A Cross-Stage Partial Network and a Cross-Attention-Based Transformer for an Electrocardiogram-Based Cardiovascular Disease Decision System
Abstract
:1. Introduction
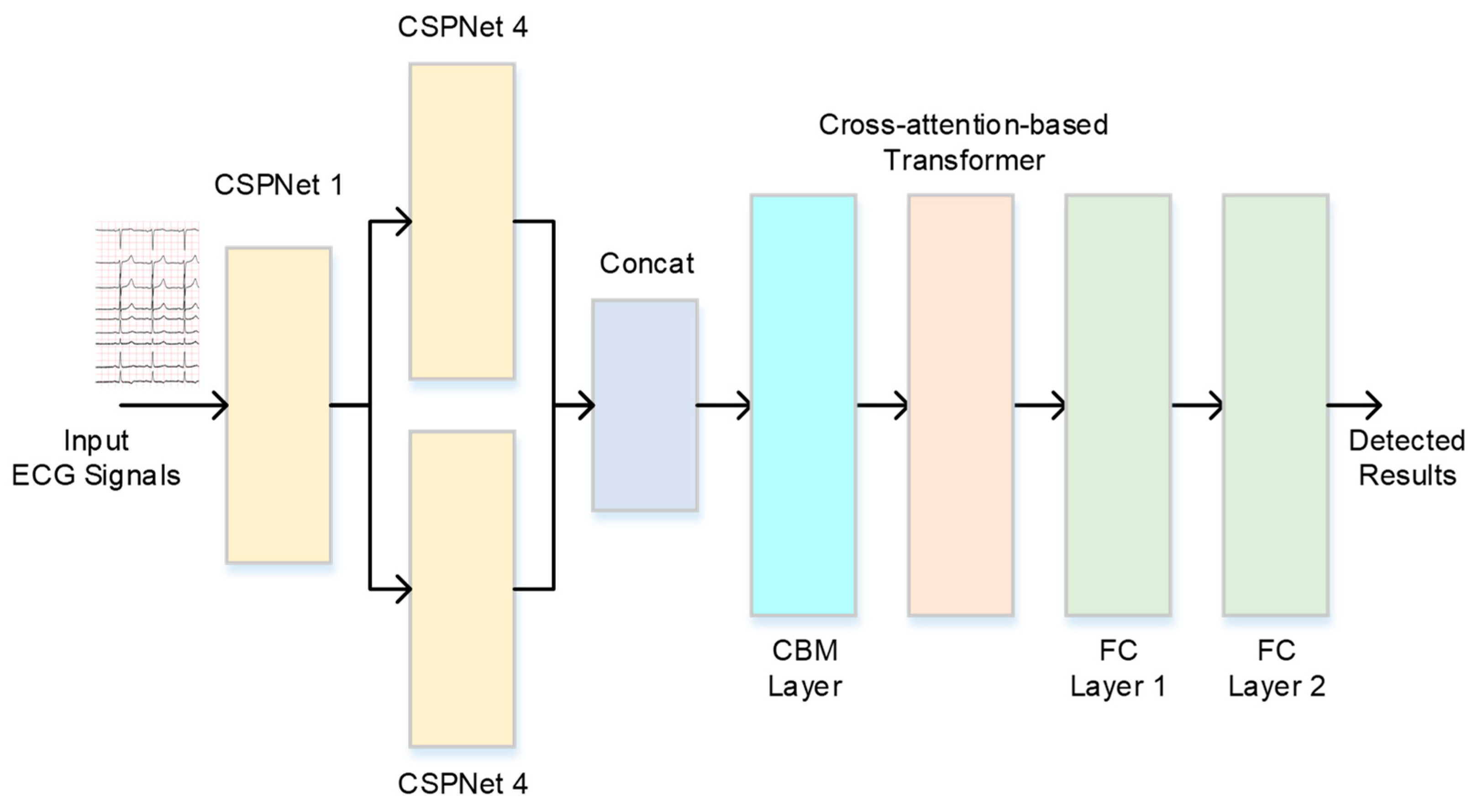
2. The ECG-Based Cardiovascular