Overt Word Reading and Visual Object Naming in Adults with Dyslexia: Electroencephalography Study in Transparent Orthography
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials and Stimuli
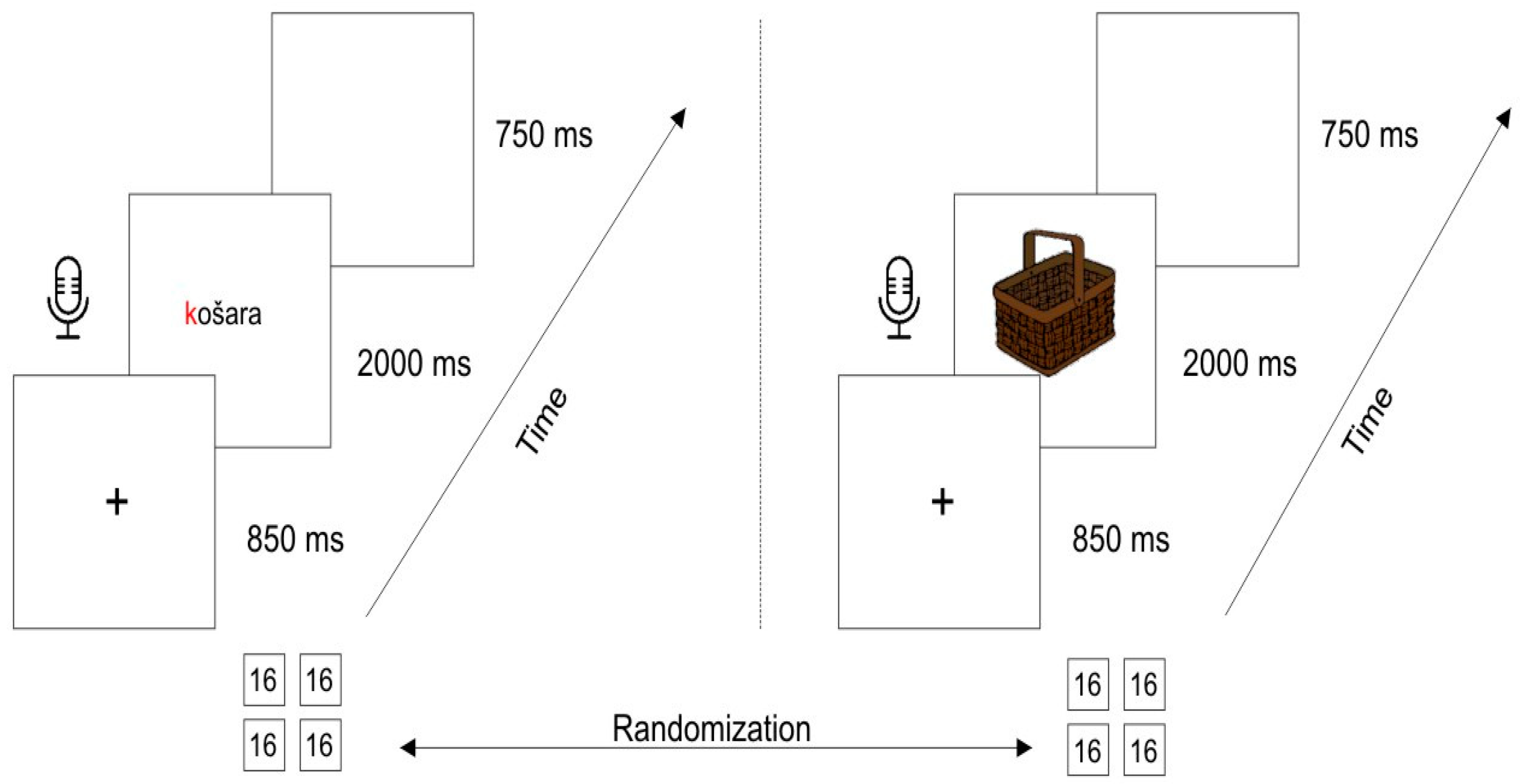
2.3. Procedure
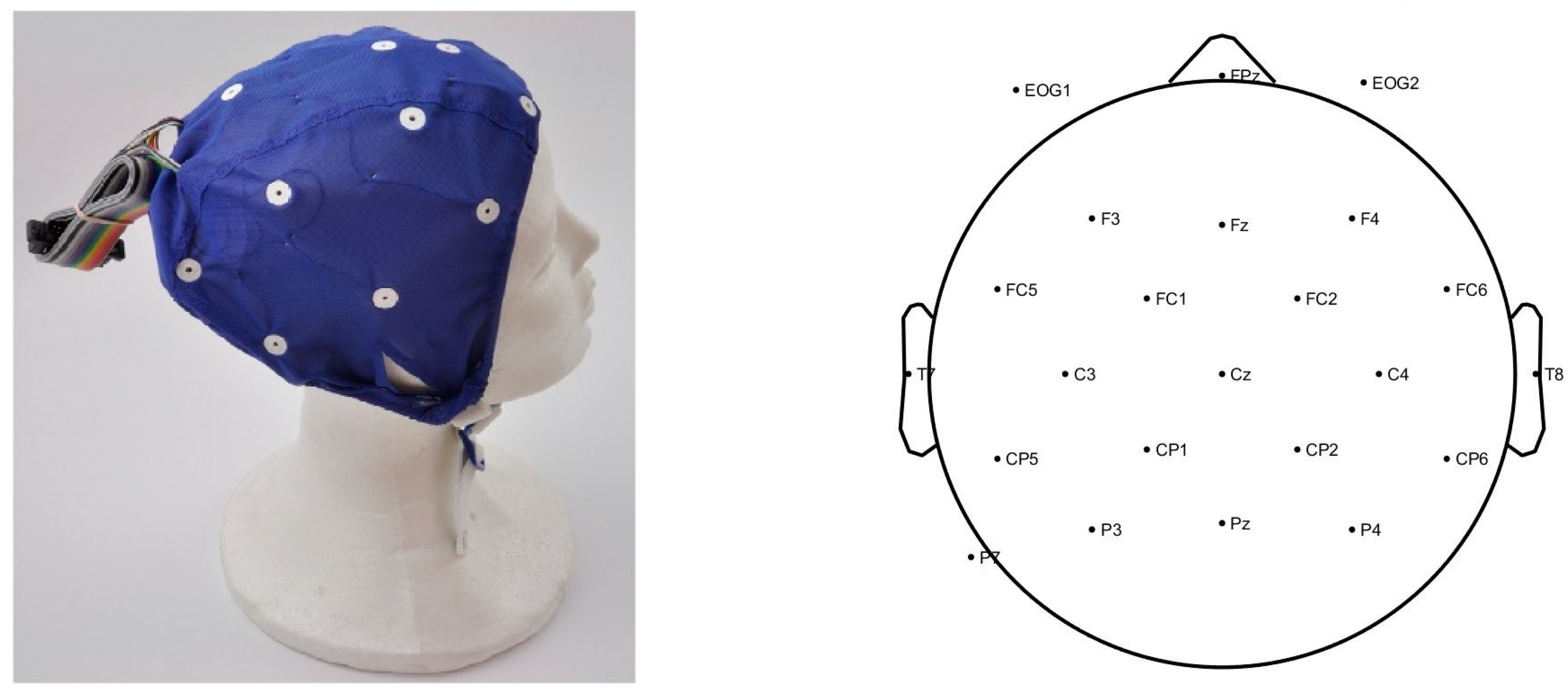
2.4. ERP Acquisition and Pre-Processing
2.5. Statistical Analysis
3. Results
3.1. Behavioral Results
3.2. ERP Results
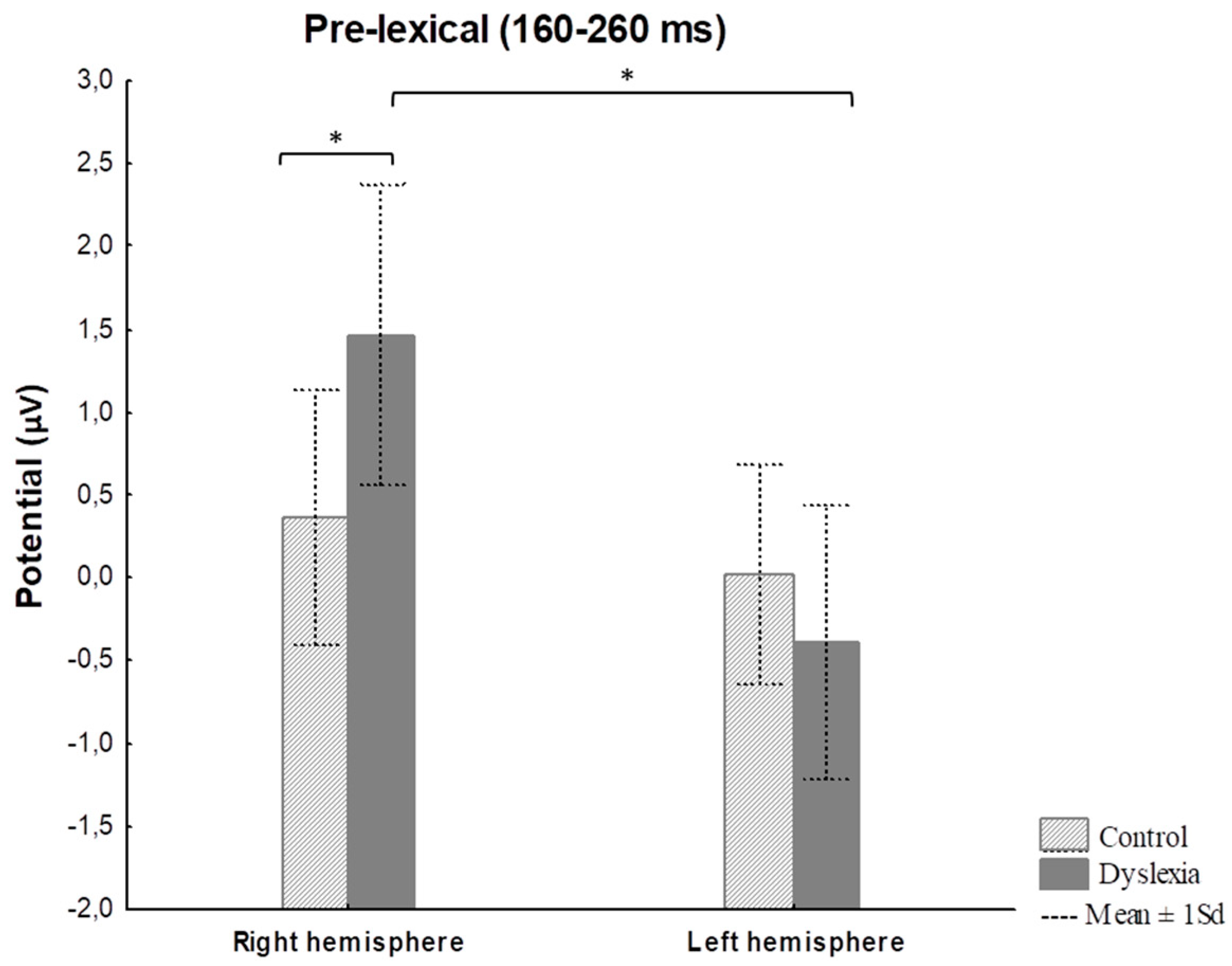
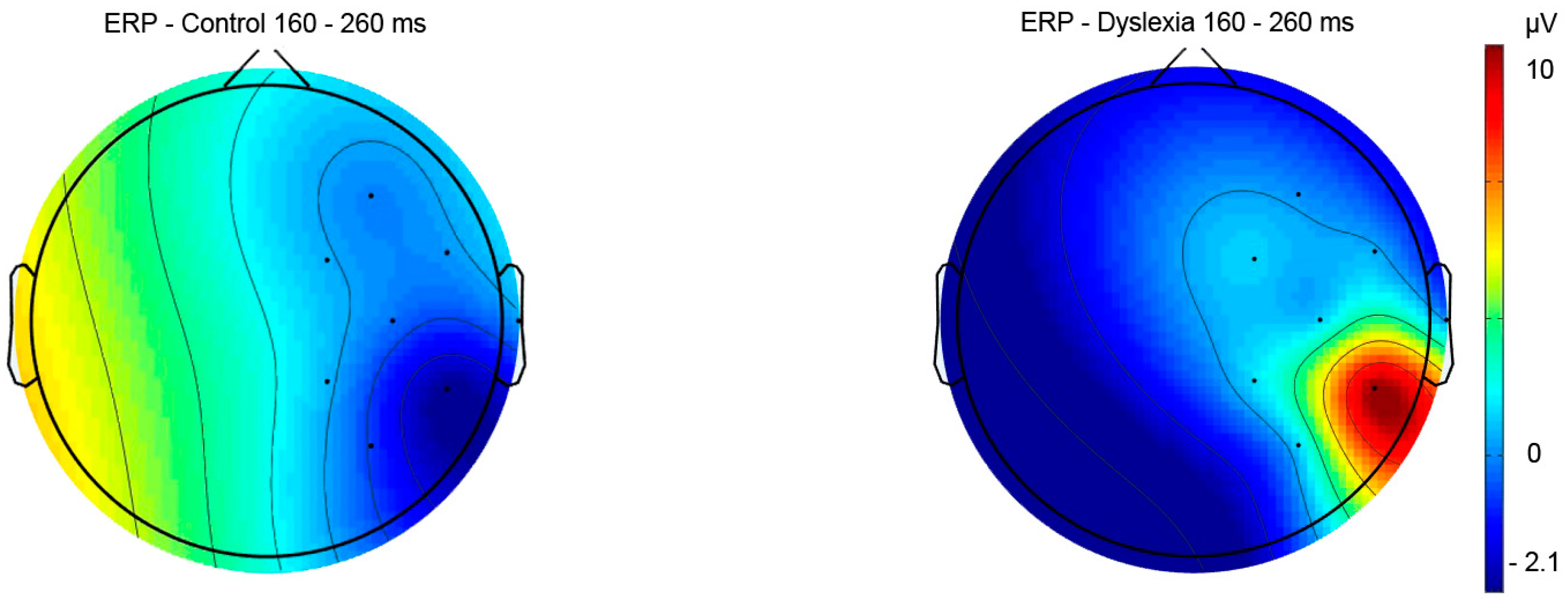
3.2.1. Reading Task—ERP Results
3.2.2. Naming Task—ERP Results
4. Discussion
4.1. Overt Reading of Words and ERP
4.2. Overt Visual Object Naming and ERP
4.3. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soriano-Ferrer, M.; Piedra Martínez, E. A review of the neurobiological basis of dyslexia in the adult population. Neurologia 2017, 32, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, S.E.; Shaywitz, J.E.; Shaywitz, B.A. Dyslexia in the 21st century. Curr. Opin. Psychiatry 2021, 34, 80–86. [Google Scholar] [CrossRef] [PubMed]
- International Dyslexia Association. Available online: https://dyslexiaida.org/definition-of-dyslexia/ (accessed on 12 December 2023).
