Polyhydroxyalkanoate Copolymer Production by Recombinant Ralstonia eutropha Strain 1F2 from Fructose or Carbon Dioxide as Sole Carbon Source
Abstract
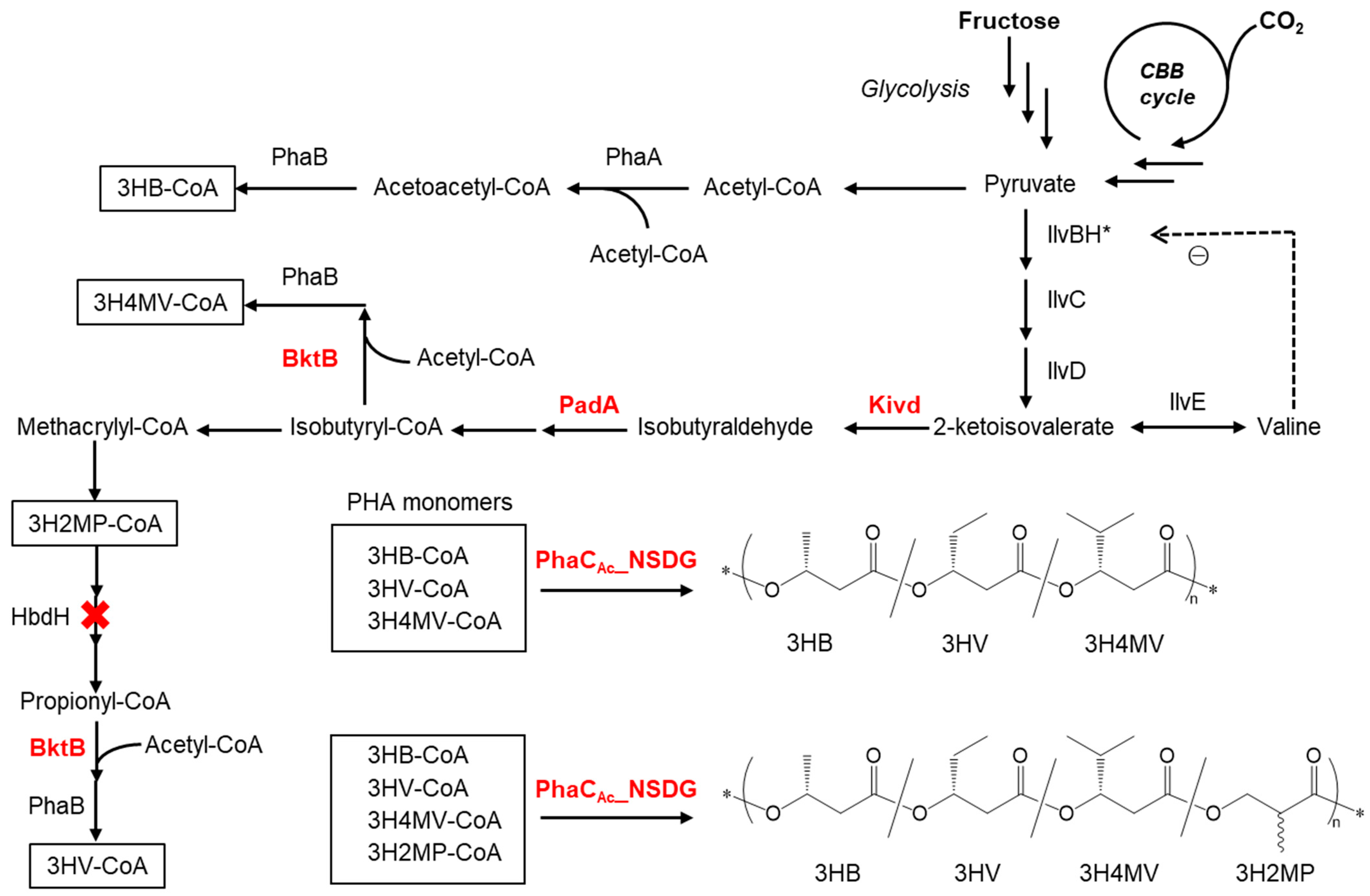
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. Plasmids Construction
2.3. Biosynthesis of PHA Copolymers from Fructose or CO2
2.4. Analysis of PHA
2.4.1. PHA Content Analysis by Gas Chromatography (GC)
2.4.2. Structure Analysis by Gas Chromatography–Mass Spectrometry (GC-MS)
2.4.3. Molecular Weight Analysis by Gel Permeation Chromatography (GPC)
2.4.4. Structure Analysis by Nuclear Magnetic Resonance (NMR)
3. Results
3.1. Biosynthesis of PHA by Recombinant R. eutropha 1F2 from Fructose
3.2. NMR Analysis of Biosynthesized PHA
3.3. Biosynthesis of 3H2MP-Containing PHA Copolymer Using the hbdH-Deficient Strain
3.4. Structural Analysis of PHA Biosynthesized by hbdH-Deficient Strains
3.5. Molecular Weight of PHA Biosynthesized from Fructose
3.6. Biosynthesis of PHA from CO2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Alves, A.A.; Siqueira, E.C.; Barros, M.P.; Silva, P.E.; Houllou, L.M. Polyhydroxyalkanoates: A review of microbial production and technology application. Int. J. Environ. Sci. Technol. 2022, 20, 3409–3420. [Google Scholar] [CrossRef]
- Fernandez-Bunster, G.; Pavez, P. Novel production methods of polyhydroxyalkanoates and their innovative uses in biomedicine and industry. Molecules 2022, 27, 8351. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Fritz, I.; Marova, I. Polyhydroxyalkanoates: Their importance and future. BioResources 2019, 14, 2468–2471. [Google Scholar]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Ragitha, V.M.; Edison, L.K. Safety issues, environmental impacts, and health effects of biopolymers. In Handbook of Biopolymers; Springer: Singapore, 2022; pp. 1469–1495. [Google Scholar]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Zhila, N.O.; Kiselev, E.G.; Sukovatyi, A.G.; Lukyanenko, A.V.; Shishatskaya, E.I. Biodegradable polyhydroxyalkanoates with a different set of valerate monomers: Chemical structure and physicochemical properties. Int. J. Mol. Sci. 2023, 24, 14082. [Google Scholar] [CrossRef]
- Furutate, S.; Kamoi, J.; Nomura, C.T.; Taguchi, S.; Abe, H.; Tsuge, T. Superior thermal stability and fast crystallization behavior of a novel, biodegradable α-methylated bacterial polyester. NPG Asia Mater. 2021, 13, 31. [Google Scholar] [CrossRef]
- Miyahara, Y.; Nakamura, T.; Mierzati, M.; Qie, Z.; Shibasaka, T.; Nomura, C.T.; Taguchi, S.; Abe, H.; Tsuge, T. Thermal and crystallization properties of a polyhydroxyalkanoate binary copolymer containing 3-hydroxybutyrate and 3-hydroxy-2-methylvalerate units. Processes 2023, 11, 1901. [Google Scholar] [CrossRef]