Disease Decision System
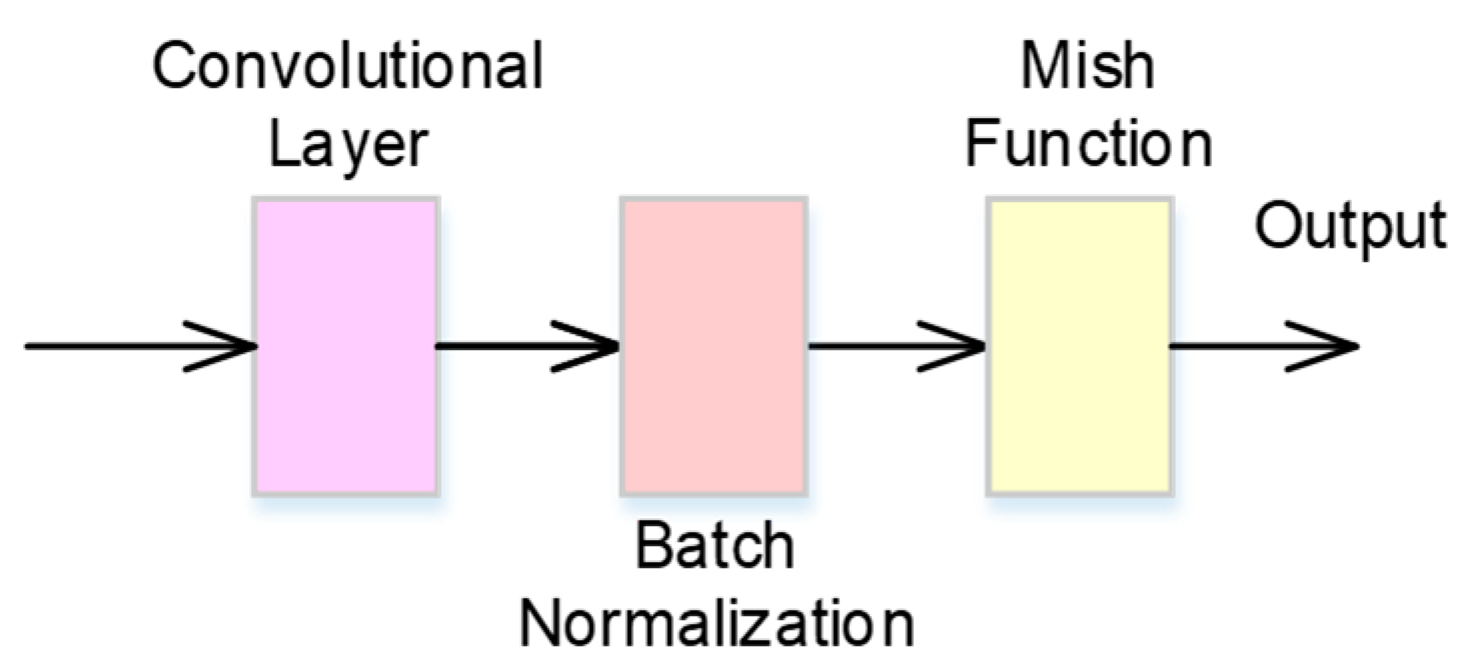
2.1. CBM Neural Network
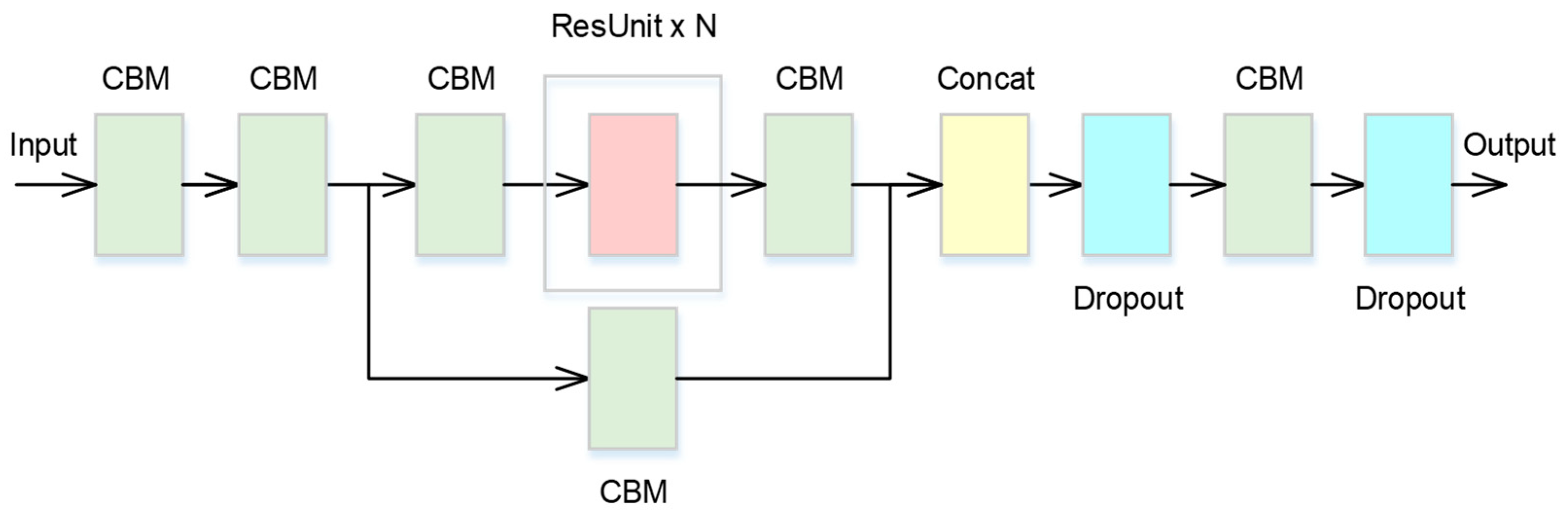
