Modern In Vitro Techniques for Modeling Hearing Loss
Abstract
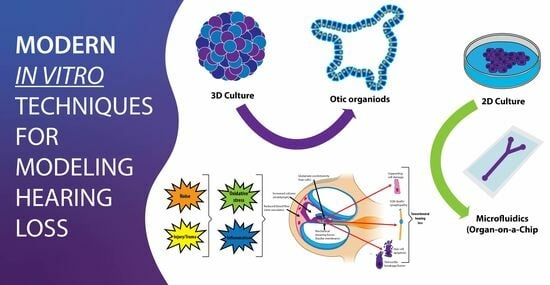
:1. Introduction
2. Methods
3. Methodological Advances in Otic Organoid Generation
4. Cellular and Molecular Characterization
5. Technological Innovations for Culture Enhancement
6. Challenges and Limitations
7. Conclusions and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Fact Sheet: Deafness and Hearing Loss. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 1 October 2023).
- Veterans Benefits Administration. Part 2 Annual Benefits Report (ABR)—Compensation—Fiscal Year 2020. 2020. Available online: https://www.benefits.va.gov/REPORTS/abr/docs/2020_compensation.pdf (accessed on 14 November 2023).
- Wells, T.S.; Seelig, A.D.; Ryan, M.A.; Jones, J.M.; Hooper, T.I.; Jacobson, I.G.; Boyko, E.J. Hearing loss associated with US military combat deployment. Noise Health 2015, 17, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Hashino, E.; Nelson, R.F. Progress in modeling and targeting inner ear disorders with pluripotent stem cells. Stem Cell Rep. 2020, 14, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Brichta, A.M. Anatomical and physiological development of the human inner ear. Hear. Res. 2016, 338, 9–21. [Google Scholar] [CrossRef]
- Bowl, M.R.; Dawson, S.J. Age-related hearing loss. Cold Spring Harb. Perspect. Med. 2019, 9, a033217. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, E.N.; Li, M.; Shah, A.; Elliott, K.L.; Cheah, K.; Xu, P.X.; Phillips, S.; Young, S.M., Jr.; Eberl, D.F.; Fritzsch, B. Using Sox2 to alleviate the hallmarks of age-related hearing loss. Ageing Res. Rev. 2020, 59, 101042. [Google Scholar] [CrossRef]
- Mizutari, K. Blast-induced hearing loss. J. Zhejiang Univ. Sci. B 2019, 20, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Fettiplace, R.; Hackney, C.M. The sensory and motor roles of auditory hair cells. Nat. Rev. Neurosci. 2006, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Géléoc, G.S.; Holt, J.R. Auditory amplification: Outer hair cells pres the issue. Trends Neurosci. 2003, 26, 115–117. [Google Scholar] [CrossRef]
- Burns, J.C.; Stone, J.S. Development and regeneration of vestibular hair cells in mammals. Semin. Cell Dev. Biol. 2017, 65, 96–105. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear. Res. 2019, 377, 44–52. [Google Scholar] [CrossRef]
- Huth, M.E.; Ricci, A.J.; Cheng, A.G. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int. J. Otolaryngol. 2011, 2011, 937861. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.E.; Perez, A.; Lin, R.; Sajjadi, A.; Ricci, A.J.; Cheng, A.G. Towards the Prevention of Aminoglycoside-Related Hearing Loss. Front. Cell. Neurosci. 2017, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Kral, A.; Dorman, M.F.; Wilson, B.S. Neuronal development of hearing and language: Cochlear implants and critical periods. Annu. Rev. Neurosci. 2019, 42, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.G.M.; Su, M.P.; Blackshaw, H.; Lustig, L.; Staecker, H.; Lenarz, T.; Safieddine, S.; Gomes-Santos, C.S.; Holme, R.; Warnecke, A. Hearing protection, restoration, and regeneration. Otol. Neurotol. 2019, 40, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Roccio, M.; Edge, A.S.B. Inner ear organoids: New tools to understand neurosensory cell development, degeneration and regeneration. Development 2019, 146, dev177188. [Google Scholar] [CrossRef] [PubMed]
- Géléoc, G.S.G.; Holt, J.R. Sound strategies for hearing restoration. Science 2014, 344, 1241062. [Google Scholar] [CrossRef] [PubMed]
- Delmaghani, S.; El-Amraoui, A. Inner ear gene therapies take off: Current promises and future challenges. J. Clin. Med. 2020, 9, 2309. [Google Scholar] [CrossRef]