- Mierzati, M.; Sakurai, T.; Ishii-Hyakutake, M.; Miyahara, Y.; Nomura, C.T.; Taguchi, S.; Abe, H.; Tsuge, T. Biosynthesis, characterization, and biodegradation of elastomeric polyhydroxyalkanoates consisting of α-dimethylated monomer units. Mater. Today Sustain. 2023, 24, 100577. [Google Scholar] [CrossRef]
- Mierzati, M.; Miyahara, Y.; Curial, B.; Nomura, C.T.; Taguchi, S.; Abe, H.; Tsuge, T. Tacticity characterization of biosynthesized polyhydroxyalkanoates containing (S)-and (R)-3-hydroxy-2-methylpropionate units. Biomacromolecules 2023, 25, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Saika, A.; Watanabe, Y.; Sudesh, K.; Abe, H.; Tsuge, T. Enhanced incorporation of 3-hydroxy-4-methylvalerate unit into biosynthetic polyhydroxyalkanoate using leucine as a precursor. AMB Express 2011, 1, 6. [Google Scholar] [CrossRef]
- Saika, A.; Ushimaru, K.; Mizuno, S.; Tsuge, T. Genome-based analysis and gene dosage studies provide new insight into 3-hydroxy-4-methylvalerate biosynthesis in Ralstonia eutropha. J. Bacteriol. 2015, 197, 1350–1359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyahara, Y.; Yamamoto, M.; Thorbecke, R.; Mizuno, S.; Tsuge, T. Autotrophic biosynthesis of polyhydroxyalkanoate by Ralstonia eutropha from non-combustible gas mixture with low hydrogen content. Biotechnol. Lett. 2020, 42, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.Y.; Park, S.; Gong, G.; Roh, S.; Yoo, J.; Ahn, J.H.; Lee, S.-M.; Um, Y.; Kim, K.H.; Ko, J.K. Enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with modulated 3-hydroxyvalerate fraction by overexpressing acetolactate synthase in Cupriavidus necator H16. Int. J. Biol. Macromol. 2023, 242, 125166. [Google Scholar] [CrossRef]
- Zhang, K.; Woodruff, A.P.; Xiong, M.; Zhou, J.; Dhande, Y.K. A synthetic metabolic pathway for production of the platform chemical isobutyric acid. ChemSusChem 2011, 4, 1068–1070. [Google Scholar] [CrossRef]
- Arenas-López, C.; Locker, J.; Orol, D.; Walter, F.; Busche, T.; Kalinowski, J.; Minton, N.P.; Kovács, K.; Winzer, K. The genetic basis of 3-hydroxypropanoate metabolism in Cupriavidus necator H16. Biotechnol. Biofuels 2019, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T.; Watanabe, S.; Shimada, D.; Abe, H.; Doi, Y.; Taguchi, S. Combination of N149S and D171G mutations in Aeromonas caviae polyhydroxyalkanoate synthase and impact on polyhydroxyalkanoate biosynthesis. FEMS Microbiol. Lett. 2007, 277, 217–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simon, R.; Priefer, U.; Pühler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Bio/Technology 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Davison, J.; Heusterspreute, M.; Chevalier, N.; Ha-Thi, V.; Brunei, F. Vectors with restriction site banks V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene 1987, 51, 275–280. [Google Scholar] [CrossRef]