- Snowling, M.J.; Hulme, C.; Nation, K. Defining and understanding dyslexia: Past, present and future. Oxf. Rev. Educ. 2020, 46, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.; Juul, H.; Elbro, C. A national test of dyslexia. Ann. Dyslexia 2023, 73, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Thongseiratch, T.; Traipidok, P.; Charleowsak, P.; Kraiwong, T.; Geater, A.F. Development and diagnostic accuracy of dyslexia early identification flowchart for pediatric practice. Asian J. Psychiatr. 2023, 89, 103795. [Google Scholar] [CrossRef]
- Reynolds, A.E.; Caravolas, M. Evaluation of the Bangor Dyslexia Test (BDT) for use with Adults. Dyslexia 2016, 22, 27–46. [Google Scholar] [CrossRef]
- Hou, F.; Qi, L.; Liu, L.; Luo, X.; Gu, H.; Xie, X.; Li, X.; Zhang, J.; Song, R. Validity and Reliability of the Dyslexia Checklist for Chinese Children. Front. Psychol. 2018, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Jap, B.A.J.; Borleffs, E.; Maassen, B.A.M. Towards identifying dyslexia in Standard Indonesian: The development of a reading assessment battery. Read. Writ. 2017, 30, 1729–1751. [Google Scholar] [CrossRef]
- Tamboer, P.; Vorst, H.C. A new self-report inventory of dyslexia for students: Criterion and construct validity. Dyslexia 2015, 21, 1–34. [Google Scholar] [CrossRef]
- Denckla, M.B.; Rudel, R.G. Rapid a ‘utomatized’ naming (R.A.N): Dyslexia differentiated from other learning disabilities. Neuropsychologia 1976, 14, 471–479. [Google Scholar] [CrossRef]
- Wolf, M. Naming speed and reading: The contribution of the cognitive neurosciences. Read. Res. Q. 1991, 26, 123–141. [Google Scholar] [CrossRef]
- Majerus, S.; Cowan, N. The nature of verbal short-term impairment in dyslexia: The importance of serial order. Front. Psychol. 2016, 3, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Coslett, H.B. Localization of sublexical speech perception components. Brain Lang. 2010, 114, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Felton, R.H.; Naylor, C.E.; Wood, F.B. Neuropsychological profile of adult dyslexics. Brain Lang. 1990, 39, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Avons, S.E.; Hanna, C. The memory-span deficit in children with specific reading disability: Is speech rate responsible? Br. J. Dev. Psychol. 1995, 13, 303–311. [Google Scholar] [CrossRef]
- Snowling, M.J.; Goulandris, N.; Defty, N. A longitudinal study of reading development in dyslexic children. J. Educ. Psychol. 1996, 88, 653–669. [Google Scholar] [CrossRef]
- Navas, A.L.; FerrazÉde, C.; Borges, J.P. Phonological processing deficits as a universal model for dyslexia: Evidence from different orthographies. Codas 2014, 26, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Coltheart, M.; Rastle, K.; Perry, C.; Langdon, R.; Ziegler, J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol. Rev. 2001, 108, 204–256. [Google Scholar] [CrossRef]
- Indefrey, P. The spatial and temporal signatures of word production components: A critical update. Front. Psychol. 2011, 2, 255. [Google Scholar] [CrossRef]
- Indefrey, P.; Levelt, W.J. The spatial and temporal signatures of word production components. Cognition 2004, 92, 101–144. [Google Scholar] [CrossRef]
- Levelt, W.J. Models of word production. Trends Cogn. Sci. 1999, 3, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.S.; Rastle, K.; Davis, M.H. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol. Bull. 2013, 139, 766–791. [Google Scholar] [CrossRef]
- Hauk, O.; Coutout, C.; Holden, A.; Chen, Y. The time-course of single word reading: Evidence from fast behavioral and brain responses. Neuroimage 2012, 60, 1462–1477. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Frost, S.J.; Menc, W.E.; Sandak, R.; Pugh, K.R. Neurobiological bases of reading comprehension: Insights from neuroimaging studies of word level and text level processing in skilled and impaired readers. Read. Writ. Q. 2013, 29, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Harm, M.W.; Seidenberg, M.S. Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychol. Rev. 2004, 111, 662–720. [Google Scholar] [CrossRef]
- Perry, C.; Ziegler, J.C.; Zorzi, M. Nested incremental modeling in the development of computational theories: The CDP+ model of reading aloud. Psychol. Rev. 2007, 114, 273–315. [Google Scholar] [CrossRef]
- Alario, F.X.; Ferrand, L.; Laganaro, M.; New, B.; Frauenfelder, U.H.; Segui, J. Predictors of picture naming speed. Behav. Res. Methods Instrum. Comput. 2004, 36, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.; Morrison, C.M.; Ellis, A.W. Naming the Snodgrass and Vanderwart Pictures: Effects of Age of Acquisition, Frequency, and Name Agreement. Q. J. Exp. Psychol. Sect. A 1997, 50, 560–585. [Google Scholar] [CrossRef]
- Caravolas, M.; Volín, J.; Hulme, C. Phoneme awareness is a key component of alphabetic literacy skills in consistent and inconsistent orthographies: Evidence from Czech and English children. J. Exp. Child. Psychol. 2005, 92, 107–139. [Google Scholar] [CrossRef]
- de Jong, P.F.; van der Leij, A. Specific contributions of phonological abilities to early reading acquisition: Results from a Dutch latent variable longitudinal study. J. Educ. Psychol. 1999, 91, 450–476. [Google Scholar] [CrossRef]
- Georgiou, G.K.; Torppa, M.; Landerl, K.; Desrochers, A.; Manolitsis, G.; de Jong, P.F.; Parrila, R. Reading and Spelling Development Across Languages Varying in Orthographic Consistency: Do Their Paths Cross? Child. Dev. 2020, 91, e266–e279. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, H.; Mayringer, H.; Landerl, K. The double-deficit hypothesis and difficulties in learning to read a regular orthography. J. Educ. Psychol. 2000, 92, 668–680. [Google Scholar] [CrossRef]
