A. V., Saxena, A. K., Popoiu, T. A., Boia, E. S., Cimpean, A. M., Rus, F. S., Dorobantu, F. R., & Chis, M. (2024). Glycosaminoglycans Modulate the Angiogenic Ability of Type I Collagen-Based Scaffolds by Acting on Vascular Network Remodeling and Maturation. Bioengineering, 11(5), 423. https://doi.org/10.3390/bioengineering11050423