- Ganesan, P.; Schmiedge, J.; Manchaiah, V.; Swapna, S.; Dhandayutham, S.; Kothandaraman, P.P. Ototoxicity: A challenge in diagnosis and treatment. J. Audiol. Otol. 2018, 22, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Roccio, M.; Senn, P.; Heller, S. Novel insights into inner ear development and regeneration for targeted hearing loss therapies. Hear. Res. 2020, 397, 107859. [Google Scholar] [CrossRef]
- Thomson, J.A. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007, 2, 3081–3089. [Google Scholar] [CrossRef]
- Kayyali, M.N.; Wright, A.C.; Ramsey, A.J.; Brant, J.A.; Stein, J.M.; O’Malley, B.W., Jr.; Li, D. Challenges and opportunities in developing targeted molecular imaging to determine inner ear defects of sensorineural hearing loss. Nanomedicine 2018, 14, 397–404. [Google Scholar] [CrossRef]
- Johnson Chacko, L.; Wertjanz, D.; Sergi, C.; Dudas, J.; Fischer, N.; Eberharter, T.; Hoermann, R.; Glueckert, R.; Fritsch, H.; Rask-Andersen, H.; et al. Growth and cellular patterning during fetal human inner ear development studied by a correlative imaging approach. BMC Dev. Biol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Kniss, J.S.; Jiang, L.; Piotrowski, T. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 2016, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Warchol, M.E.; Corwin, J.T. Regenerative proliferation in organ cultures of the avian cochlea: Identification of the initial progenitors and determination of the latency of the proliferative response. J. Neurosci. 1996, 16, 5466–5477. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Corrales, C.E.; Liberman, M.C.; Edge, A.S.B. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci. 2007, 26, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jongkamonwiwat, N.; Abbas, L.; Eshtan, S.J.; Johnson, S.L.; Kuhn, S.; Milo, M.; Thurlow, J.K.; Andrews, P.W.; Marcotti, W.; et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 2012, 490, 278–282. [Google Scholar] [CrossRef]
- Gunewardene, N.; Bergen, N.V.; Crombie, D.; Needham, K.; Dottori, M.; Nayagam, B.A. Directing human induced pluripotent stem cells into a neurosensory lineage for auditory neuron replacement. BioRes. Open Access 2014, 3, 162–175. [Google Scholar] [CrossRef]
- Ronaghi, M.; Nasr, M.; Ealy, M.; Durruthy-Durruthy, R.; Waldhaus, J.; Diaz, G.H.; Joubert, L.M.; Oshima, K.; Heller, S. Inner ear hair cell-like cells from human embryonic stem cells. Stem Cells Dev. 2014, 23, 1275–1284. [Google Scholar] [CrossRef]
- Ohnishi, H.; Skerleva, D.; Kitajiri, S.; Sakamoto, T.; Yamamoto, N.; Ito, J.; Nakagawa, T. Limited hair cell induction from human induced pluripotent stem cells using a simple stepwise method. Neurosci. Lett. 2015, 599, 49–54. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Z.; Chen, J.; Shi, H.; Chen, J.; Wang, C.; Zhang, C.; Li, L.; Chen, P.; Wang, J. Induction of differentiation of human embryonic stem cells into functional hair-cell-like cells in the absence of stromal cells. Int. J. Biochem. Cell Biol. 2016, 81, 208–222. [Google Scholar] [CrossRef]
- Ealy, M.; Ellwanger, D.C.; Kosaric, N.; Stapper, A.P.; Heller, S. Single-cell analysis delineates a trajectory toward the human early otic lineage. Proc. Natl. Acad. Sci. USA 2016, 113, 8508–8513. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, A.J.; Morrissey, Z.D.; Zhang, C.; Homma, K.; Belmadani, A.; Miller, C.A.; Chadly, D.M.; Kobayashi, S.; Edelbrock, A.N.; Tanaka-Matakatsu, M.; et al. Directed differentiation of human embryonic stem cells toward placode-derived spiral Ganglion-like sensory neurons. Stem Cell Transl. Med. 2016, 6, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Fujioka, M.; Sone, T.; Okamoto, S.; Akamatsu, W.; Ukai, H.; Ueda, H.R.; Ogawa, K.; Matsunaga, T.; Okano, H. Cochlear cell modeling using disease-specific iPSCs unveils a degenerative phenotype and suggests treatments for congenital progressive hearing loss. Cell Rep. 2017, 18, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.R.; Nie, J.; Longworth-Mills, E.; Liu, X.P.; Lee, J.; Holt, J.R.; Hashino, E. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 2017, 35, 583–589. [Google Scholar] [CrossRef]