- Trauzettel-Klosinski, S.; Dürrwächter, U.; Klosinski, G.; Braun, C. Cortical activation during word reading and picture naming in dyslexic and non-reading-impaired children. Clin. Neurophysiol. 2006, 117, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, F.; Meyler, A.; Hernandez, A.; Juel, C.; Taylor-Hill, H.; Martindale, J.L.; McMillon, G.; Kolchugina, G.; Black, J.M.; Faizi, A.; et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. USA 2007, 104, 4234–4239. [Google Scholar] [CrossRef] [PubMed]
- McCrory, E.J.; Mechelli, A.; Frith, U.; Price, C.J. More than words: A common neural basis for reading and naming deficits in developmental dyslexia? Brain 2005, 128 Pt 2, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Paulesu, E.; Frith, C.D.; Frackowiak, R.S. The neural correlates of the verbal component of working memory. Nature 1993, 362, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Structural abnormalities in the dyslexic brain: A meta-analysis of voxel-based morphometry studies. Hum. Brain Mapp. 2013, 34, 3055–3065. [Google Scholar] [CrossRef] [PubMed]
- Saygin, Z.M.; Norton, E.S.; Osher, D.E.; Beach, S.D.; Cyr, A.B.; Ozernov-Palchik, O.; Yendiki, A.; Fischl, B.; Gaab, N.; Gabrieli, J.D. Tracking the roots of reading ability: White matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013, 33, 13251–13258. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.; Faísca, L.; Bramão, I.; Reis, A.; Petersson, K.M. Lexical and sublexical orthographic processing: An ERP study with skilled and dyslexic adult readers. Brain Lang. 2015, 141, 16–27. [Google Scholar] [CrossRef]
- Pugh, K.R.; Mencl, W.E.; Jenner, A.R.; Katz, L.; Frost, S.J.; Lee, J.R.; Shaywitz, S.E.; Shaywitz, B.A. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 207–213. [Google Scholar] [CrossRef]
- Shaywitz, B.A.; Shaywitz, S.E.; Blachman, B.A.; Pugh, K.R.; Fulbright, R.K.; Skudlarski, P.; Mencl, W.E.; Constable, R.T.; Holahan, J.M.; Marchione, K.E.; et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biol. Psychiatry 2004, 55, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Cohen, L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011, 15, 254–262. [Google Scholar] [CrossRef]
- Norton, E.S.; Beach, S.D.; Gabrieli, J.D. Neurobiology of dyslexia. Curr. Opin. Neurobiol. 2015, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F.; Altarelli, I.; Jednoróg, K.; Zhao, J.; Scotto di Covella, L. Neuroanatomy of developmental dyslexia: Pitfalls and promise. Neurosci. Biobehav. Rev. 2018, 84, 434–452. [Google Scholar] [CrossRef]
- Kim, S.K. Recent update on reading disability (dyslexia) focused on neurobiology. Clin. Exp. Pediatr. 2021, 64, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vásquez, R.; Córdova García, U.; Barreto, A.M.B.; Rojas, M.L.R.; Ponce-Meza, J.; Saavedra-López, M. An Overview on Electrophysiological and Neuroimaging Findings in Dyslexia. Iran. J. Psychiatry 2023, 18, 503–509. [Google Scholar] [CrossRef]
- Cainelli, E.; Vedovelli, L.; Carretti, B.; Bisiacchi, P. EEG correlates of developmental dyslexia: A systematic review. Ann. Dyslexia 2023, 73, 184–213. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.S.; Shen, X.; Holahan, J.M.; Scheinost, D.; Lacadie, C.; Papademetris, X.; Shaywitz, S.E.; Shaywitz, B.A.; Constable, R.T. Disruption of functional networks in dyslexia: A whole-brain, data-driven analysis of connectivity. Biol. Psychiatry 2014, 76, 397–404. [Google Scholar] [CrossRef]
- Shaywitz, B.A.; Shaywitz, S.E.; Pugh, K.R.; Mencl, W.E.; Fulbright, R.K.; Skudlarski, P.; Constable, R.T.; Marchione, K.E.; Fletcher, J.M.; Lyon, G.R.; et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry 2002, 52, 101–110. [Google Scholar] [CrossRef]
- Pagnotta, M.F.; Zouridakis, G.; Li, L.; Lizarazu, M.; Lallier, M.; Molinaro, N.; Carreiras, M. Low frequency overactivation in dyslexia: Evidence from resting state Magnetoencephalography. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6959–6962. [Google Scholar]
- Paz-Alonso, P.M.; Oliver, M.; Lerma-Usabiaga, G.; Caballero-Gaudes, C.; Quiñones, I.; Suárez-Coalla, P.; Duñabeitia, J.A.; Cuetos, F.; Carreiras, M. Neural correlates of phonological, orthographic and semantic reading processing in dyslexia. Neuroimage Clin. 2018, 20, 433–447. [Google Scholar] [CrossRef]
- Chiarenza, G.A.; Olgiati, P.; Trevisan, C.; Marchi, I.D.; Casarotto, S. Reading aloud: A psychophysiological investigation in children. Neuropsychologia 2013, 51, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Bakos, S.; Mehlhase, H.; Landerl, K.; Bartling, J.; Schulte-Körne, G.; Moll, K. Naming processes in reading and spelling disorders: An electrophysiological investigation. Clin. Neurophysiol. 2020, 131, 351–360. [Google Scholar] [CrossRef]
- Ganushchak, L.Y.; Christoffels, I.K.; Schiller, N.O. The use of electroencephalography in language production research: A review. Front. Psychol. 2011, 202, 208. [Google Scholar] [CrossRef] [PubMed]
- Mahé, G.; Pont, C.; Zesiger, P.; Laganaro, M. The electrophysiological correlates of developmental dyslexia: New insights from lexical decision and reading aloud in adults. Neuropsychologia 2018, 121, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Strijkers, K.; Holcomb, P.J.; Costa, A. Conscious intention to speak proactively facilitates lexical access during overt object naming. J. Mem. Lang. 2012, 65, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.; Fize, D.; Marlot, C. Speed of processing in the human visual system. Nature 1996, 381, 520. [Google Scholar] [CrossRef] [PubMed]
- Strijkers, K.; Costa, A. Riding the lexical speedway: A critical review on the time course of lexical selection in speech production. Front. Psychol. 2011, 2, 356. [Google Scholar] [CrossRef]
- Dirani, J.; Pylkkänen, L. The time course of cross-modal representations of conceptual categories. Neuroimage 2023, 277, 120254. [Google Scholar] [CrossRef]
- Stephan, F.; Saalbach, H.; Rossi, S. The Brain Differentially Prepares Inner and Overt Speech Production: Electrophysiological and Vascular Evidence. Brain Sci. 2020, 10, 148. [Google Scholar] [CrossRef]