- Kovach, M.E.; Elzer, P.H.; Steven Hill, D.; Robertson, G.T.; Farris, M.A.; Roop, R.M.; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef] [PubMed]
- DelaPlaza, M.; Fernández de Palencia, P.; Peláez, C.; Requena, T. Biochemical and molecular characterization of alpha-ketoisovalerate decarboxylase, an enzyme involved in the formation of aldehydes from amino acids by Lactococcus lactis. FEMS Microbiol. Lett. 2004, 238, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Slater, S.; Houmiel, K.L.; Tran, M.; Mitsky, T.A.; Taylor, N.B.; Padgette, S.R.; Gruys, K.J. Multiple β-ketothiolases mediate poly(β-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J. Bacteriol. 1998, 180, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ichinomiya, Y.; Shimada, D.; Saika, A.; Abe, H.; Taguchi, S.; Tsuge, T. Development and validation of an HPLC-based screening method to acquire polyhydroxyalkanoate synthase mutants with altered substrate specificity. J. Biosci. Bioeng. 2012, 113, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Wang, C.-T.; Ishii-Hyakutake, M.; Tsuge, T. Continuous supply of non-combustible gas mixture for safe autotrophic culture to produce polyhydroxyalkanoate by hydrogen-oxidizing bacteria. Bioengineering 2022, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, H.; Sato, S.; Fujiki, T.; Matsumoto, K. Simple and rapid method for isolation and quantitation of polyhydroxyalkanoate by SDS-sonication treatment. J. Biosci. Bioeng. 2017, 124, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Yu, P.; Wang, J.; Zhang, K. Improving engineered Escherichia coli strains for high-level biosynthesis of isobutyrate. AIMS Bioeng. 2015, 2, 60–74. [Google Scholar] [CrossRef]
- Sherkhanov, S.; Korman, T.P.; Chan, S.; Faham, S.; Liu, H.; Sawaya, M.R.; Hsu, W.-T.; Vikram, E.; Cheng, T.; Bowie, J.U. Isobutanol production freed from biological limits using synthetic biochemistry. Nat. Commun. 2020, 11, 4292. [Google Scholar] [CrossRef]
- Miao, R.; Xie, H.; Ho, M.F.; Lindblad, P. Protein engineering of α-ketoisovalerate decarboxylase for improved isobutanol production in Synechocystis PCC 6803. Metab. Eng. 2018, 47, 42–48. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, Y.; Yuan, Z.; Liu, G.; Sun, Z.; Du, S.; Liu, H.; Li, Y.; Liu, H.; Zhou, Z. Evaluation of metabolic engineering strategies on 2-ketoisovalerate production by Escherichia coli. Appl. Environ. Microbiol. 2022, 88, e00976-22. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Aoyagi, Y.; Fujita, M.; Yamane, H.; Doi, Y.; Suzuki, Y.; Takeuchi, A.; Uesugi, K. Processing of a strong biodegradable poly[(R)-3-hydroxybutyrate] fiber and a new fiber structure revealed by micro-beam X-ray diffraction with synchrotron radiation. Macromol. Rapid Commun. 2004, 25, 1100–1104. [Google Scholar] [CrossRef]