- Jeong, M.; O’Reilly, M.; Kirkwood, N.K.; Al-Aama, J.; Lako, M.; Kros, C.J.; Armstrong, L. Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells. Cell Death Dis. 2018, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, H.; Nivet, E.; Lopez-Juarez, A.; Fontbonne, A.; Assou, S.; Zine, A. Enriched differentiation of human otic sensory progenitor cells derived from induced pluripotent stem cells. Front. Mol. Neurosci. 2018, 11, 452. [Google Scholar] [CrossRef]
- Mattei, C.; Lim, R.; Drury, H.; Nasr, B.; Li, Z.; Tadros, M.A.; D‘Abaco, G.M.; Stok, K.S.; Nayagam, B.A.; Dottori, M. Generation of vestibular tissue-like organoids from human pluripotent stem cells using the rotary cell culture System. Front. Cell Dev. Biol. 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Senn, P.; Mina, A.; Volkenstein, S.; Kranebitter, V.; Oshima, K.; Heller, S. Progenitor cells from the adult human inner ear. Anat. Rec. 2020, 303, 461–470. [Google Scholar] [CrossRef]
- Chen, W.; Johnson, S.L.; Marcotti, W.; Andrews, P.W.; Moore, H.D.; Rivolta, M.N. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells 2009, 27, 1196–1204. [Google Scholar] [CrossRef]
- Roccio, M.; Perny, M.; Ealy, M.; Widmer, H.R.; Heller, S.; Senn, P. Molecular characterization and prospective isolation of human fetal cochlear hair cell progenitors. Nat. Commun. 2018, 9, 4027. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.R.; Jagger, D.J.; Saeed, S.R.; Axon, P.; Donnelly, N.; Tysome, J.; Moffatt, D.; Irving, R.; Monksfield, P.; Coulson, C.; et al. Characterizing human vestibular sensory epithelia for experimental studies: New hair bundles on old tissue and implications for therapeutic interventions in ageing. Neurobiol. Aging 2015, 36, 2068–2084. [Google Scholar] [CrossRef]
- Taylor, R.R.; Filia, A.; Paredes, U.; Asai, Y.; Holt, J.R.; Lovett, M.; Forge, A. Regenerating hair cells in vestibular sensory epithelia from humans. eLife 2018, 7, e34817. [Google Scholar] [CrossRef]
- Czajkowski, A.; Mounier, A.; Delacroix, L.; Malgrange, B. Pluripotent stem cell-derived cochlear cells: A challenge in constant progress. Cell. Mol. Life Sci. 2019, 76, 627–635. [Google Scholar] [CrossRef]
- Trainor, P.A.; Tam, P.P. Cranial paraxial mesoderm and neural crest cells of the mouse embryo: Co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development 1995, 121, 2569. [Google Scholar] [CrossRef]
- Boddy, S.L.; Romero-Guevara, R.; Ji, A.R.; Unger, C.; Corns, L.; Marcotti, W.; Rivolta, M.N. Generation of otic lineages from integration-free human-induced pluripotent stem cells reprogrammed by mRNAs. Stem Cells Int. 2020, 2020, 3692937. [Google Scholar] [CrossRef] [PubMed]
- Munnamalai, V.; Fekete, D.M. Building the human inner ear in an organoid. Nat. Biotechnol. 2017, 35, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Longworth-Mills, E.; Koehler, K.R.; Hashino, E. Generating inner ear organoids from mouse embryonic stem cells. Methods Mol. Biol. 2016, 1341, 391–406. [Google Scholar]
- Koehler, K.R.; Hashino, E. 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat. Protoc. 2014, 9, 1229–1244. [Google Scholar] [CrossRef]
- Doda, D.; Alonso Jimenez, S.; Rehrauer, H.; Carreño, J.F.; Valsamides, V.; Di Santo, S.; Widmer, H.R.; Edge, A.; Locher, H.; van der Valk, W.H.; et al. Human pluripotent stem cell-derived inner ear organoids recapitulate otic development in vitro. Development 2023, 150, dev201865. [Google Scholar] [CrossRef]