- Strijkers, K.; Costa, A.; Pulvermüller, F. The cortical dynamics of speaking: Lexical and phonological knowledge simultaneously recruit the frontal and temporal cortex within 200 ms. Neuroimage 2017, 163, 206–219. [Google Scholar] [CrossRef]
- Eulitz, C.; Hauk, O.; Cohen, R. Electroencephalographic activity over temporal brain areas during phonological encoding in picture naming. Clin. Neurophysiol. 2000, 111, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Maguire, M.J.; Sizemore, M.L. Neural patterns elicited by lexical processing in adolescents with specific language impairment: Support for the procedural deficit hypothesis? J. Neurodev. Disord. 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Paulitzki, J.R.; Risko, E.F.; O’Malley, S.; Stolz, J.A.; Besner, D. On the role of set when reading aloud: A dissociation between prelexical and lexical processing. Conscious. Cogn. 2009, 18, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.M.; Münte, T.F.; Kutas, M. Electrophysiological estimates of the time course of semantic and phonological encoding during implicit picture naming. Psychophysiology 2000, 37, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Strijkers, K.; Martin, C.; Thierry, G. The time course of word retrieval revealed by event-related brain potentials during overt speech. Proc. Natl. Acad. Sci. USA 2009, 106, 21442–21446. [Google Scholar] [CrossRef] [PubMed]
- Graves, W.W.; Grabowski, T.J.; Mehta, S.; Gordon, J.K. A neural signature of phonological access: Distinguishing the effects of word frequency from familiarity and length in overt picture naming. J. Cogn. Neurosci. 2007, 19, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Kan, I.P.; Thompson-Schill, S.L. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cogn. Affect. Behav. Neurosci. 2004, 4, 43–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koester, D.; Schiller, N.O. Morphological priming in overt language production: Electrophysiological evidence from Dutch. Neuroimage 2008, 42, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Krott, A.; Medaglia, M.T.; Porcaro, C. Early and Late Effects of Semantic Distractors on Electroencephalographic Responses During Overt Picture Naming. Front. Psychol. 2019, 10, 696. [Google Scholar] [CrossRef]
- Piai, V.; Roelofs, A.; van der Meij, R. Event-related potentials and oscillatory brain responses associated with semantic and Stroop-like interference effects in overt naming. Brain Res. 2012, 1450, 87–101. [Google Scholar] [CrossRef]
- Verhoef, K.; Roelofs, A.; Chwilla, D.J. Role of inhibition in language switching: Evidence from event-related brain potentials in overt picture naming. Cognition 2009, 110, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, K.M.; Roelofs, A.; Chwilla, D.J. Electrophysiological evidence for endogenous control of attention in switching between languages in overt picture naming. J. Cogn. Neurosci. 2010, 22, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Schiller, N.O. Lexico-syntactic features are activated but not selected in bare noun production: Electrophysiological evidence from overt picture naming. Cortex 2019, 116, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Taylor, K.I.; Devereux, B.; Randall, B.; Tyler, L.K. From perception to conception: How meaningful objects are processed over time. Cereb. Cortex 2013, 23, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Taylor, K.I.; Tyler, L.K. The evolution of meaning: Spatio-temporal dynamics of visual object recognition. J. Cogn. Neurosci. 2011, 23, 1887–1899. [Google Scholar] [CrossRef]
- Scholl, C.A.; Jiang, X.; Martin, J.G.; Riesenhuber, M. Time course of shape and category selectivity revealed by EEG rapid adaptation. J. Cogn. Neurosci. 2014, 26, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Branzi, F.M.; Martin, C.D.; Biau, E. Activating words without language: Beta and theta oscillations reflect lexical access and control processes during verbal and non-verbal object recognition tasks. Cereb. Cortex 2023, 33, 6228–6240. [Google Scholar] [CrossRef]
- Bermúdez-Margaretto, B.; Beltrán, D.; Domínguez, A.; Cuetos, F. Repeated Exposure to “meaningless” Pseudowords Modulates LPC, but Not N(FN)400. Brain Topogr. 2015, 28, 838–851. [Google Scholar] [CrossRef]
- Rüsseler, J.; Probst, S.; Johannes, S.; Münte, T. Recognition memory for high- and low-frequency words in adult normal and dyslexic readers: An event-related brain potential study. J. Clin. Exp. Neuropsychol. 2003, 25, 815–829. [Google Scholar] [CrossRef]
- Perfetti, C.A.; Wlotko, E.W.; Hart, L.A. Word learning and individual differences in word learning reflected in event-related potentials. J. Exp. Psychol. Learn. Mem. Cogn. 2005, 31, 1281–1292. [Google Scholar] [CrossRef]
- Liotti, M.; Woldorff, M.G.; Perez, R.; Mayberg, H.S. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia 2000, 38, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Araújo, S.; Morais, I.S.; Faísca, L. Reading and reading-related skills in adults with dyslexia from different orthographic systems: A review and meta-analysis. Ann. Dyslexia 2020, 70, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Salmelin, R.; Hari, R.; Lounasmaa, O.V.; Sams, M. Dynamics of brain activation during picture naming. Nature 1994, 368, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Mahé, G.; Zesiger, P.; Laganaro, M. Beyond the initial 140 ms, lexical decision and reading aloud are different tasks: An ERP study with topographic analysis. Neuroimage 2015, 122, 65–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahé, G.; Bonnefond, A.; Gavens, N.; Dufour, A.; Doignon-Camus, N. Impaired visual expertise for print in French adults with dyslexia as shown by N170 tuning. Neuropsychologia 2012, 50, 3200–3206. [Google Scholar] [CrossRef]