- Tanaka, T.; Fujita, M.; Takeuchi, A.; Suzuki, Y.; Uesugi, K.; Ito, K.; Fujisawa, T.; Doi, Y.; Iwata, T. Formation of highly ordered structure in poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] high-strength fibers. Macromolecules 2006, 39, 2940–2946. [Google Scholar] [CrossRef]
- Lee, H.M.; Jeon, B.Y.; Oh, M.K. Microbial production of ethanol from acetate by engineered Ralstonia eutropha. Biotechnol. Bioprocess Eng. 2016, 21, 402–407. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Doi, Y. Kinetics and mechanism of synthesis and degradation of poly(3-hydroxybutyrate) in Alcaligenes eutrophus. Macromolecules 1992, 25, 2324–2329. [Google Scholar] [CrossRef]
- Madden, L.A.; Anderson, A.J.; Shah, D.T.; Asrar, J. Chain termination in polyhydroxyalkanoate synthesis: Involvement of exogenous hydroxy-compounds as chain transfer agents. Int. J. Biol. Macromol. 1999, 25, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T. Fundamental factors determining the molecular weight of polyhydroxyalkanoate during biosynthesis. Polym. J. 2016, 48, 1051–1057. [Google Scholar] [CrossRef]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
- Thorbecke, R.; Yamamoto, M.; Miyahara, Y.; Oota, M.; Mizuno, S.; Tsuge, T. The gene dosage effect of carbonic anhydrase on the biosynthesis of poly(3-hydroxybutyrate) under autotrophic and mixotrophic culture conditions. Polym. J. 2021, 53, 209–213. [Google Scholar] [CrossRef]
- Tu, W.; Xu, J.; Thompson, I.P.; Huang, W.E. Engineering artificial photosynthesis based on rhodopsin for CO2 fixation. Nat. Commun. 2023, 14, 8012. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, G.; Orita, I.; Nakamura, R.; Fukui, T. Gas fermentation combined with water electrolysis for production of polyhydroxyalkanoate copolymer from carbon dioxide by engineered Ralstonia eutropha. Bioresour. Technol. 2024, 394, 130266. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.S.; Lu, J.; Brigham, C.J.; Bernardi, A.C.; Sinskey, A.J. Insights into bacterial CO2 metabolism revealed by the characterization of four carbonic anhydrases in Ralstonia eutropha H16. AMB Express 2014, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xin, X.; Xiong, B.; Zhao, D.; Zhang, X.; Bi, C. Engineering the Calvin–Benson–Bassham cycle and hydrogen utilization pathway of Ralstonia eutropha for improved autotrophic growth and polyhydroxybutyrate production. Microb. Cell Fact. 2020, 19, 228. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, Y.; Xiu, S.; Zheng, S.; Huang, Y.; Zhou, Z.; Hou, Y.; Yang, B.; Lei, L.; Li, Z. Efficient CO2 conversion by biocompatible N-doped carbon nanosheets coupled with Ralstonia eutropha: Synergistic interactions between microbial and inorganic catalysts. Green Chem. 2023, 25, 4760–4768. [Google Scholar] [CrossRef]