- Liu, X.-P.; Koehler, K.R.; Mikosz, A.M.; Hashino, E.; Holt, J.R. Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat. Commun. 2016, 7, 11508. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Alex, A.L.; Nie, J.; Lee, J.; Roth, A.A.; Booth, K.T.; Koehler, K.R.; Hashino, E.; Nelson, R.F. Defective Tmprss3-associated hair cell degeneration in inner ear Organoids. Stem Cell Rep. 2019, 13, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Libby, R.T.; Steel, K.P. The roles of unconventional myosins in hearing and deafness. Essays Biochem. 2000, 35, 159–174. [Google Scholar] [PubMed]
- Tang, Z.H.; Chen, J.R.; Zheng, J.; Shi, H.S.; Ding, J.; Qian, X.D.; Zhang, C.; Chen, J.L.; Wang, C.C.; Li, L.; et al. Genetic correction of induced pluripotent stem cells from a deaf patient with MYO7A mutation results in morphologic and functional recovery of the derived hair cell-like cells. Stem Cells Transl. Med. 2016, 5, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Tang, Z.H.; Zheng, J.; Shi, H.S.; Ding, J.; Qian, X.D.; Zhang, C.; Chen, J.L.; Wang, C.C.; Li, L.; et al. Effects of genetic correction on the differentiation of hair cell-like cells from iPSCs with MYO15A mutation. Cell Death Differ. 2016, 23, 1347–1357. [Google Scholar] [CrossRef]
- Rossant, J.; Papaioannou, V.E. The relationship between embryonic, embryonal carcinoma and embryo-derived stem cells. Cell Differ. 1984, 15, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Benvenisty, N. Defining human pluripotency. Cell Stem Cell 2019, 25, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.Z.; Hockemeyer, D. Human stem cell-based disease modeling: Prospects and challenges. Curr. Opin. Cell Biol. 2015, 37, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, A.; Mellott, A.J.; Römer, A.; Lenarz, T.; Staecker, H. Advances in translational inner ear stem cell research. Hear. Res. 2017, 353, 76–86. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Forge, A.; Li, L.; Corwin, J.T.; Nevill, G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 1993, 259, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Warchol, M.E.; Lambert, P.R.; Goldstein, B.J.; Forge, A.; Corwin, J.T. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 1993, 259, 1619–1622. [Google Scholar] [CrossRef]
- Bramhall, N.F.; Shi, F.; Arnold, K.; Hochedlinger, K.; Edge, A.S. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Rep. 2014, 2, 311–322. [Google Scholar] [CrossRef]
- Burns, J.C.; Cox, B.C.; Thiede, B.R.; Zuo, J.; Corwin, J.T. In vivo proliferative regeneration of balance hair cells in newborn mice. J. Neurosci. 2012, 32, 6570–6577. [Google Scholar] [CrossRef]
- Hu, L.; Lu, J.; Chiang, H.; Wu, H.; Edge, A.S.; Shi, F. Diphtheria Toxin-Induced Cell Death Triggers Wnt-Dependent Hair Cell Regeneration in Neonatal Mice. J. Neurosci. 2016, 36, 9479–9489. [Google Scholar] [CrossRef]
- Steventon, B.; Mayor, R.; Streit, A. Neural crest and placode interaction during the development of the cranial sensory system. Dev. Biol. 2014, 389, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Tambalo, M.; Anwar, M.; Ahmed, M.; Streit, A. Enhancer activation by FGF signalling during otic induction. Dev. Biol. 2020, 457, 69–82. [Google Scholar] [CrossRef]
- Schlosser, G.; Patthey, C.; Shimeld, S.M. The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning. Dev. Biol. 2014, 389, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Patthey, C.; Gunhaga, L. Signaling pathways regulating ectodermal cell fate choices. Exp. Cell Res. 2014, 321, 11–16. [Google Scholar] [CrossRef]
- Ladher, R.K.; Anakwe, K.U.; Gurney, A.L.; Schoenwolf, G.C.; Francis-West, P.H. Identification of synergistic signals initiating inner ear development. Science 2000, 290, 1965–1967. [Google Scholar] [CrossRef]
- Ohyama, T.; Groves, A.K. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 2004, 38, 195–199. [Google Scholar] [CrossRef]
- Ohyama, T.; Mohamed, O.A.; Taketo, M.M.; Dufort, D.; Groves, A.K. Wnt signals mediate a fate decision between otic placode and epidermis. Development 2006, 133, 865–875. [Google Scholar] [CrossRef]