- Amora, K.K.; Tretow, A.; Verwimp, C.; Tijms, J.; Leppänen, P.H.T.; Csépe, V. Typical and Atypical Development of Visual Expertise for Print as Indexed by the Visual Word N1 (N170w): A Systematic Review. Front. Neurosci. 2022, 16, 898800. [Google Scholar] [CrossRef]
- Silva, P.B.; Oliveira, D.G.; Cardoso, A.D.; Laurence, P.G.; Boggio, P.S.; Macedo, E.C. Event-related potential and lexical decision task in dyslexic adults: Lexical and lateralization effects. Front. Psychol. 2022, 13, 852219. [Google Scholar] [CrossRef]
- Helenius, P.; Parviainen, T.; Paetau, R.; Salmelin, R. Neural processing of spoken words in specific language impairment and dyslexia. Brain 2009, 132 Pt 7, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Premeti, A.; Bucci, M.P.; Isel, F. Evidence from ERP and Eye Movements as Markers of Language Dysfunction in Dyslexia. Brain Sci. 2022, 12, 73. [Google Scholar] [CrossRef]
- Egan, C.; Payne, J.S.; Jones, M.W. The impact of phonological relatedness on semantic congruency judgements in readers with dyslexia: Evidence from behavioural judgements, event related potentials and pupillometry. Neuropsychologia 2023, 184, 108548. [Google Scholar] [CrossRef]
- Friedman, D.; Johnson, R., Jr. Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc. Res. Tech. 2000, 51, 6–28. [Google Scholar] [CrossRef]
- Emerson, S.N.; Conway, C.M.; Özçalışkan, Ş. Semantic P600-but not N400-effects index crosslinguistic variability in speakers’ expectancies for expression of motion. Neuropsychologia 2020, 149, 107638. [Google Scholar] [CrossRef] [PubMed]
- Wachinger, C.; Volkmer, S.; Bublath, K.; Bruder, J.; Bartling, J.; Schulte-Körne, G. Does the late positive component reflect successful reading acquisition? A longitudinal ERP study. Neuroimage Clin. 2017, 17, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Riès, S.; Legou, T.; Burle, B.; Alario, F.X.; Malfait, N. Corrigendum to “Why does picture naming take longer than word naming? The contribution of articulatory processes”. Psychon. Bull. Rev. 2015, 22, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; McCrory, E.; Noppeney, U.; Mechelli, A.; Moore, C.J.; Biggio, N.; Devlin, J.T. How reading differs from object naming at the neuronal level. Neuroimage 2006, 29, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Aristei, S.; Melinger, A.; Abdel Rahman, R. Electrophysiological chronometry of semantic context effects in language production. J. Cogn. Neurosci. 2011, 23, 1567–1586. [Google Scholar] [CrossRef] [PubMed]
- Schendan, H.E.; Maher, S.M. Object knowledge during entry-level categorization is activated and modified by implicit memory after 200 ms. Neuroimage 2009, 44, 1423–1438. [Google Scholar] [CrossRef]
- Schendan, H.E.; Lucia, L.C. Object-sensitive activity reflects earlier perceptual and later cognitive processing of visual objects between 95 and 500 ms. Brain Res. 2010, 1329, 124–141. [Google Scholar] [CrossRef]
- von Seth, J.; Nicholls, V.I.; Tyler, L.K.; Clarke, A. Recurrent connectivity supports higher-level visual and semantic object representations in the brain. Commun. Biol. 2023, 6, 1207. [Google Scholar] [CrossRef]
- Wong, A.W.; Wang, J.; Ng, T.Y.; Chen, H.C. Syllabic encoding during overt speech production in Cantonese: Evidence from temporal brain responses. Brain Res. 2016, 1648 Pt A, 101–109. [Google Scholar] [CrossRef]
- Laganaro, M.; Valente, A.; Perret, C. Time course of word production in fast and slow speakers: A high density ERP topographic study. Neuroimage 2012, 59, 3881–3888. [Google Scholar] [CrossRef] [PubMed]
- Kast, M.; Elmer, S.; Jancke, L.; Meyer, M. ERP differences of pre-lexical processing between dyslexic and non-dyslexic children. Int. J. Psychophysiol. 2010, 77, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Mayseless, N.; Breznitz, Z. Brain activity during processing objects and pseudo-objects: Comparison between adult regular and dyslexic readers. Clin. Neurophysiol. 2011, 122, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Sučević, Đ.; Momirović, A.; Goran Fruk, G.; Auguštin, B. Kognitivni Neverbalni Test—KNT; Naklada Slap: Zagreb, Croatia, 2004. [Google Scholar]
- Rossion, B.; Pourtois, G. Revisiting Snodgrass and Vanderwart’s object pictorial set: The role of surface detail in basic-level object recognition. Perception 2004, 33, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Corel GalleryTMMagic 65,000 Software, version revised September 1998; Corel Corporation: Ottawa, Canada, 1998.
- Moguš, M.; Bratanić, M.; Tadić, M. Hrvatski Čestotni Rječnik; Školska knjiga d.d.; Zavod za lingvistiku Filozofskog fakulteta Sveučilišta u Zagrebu: Zagrebu, Croatia, 1999. [Google Scholar]
- Lenček, M. Assessment of Dyslexia in Croatian: Some Characteristics of Reading and Writing in Students with Dyslexia. Hrvat. Rev. Za Rehabil. Istraživanja 2012, 48, 11–26. [Google Scholar]
- Presentation® Software, Version 20.0; Neurobehavioral Systems, Inc.: Berkeley, CA, USA, 2017. Available online: www.neurobs.com (accessed on 15 January 2018).
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- MATLAB, version: 9.4.0.949201 (R2018a); The MathWorks Inc.: Natick, MA, USA, 2018.
- Makeig, S.; Bell, A.J.; Jung, T.P.; Sejnowski, T.J. Independent component analysis of electroencephalographic data. Adv. Neural Inf. Process Syst. 1996, 8, 145–151. [Google Scholar]
- Stekić, K.; Ilić, O.; Ković, V.; Savić, A.M. ERP Indicators of Phonological Awareness Development in Children: A Systematic Review. Brain Sci. 2023, 13, 290. [Google Scholar] [CrossRef]
- Basma, B.; Savage, R.; Bertone, A. The N400 in readers with dyslexia: A systematic review and meta-analysis. Int. J. Psychophysiol. 2024, 196, 112283. [Google Scholar] [CrossRef]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer [Computer Program]. Version 5.3.56. Available online: http://www.praat.org/ (accessed on 2 May 2023).