- Liu, C.; Colón, B.C.; Ziesack, M.; Silver, P.A.; Nocera, D.G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 2016, 352, 1210–1213. [Google Scholar] [CrossRef]




| Entry | Genome | Plasmid-Based Expression | Dry Cell wt. (g/L) | PHA Content (wt.%) | PHA (g/L) | PHA Composition (mol%) a | Mw (×105) b | PDI b | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 3H4MV | 3H2MP | ||||||||
| 1 | - | - c | 1.59 ± 0.04 | 54.4 ± 1.3 | 0.86 ± 0.04 | 98.5 | 1.2 | 0.3 | 0 | 4.62 | 1.86 |
| 2 | - | bktB | 1.93 ± 0.05 | 66.9 ± 2.1 | 1.29 ± 0.05 | 96.0 | 3.6 | 0.4 | 0 | 4.89 | 1.92 |
| 3 | - | bktB, kivd, padA | 1.67 ± 0.03 | 55.9 ± 1.8 | 0.93 ± 0.03 | 85.6 | 13.9 | 0.5 | 0 | 3.40 | 1.95 |
| 4 | ΔhbdH | - c | 2.10 ± 0.01 | 58.2 ± 2.0 | 1.22 ± 0.04 | 97.8 | 1.1 | 0.2 | 0.9 | 32.50 | 3.64 |
| 5 | ΔhbdH | bktB | 1.97 ± 0.07 | 60.5 ± 2.0 | 1.19 ± 0.07 | 97.6 | 0.9 | 0.4 | 1.1 | 14.80 | 2.89 |
| 6 | ΔhbdH | bktB, kivd, padA | 0.97 ± 0.02 | 7.8 ± 0.6 | 0.08 ± 0.04 | 94.8 | 2.2 | 1.3 | 1.7 | 3.97 | 2.11 |
| Entry | Genome | Plasmid-Based Expression | Dry Cell wt. (g/L) | PHA Content (wt.%) | PHA (g/L) | PHA Composition (mol%) a | Mw (×105) b | PDI b | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 3H4MV | 3H2MP | ||||||||
| 7 | - | bktB, kivd, padA | 0.72 | 28.4 | 0.20 | 92.7 | 6.4 | 0.9 | 0 | 1.88 | 2.04 |
| 8 | ΔhbdH | - | 1.20 | 49.7 | 0.60 | 99.3 | 0 | 0.7 | trace | 3.56 | 2.24 |
| 9 | ΔhbdH | bktB | 1.14 | 49.0 | 0.55 | 97.4 | 0 | 1.2 | 1.4 | 0.59 | 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-T.; Sivashankari, R.M.; Miyahara, Y.; Tsuge, T. Polyhydroxyalkanoate Copolymer Production by Recombinant Ralstonia eutropha Strain 1F2 from Fructose or Carbon Dioxide as Sole Carbon Source. Bioengineering 2024, 11, 455. https://doi.org/10.3390/bioengineering11050455
Wang C-T, Sivashankari RM, Miyahara Y, Tsuge T. Polyhydroxyalkanoate Copolymer Production by Recombinant Ralstonia eutropha Strain 1F2 from Fructose or Carbon Dioxide as Sole Carbon Source. Bioengineering. 2024; 11(5):455. https://doi.org/10.3390/bioengineering11050455
Chicago/Turabian StyleWang, Chih-Ting, Ramamoorthi M Sivashankari, Yuki Miyahara, and Takeharu Tsuge. 2024. "Polyhydroxyalkanoate Copolymer Production by Recombinant Ralstonia eutropha Strain 1F2 from Fructose or Carbon Dioxide as Sole Carbon Source" Bioengineering 11, no. 5: 455. https://doi.org/10.3390/bioengineering11050455
APA StyleWang, C.-T., Sivashankari, R. M., Miyahara, Y., & Tsuge, T. (2024). Polyhydroxyalkanoate Copolymer Production by Recombinant Ralstonia eutropha Strain 1F2 from Fructose or Carbon Dioxide as Sole Carbon Source. Bioengineering, 11(5), 455. https://doi.org/10.3390/bioengineering11050455