- Jayasena, C.S.; Ohyama, T.; Segil, N.; Groves, A.K. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 2008, 135, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Freter, S.; Muta, Y.; Mak, S.-S.; Rinkwitz, S.; Ladher, R.K. Progressive restriction of otic fate: The role of FGF and Wnt in resolving inner ear potential. Development 2008, 135, 3415–3424. [Google Scholar] [CrossRef] [PubMed]
- Freyer, L.; Aggarwal, V.; Morrow, B.E. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development 2011, 138, 5403–5414. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.E.; Martin, D.M. Neural crest contributions to the ear: Implications for congenital hearing disorders. Hear. Res. 2018, 376, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Groves, A.K. The molecular basis of craniofacial placode development. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 363–376. [Google Scholar] [CrossRef]
- Nie, J.; Hashino, E. Generation of inner ear organoids from human pluripotent stem cells. Methods Cell Biol. 2020, 159, 303–321. [Google Scholar] [PubMed]
- Chizhikov, V.V.; Iskusnykh, I.Y.; Fattakhov, N.; Fritzsch, B. Lmx1a and Lmx1b are redundantly required for the development of multiple components of the mammalian auditory system. Neuroscience 2021, 452, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Evsen, L.; Sugahara, S.; Uchikawa, M.; Kondoh, H.; Wu, D.K. Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. J. Neurosci. 2013, 33, 3879–3890. [Google Scholar] [CrossRef]
- Dabdoub, A.; Puligilla, C.; Jones, J.M.; Fritzsch, B.; Cheah, K.S.; Pevny, L.H.; Kelley, M.W. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA 2008, 105, 18396–18401. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Macova, I.; Bohuslavova, R.; Anderova, M.; Fritzsch, B.; Pavlinkova, G. Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev. Biol. 2020, 457, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.T.; Nakamura, T.; Nie, J.; Solivais, A.J.; Aristizábal-Ramírez, I.; Ueda, Y.; Manikandan, M.; Reddy, V.S.; Romano, D.R.; Hoffman, J.R.; et al. Generating high-fidelity cochlear organoids from human pluripotent stem cells. Cell Stem Cell 2023, 30, 950–961.e7. [Google Scholar] [CrossRef] [PubMed]
- White, H.J.; Helwany, M.; Biknevicius, A.R.; Peterson, D.C. Anatomy, Head and Neck, Ear Organ of Corti; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538335/ (accessed on 15 January 2024).
- Basch, M.L.; Brown, R.M., 2nd; Jen, H.I.; Groves, A.K. Where hearing starts: The development of the mammalian cochlea. J. Anat. 2016, 228, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.C.; Chen, P. Development of form and function in the mammalian cochlea. Curr. Opin. Neurobiol. 2009, 19, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, A.E.; Pelling, A.L.; Leung, K.K.; Tang, A.S.; Bell, D.M.; Tease, C.; Lovell-Badge, R.; Steel, K.P.; Cheah, K.S. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005, 434, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Basch, M.L.; Mishina, Y.; Lyons, K.M.; Segil, N.; Groves, A.K. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 2010, 30, 15044–15051. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Sanchez-Guardado, L.; Juniat, S.; Gale, J.E.; Daudet, N.; Henrique, D. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development 2015, 142, 1948–1959. [Google Scholar] [CrossRef]
- Menendez, L.; Trecek, T.; Gopalakrishnan, S.; Tao, L.; Markowitz, A.L.; Yu, H.V.; Wang, X.; Llamas, J.; Huang, C.; Lee, J.; et al. Generation of inner ear hair cells by direct lineage conversion of primary somatic cells. eLife 2020, 9, e55249. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, X.; Shao, Y.; Wang, S.; Esfahani, S.N.; Li, Z.; Muncie, J.M.; Lakins, J.N.; Weaver, V.M.; Gumucio, D.L.; et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 2019, 573, 421–425. [Google Scholar] [CrossRef]