- Carioti, D.; Masia, M.F.; Travellini, S.; Berlingeri, M. Orthographic depth and developmental dyslexia: A meta-analytic study. Ann. Dyslexia 2021, 71, 399–438. [Google Scholar] [CrossRef] [PubMed]
- Bentin, S.; Mouchetant-Rostaing, Y.; Giard, M.H.; Echallier, J.F.; Pernier, J. ERP manifestations of processing printed words at different psycholinguistic levels: Time course and scalp distribution. J. Cogn. Neurosci. 1999, 11, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, T.; Etienne, Y.; Contentin, C.; Bernard, C.; Largy, P.; Mellier, D.; Lalonde, R.; Rebaï, M. Behavioral performances in participants with phonological dyslexia and different patterns on the N170 component. Brain Cogn. 2011, 75, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vitevitch, M.S.; Luce, P.A. Phonological neighborhood effects in spoken word perception and production. Annu. Rev. Linguist. 2016, 2, 75–94. [Google Scholar] [CrossRef]
- Araújo, S.; Fernandes, T.; Huettig, F. Learning to read facilitates the retrieval of phonological representations in rapid automatized naming: Evidence from unschooled illiterate, ex-illiterate, and schooled literate adults. Dev. Sci. 2019, 22, e12783. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, A.K.; Närhi, V.M.; Eklund, K.M.; Ahonen, T.P.S.; Aro, T.I. Resolving reading disability-Childhood predictors and adult-age outcomes. Dyslexia 2019, 25, 20–37. [Google Scholar] [CrossRef]
- Valdois, S. The visual-attention span deficit in developmental dyslexia: Review of evidence for a visual-attention-based deficit. Dyslexia 2022, 28, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Werth, R. Dyslexia Due to Visual Impairments. Biomedicines 2023, 11, 2559. [Google Scholar] [CrossRef] [PubMed]
- Premeti, A.; Bucci, M.P.; Heidlmayr, K.; Vigneron, P.; Isel, F. Neurodynamics of selected language processes involved in word reading: An EEG study with French dyslexic adults. J. Neurolinguist. 2024, 71, 101201. [Google Scholar] [CrossRef]
- Denis-Noël, A.; Colé, P.; Bolger, D.; Pattamadilok, C. How do adults with dyslexia recognize spoken words? Evidence from behavioral and EEG data. Sci. Stud. Read. 2024, 28, 21–41. [Google Scholar] [CrossRef]
- Perera, H.; Shiratuddin, M.F.; Wong, K.W.; Fullarton, K. EEG signal analysis of passage reading and rapid automatized naming between adults with dyslexia and normal controls. In Proceedings of the 8th IEEE International Conference on Software Engineering and Service Science, Beijing, China, 24–26 November 2017; pp. 104–108. [Google Scholar]
- Zoccolotti, P. Success is not the entire story for a scientific theory: The case of the Phonological Deficit Theory of dyslexia. Brain Sci. 2022, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Werth, R. Dyslexia: Causes and Concomitant Impairments. Brain Sci. 2023, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Mousikou, P.; Rastle, K. Lexical frequency effects on articulation: A comparison of picture naming and reading aloud. Front. Psychol. 2015, 15, 1571. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.; Faísca, L.; Reis, A.; Marques, J.F.; Petersson, K.M. Visual naming deficits in dyslexia: An ERP investigation of different processing domains. Neuropsychologia 2016, 91, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Rugg, M.D.; Curran, T. Event-related potentials and recognition memory. Trends Cogn. Sci. 2007, 11, 251–257. [Google Scholar] [CrossRef]
- Azizian, A.; Watson, T.D.; Parvaz, M.A.; Squires, N.K. Time course of processes underlying picture and word evaluation: An event-related potential approach. Brain Topogr. 2006, 18, 213–222. [Google Scholar] [CrossRef]