- Manfrin, A.; Tabata, Y.; Paquet, E.R.; Vuaridel, A.R.; Rivest, F.R.; Naef, F.; Lutolf, M.P. Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat. Methods 2019, 16, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, F.; Rookmaaker, M.B.; Margaritis, T.; Rios, A.; Ammerlaan, C.; Jansen, J.; Gijzen, L.; Vormann, M.; Vonk, A.; Viveen, M.; et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol. 2019, 37, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Hoehnel, S.; Kuttler, F.; Homicsko, K.; Ceroni, C.; Ringel, T.; Gjorevski, N.; Schwank, G.; Coukos, G.; Turcatti, G.; et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020, 4, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Abboud, N.; Fontbonne, A.; Watabe, I.; Tonetto, A.; Brezun, J.M.; Feron, F.; Zine, A. Culture conditions have an impact on the maturation of traceable, transplantable mouse embryonic stem cell-derived otic progenitor cells. J. Tissue Eng. Regen. Med. 2017, 11, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Roblin, G.; Liu, H.; Heller, S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 13495–13500. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Shin, K.; Diensthuber, M.; Peng, A.W.; Ricci, A.J.; Heller, S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 2010, 141, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Ouji, Y.; Ishizaka, S.; Nakamura-Uchiyama, F.; Yoshikawa, M. In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium. Cell Death Dis. 2012, 3, e314. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.R.; Mikosz, A.M.; Molosh, A.I.; Patel, D.; Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 2013, 500, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.A.; Higashi, A.Y.; Loomis, B.; Schrepfer, T.; Wan, G.; Corfas, G.; Dressler, G.R.; Duncan, R.K. From otic induction to hair cell production: Pax2EGFP cell line illuminates key stages of development in mouse inner ear organoid model. Stem Cells Dev. 2018, 27, 237–251. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef]
- Schutgens, F.; Clevers, H. Human organoids: Tools for understanding biology and treating diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, M.; Abdollahi, N.; Graf, L.; Deigendesch, N.; Puche, R.; Bodmer, D.; Petkovic, V. Human blood-labyrinth barrier on a chip: A unique in vitro tool for investigation of BLB properties. RSC Adv. 2023, 13, 25508–25517. [Google Scholar] [CrossRef]
- Ishiyama, A.; Mowry, S.E.; Lopez, I.A.; Ishiyama, G. Immunohistochemical distribution of basement membrane proteins in the human inner ear from older subjects. Hear. Res. 2009, 254, 1–14. [Google Scholar] [CrossRef]
- Santi, P.A.; Johnson, S.B. Decellularized ear tissues as scaffolds for stem cell differentiation. J. Assoc. Res. Otolaryngol. 2013, 14, 3–15. [Google Scholar] [CrossRef]
- Mellott, A.J.; Shinogle, H.E.; Nelson-Brantley, J.G.; Detamore, M.S.; Staecker, H. Exploiting decellularized cochleae as scaffolds for inner ear tissue engineering. Stem Cell Res. Ther. 2017, 8, 41. [Google Scholar] [CrossRef]
- Chen, J.; Hong, F.; Zhang, C.; Li, L.; Wang, C.; Shi, H.; Fu, Y.; Wang, J. Differentiation and transplantation of human induced pluripotent stem cell-derived otic epithelial progenitors in mouse cochlea. Stem Cell Res. Ther. 2018, 9, 230. [Google Scholar] [CrossRef]
- Lopez-Juarez, A.; Lahlou, H.; Ripoll, C.; Cazals, Y.; Brezun, J.M.; Wang, Q.; Edge, A.; Zine, A. Engraftment of human stem cell-derived otic progenitors in the damaged cochlea. Mol. Ther. 2019, 27, 1101–1113. [Google Scholar] [CrossRef]
- Takeda, H.; Hosoya, M.; Fujioka, M.; Saegusa, C.; Saeki, T.; Miwa, T.; Okano, H.; Minoda, R. Engraftment of human pluripotent stem cell-derived progenitors in the inner ear of prenatal mice. Sci. Rep. 2018, 8, 1941. [Google Scholar] [CrossRef]
- Balikov, D.A.; Neal, E.H.; Lippmann, E.S. Organotypic neurovascular models: Past results and future directions. Trends Mol. Med. 2020, 26, 273–284. [Google Scholar] [CrossRef]
- Takebe, T.; Wells, J.M. Organoids by design. Science 2019, 364, 956–959. [Google Scholar] [CrossRef]
- Zou, B.; Mittal, R.; Grati, M.H.; Lu, Z.; Shu, Y.; Tao, Y.; Feng, Y.; Xie, D.; Kong, W.; Yang, S.; et al. The application of genome editing in studying hearing loss. Hear Res. 2015, 327, 102–108. [Google Scholar] [CrossRef]
- Malgrange, B.; Belachew, S.; Thiry, M.; Nguyen, L.; Rogister, B.; Alvarez, M.L.; Rigo, J.M.; Van De Water, T.R.; Moonen, G.; Lefebvre, P.P. Proliferative generation of mammalian auditory hair cells in culture. Mech. Dev. 2002, 112, 79–88. [Google Scholar] [CrossRef]
- Oshima, K.; Grimm, C.M.; Corrales, C.E.; Senn, P.; Martinez Monedero, R.; Géléoc, G.S.; Edge, A.; Holt, J.R.; Heller, S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J. Assoc. Res. Otolaryngol. 2007, 8, 18–31. [Google Scholar] [CrossRef]
- McLean, W.J.; Yin, X.; Lu, L.; Lenz, D.R.; McLean, D.; Langer, R.; Karp, J.M.; Edge, A.S.B. Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Rep. 2017, 18, 1917–1929. [Google Scholar] [CrossRef]
- Nie, J.; Hashino, E. Organoid technologies meet genome engineering. EMBO Rep. 2017, 18, 367–376. [Google Scholar] [CrossRef]
- Tchieu, J.; Zimmer, B.; Fattahi, F.; Amin, S.; Zeltner, N.; Chen, S.; Studer, L. A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell 2017, 21, 399–410. [Google Scholar] [CrossRef]
- Leung, A.W.; Murdoch, B.; Salem, A.F.; Prasad, M.S.; Gomez, G.A.; García-Castro, M.I. WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 2016, 143, 398–410. [Google Scholar] [CrossRef]
- Gomez, G.A.; Prasad, M.S.; Sandhu, N.; Shelar, P.B.; Leung, A.W.; García-Castro, M.I. Human neural crest induction by temporal modulation of WNT activation. Dev. Biol. 2019, 449, 99–106. [Google Scholar] [CrossRef]
- Scheper, V.; Hoffmann, A.; Gepp, M.M.; Schulz, A.; Hamm, A.; Pannier, C.; Hubka, P.; Lenarz, T.; Schwieger, J. Stem cell based drug delivery for protection of auditory neurons in a Guinea Pig model of cochlear implantation. Front. Cell Neurosci. 2019, 13, 177. [Google Scholar] [CrossRef]
- Roemer, A.; Köhl, U.; Majdani, O.; Klöß, S.; Falk, C.; Haumann, S.; Lenarz, T.; Kral, A.; Warnecke, A. Biohybrid cochlear implants in human neurosensory restoration. Stem Cell Res. Ther. 2016, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Nguyen, D.; Patel, A.P.; Debs, L.H.; Mittal, J.; Yan, D.; Eshraghi, A.A.; Van De Water, T.R.; Liu, X.Z. Recent Advancements in the regeneration of auditory hair cells and hearing restoration. Front. Mol. Neurosci. 2017, 10, 236. [Google Scholar] [CrossRef]
- Xia, M.; Ma, J.; Wu, M.; Guo, L.; Chen, Y.; Li, G.L.; Sun, S.; Chai, R.; Li, H.; Li, W. Generation of innervated cochlear organoid recapitulates early development of auditory unit. Stem Cell Rep. 2023, 18, 319–336. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.J.; Jimenez-Jaramillo, C.A.; Lybrand, Z.R.; Yuan, T.T.; Erbele, I.D. Modern In Vitro Techniques for Modeling Hearing Loss. Bioengineering 2024, 11, 425. https://doi.org/10.3390/bioengineering11050425
Shah JJ, Jimenez-Jaramillo CA, Lybrand ZR, Yuan TT, Erbele ID. Modern In Vitro Techniques for Modeling Hearing Loss. Bioengineering. 2024; 11(5):425. https://doi.org/10.3390/bioengineering11050425
Chicago/Turabian StyleShah, Jamie J., Couger A. Jimenez-Jaramillo, Zane R. Lybrand, Tony T. Yuan, and Isaac D. Erbele. 2024. "Modern In Vitro Techniques for Modeling Hearing Loss" Bioengineering 11, no. 5: 425. https://doi.org/10.3390/bioengineering11050425
APA StyleShah, J. J., Jimenez-Jaramillo, C. A., Lybrand, Z. R., Yuan, T. T., & Erbele, I. D. (2024). Modern In Vitro Techniques for Modeling Hearing Loss. Bioengineering, 11(5), 425. https://doi.org/10.3390/bioengineering11050425