- Schulte-Körne, G.; Deimel, W.; Bartling, J.; Remschmidt, H. Neurophysiological correlates of word recognition in dyslexia. J. Neural Transm. 2004, 111, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Hasko, S.; Groth, K.; Bruder, J.; Bartling, J.; Schulte-Körne, G. The time course of reading processes in children with and without dyslexia: An ERP study. Front. Hum. Neurosci. 2013, 7, 1–19. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Zani, A. Time course of brain activation during graphemic/phonologic processing in reading: An ERP study. Brain Lang. 2003, 87, 412–420. [Google Scholar] [CrossRef]



| Qualitative Errors | Control n(%) | Dyslexia n(%) | χ2 | p | |
|---|---|---|---|---|---|
| Reading | No response | 0 (0) | 3 (0.39) | 3.01 | 0.08 |
| Semantic error | 0 (0) | 0 (0) | - | - | |
| Phonological error | 3 (0.39) | 11 (1.43) | 4.61 | 0.03 * | |
| Hesitation | 1 (0.13) | 5 (0.65) | 2.68 | 0.10 | |
| Total | 4 (0.52) | 19 (2.47) | 9.93 | 0.002 | |
| Visual object naming | No response | 13 (1.72) | 77 (10.02) | 48.34 | <0.001 |
| Semantic error | 125 (16.28) | 121 (15.75) | 0.08 | 0.78 | |
| Phonological error | 14 (1.82) | 26 (3.38) | 3.70 | 0.05 | |
| Hesitation | 37 (4.81) | 49 (6.38) | 1.77 | 0.18 | |
| Total | 189 (24.60) | 273 (35.54) | 21.84 | <0.001 |
| Control | Dyslexia | t | p | |
|---|---|---|---|---|
| M ± SD | M ± SD | |||
| Reading latency | 663.63 ± 37.09 | 892.89 ± 96.05 | 17.81 | <0.001 |
| Naming latency | 794.44 ± 298.75 | 803.38 ± 302.03 | 0.16 | 0.86 |
| Time Window | Source of Variance | SS | F | p | Post hoc Test |
|---|---|---|---|---|---|
| Pre-lexical 160–260 ms | Group | 8.64 | 0.54 | 0.46 | HCright-PDright p = 0.02 PDright-PDleft p < 0.001 |
| Hemisphere | 88.56 | 13.09 | <0.001 | ||
| Group x Hemisphere | 41.59 | 4.79 | 0.03 | ||
| Lexical 450–700 ms | Group | 0.23 | 0.02 | 0.88 | HCright-HCleft p = 0.01 PDright-PDleft p = 0.01 |
| Hemisphere | 86.32 | 12.50 | <0.001 | ||
| Group x Hemisphere | 0.01 | 0.002 | 0.96 | ||
| Post-lexical 750–900 ms | Group | 0.04 | 0.004 | 0.94 | HCright-HCleft p = 0.004 |
| Hemisphere | 57.88 | 6.82 | 0.009 | ||
| Group x Hemisphere | 15.85 | 2.07 | 0.15 |
| Time Window | Source of Variance | SS | F | p | Post hoc |
|---|---|---|---|---|---|
|
Pre-lexical 150–200 ms | Group | 8.07 | 0.32 | 0.56 |
HCright-HCleft p = 0.04 PDright-PDleft p < 0.001 |
| Hemisphere | 220.37 | 17.03 | <0.001 | ||
| Group x Hemisphere | 25.27 | 2.12 | 0.14 | ||
|
Lexical 280–440 ms | Group | 1.68 | 0.08 | 0.77 | PDright-PDleft p = 0.02 |
| Hemisphere | 77.05 | 7.94 | 0.006 | ||
| Group x Hemisphere | 0.80 | 0.08 | 0.76 | ||
|
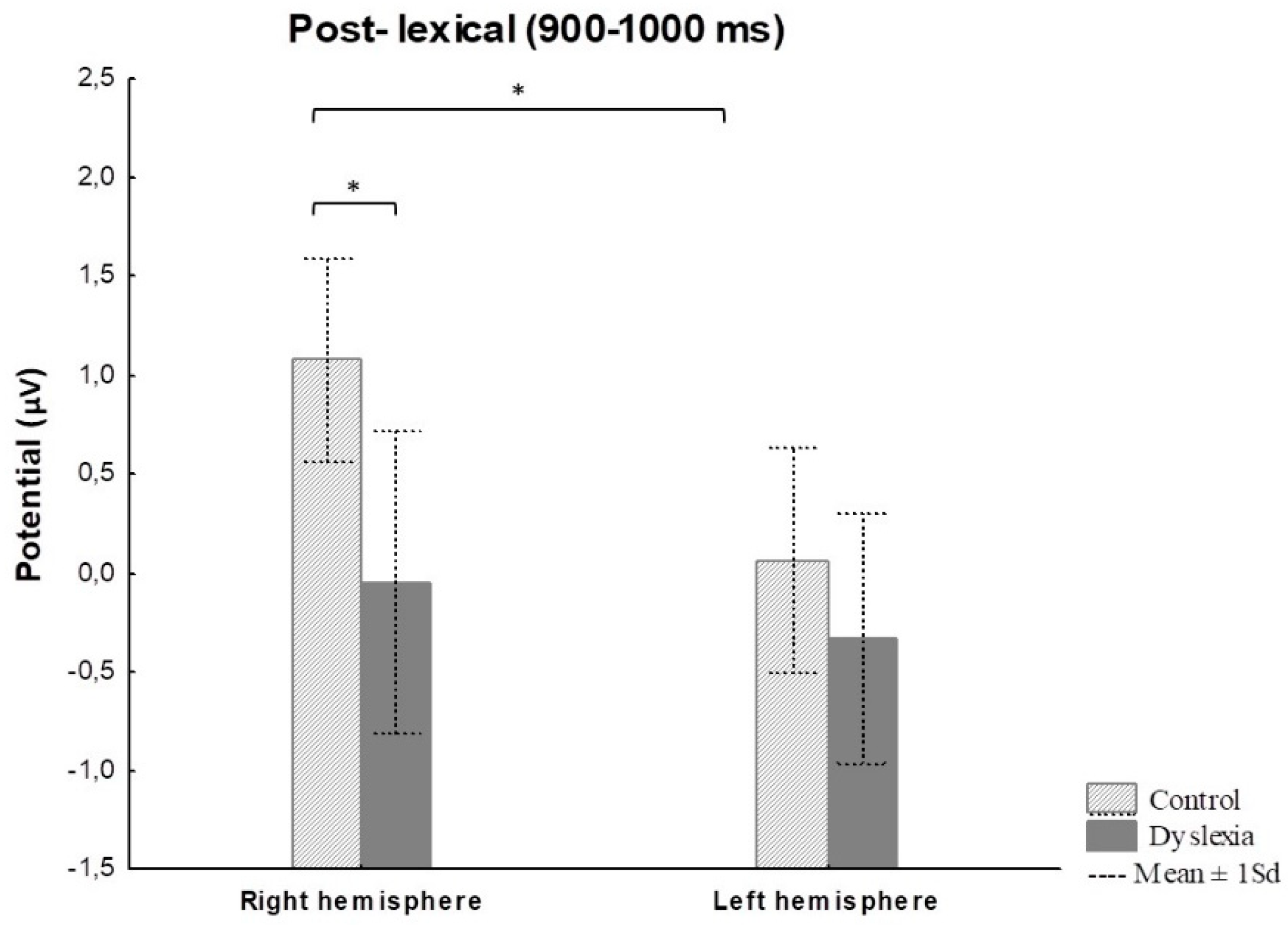
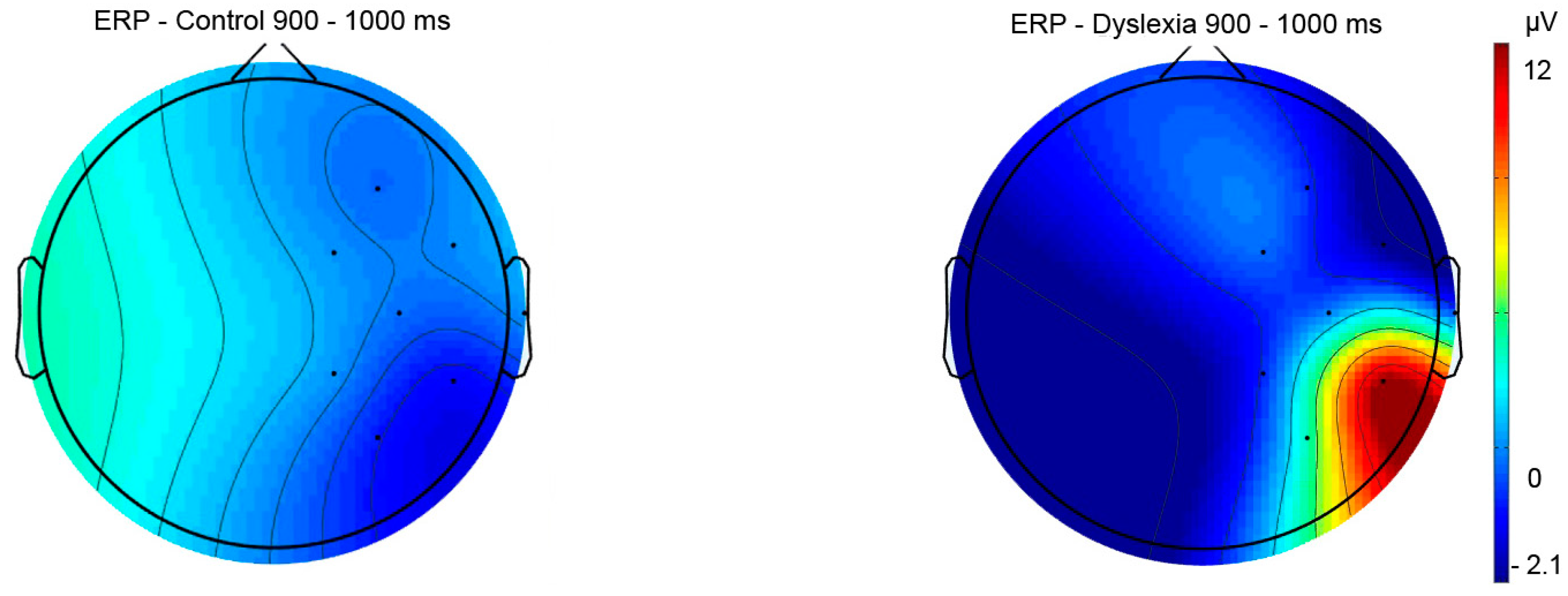
Post-lexical 900–1000 ms | Group | 41.77 | 6.19 | 0.01 |
HCright-HCleft p = 0.04 HCright-PDright p = 0.02 |
| Hemisphere | 30.87 | 5.62 | 0.02 | ||
| Group x Hemisphere | 9.83 | 1.44 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkušić Čović, M.; Vujović, I.; Šoda, J.; Palmović, M.; Rogić Vidaković, M. Overt Word Reading and Visual Object Naming in Adults with Dyslexia: Electroencephalography Study in Transparent Orthography. Bioengineering 2024, 11, 459. https://doi.org/10.3390/bioengineering11050459
Perkušić Čović M, Vujović I, Šoda J, Palmović M, Rogić Vidaković M. Overt Word Reading and Visual Object Naming in Adults with Dyslexia: Electroencephalography Study in Transparent Orthography. Bioengineering. 2024; 11(5):459. https://doi.org/10.3390/bioengineering11050459
Chicago/Turabian StylePerkušić Čović, Maja, Igor Vujović, Joško Šoda, Marijan Palmović, and Maja Rogić Vidaković. 2024. "Overt Word Reading and Visual Object Naming in Adults with Dyslexia: Electroencephalography Study in Transparent Orthography" Bioengineering 11, no. 5: 459. https://doi.org/10.3390/bioengineering11050459
APA StylePerkušić Čović, M., Vujović, I., Šoda, J., Palmović, M., & Rogić Vidaković, M. (2024). Overt Word Reading and Visual Object Naming in Adults with Dyslexia: Electroencephalography Study in Transparent Orthography. Bioengineering, 11(5), 459. https://doi.org/10.3390/bioengineering11050459