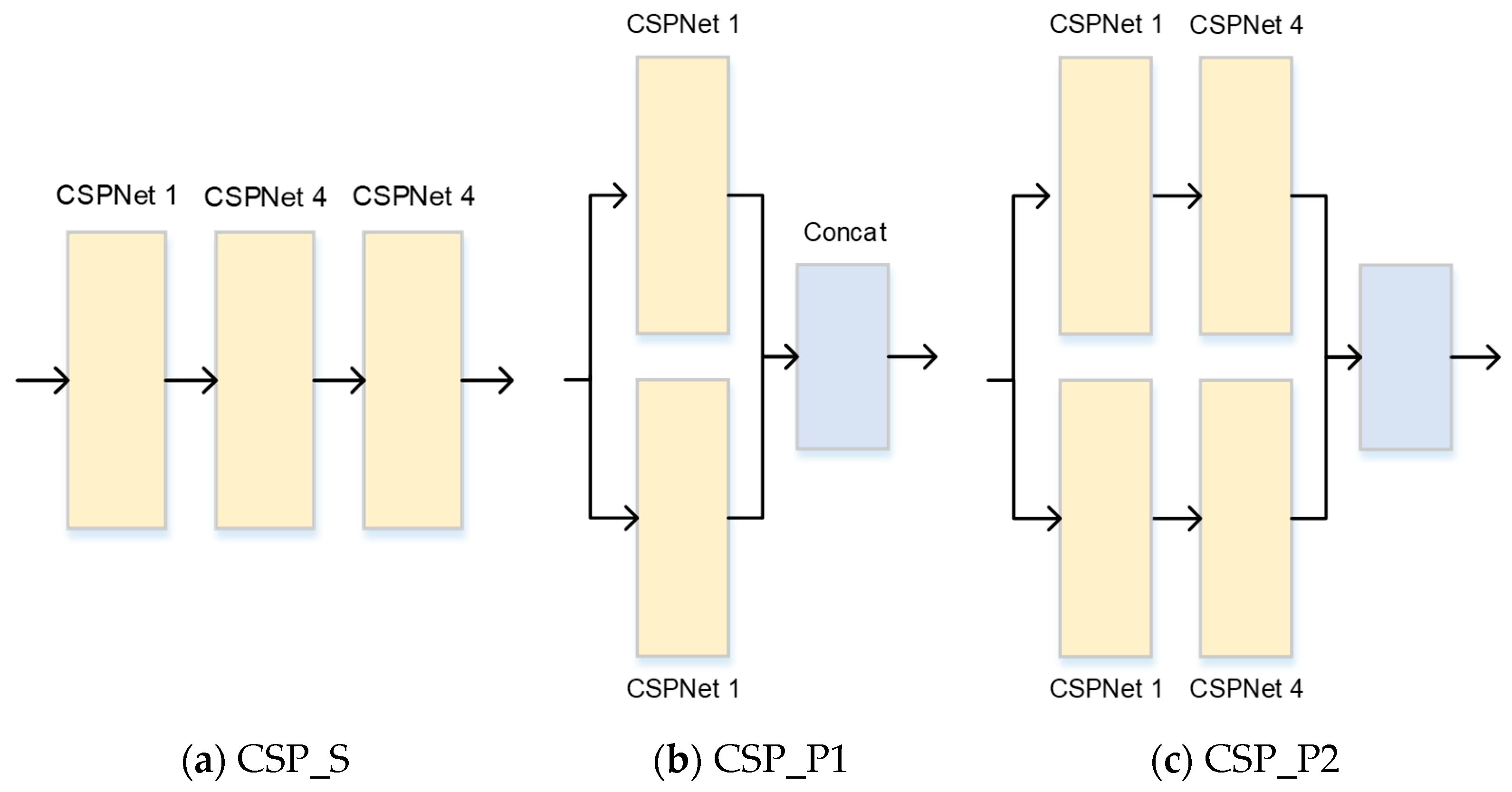
2.2. Cross-Stage Partial Network
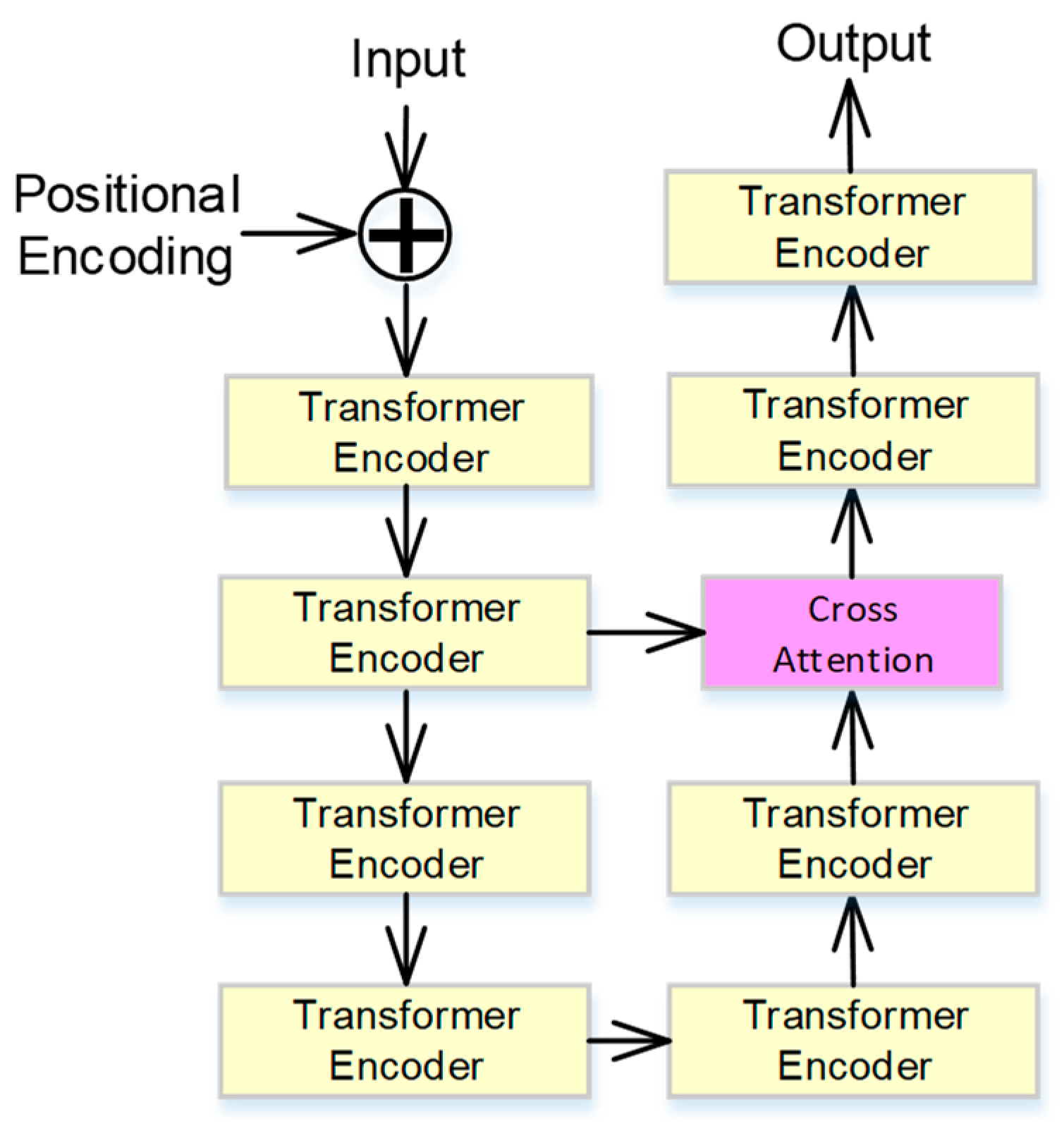
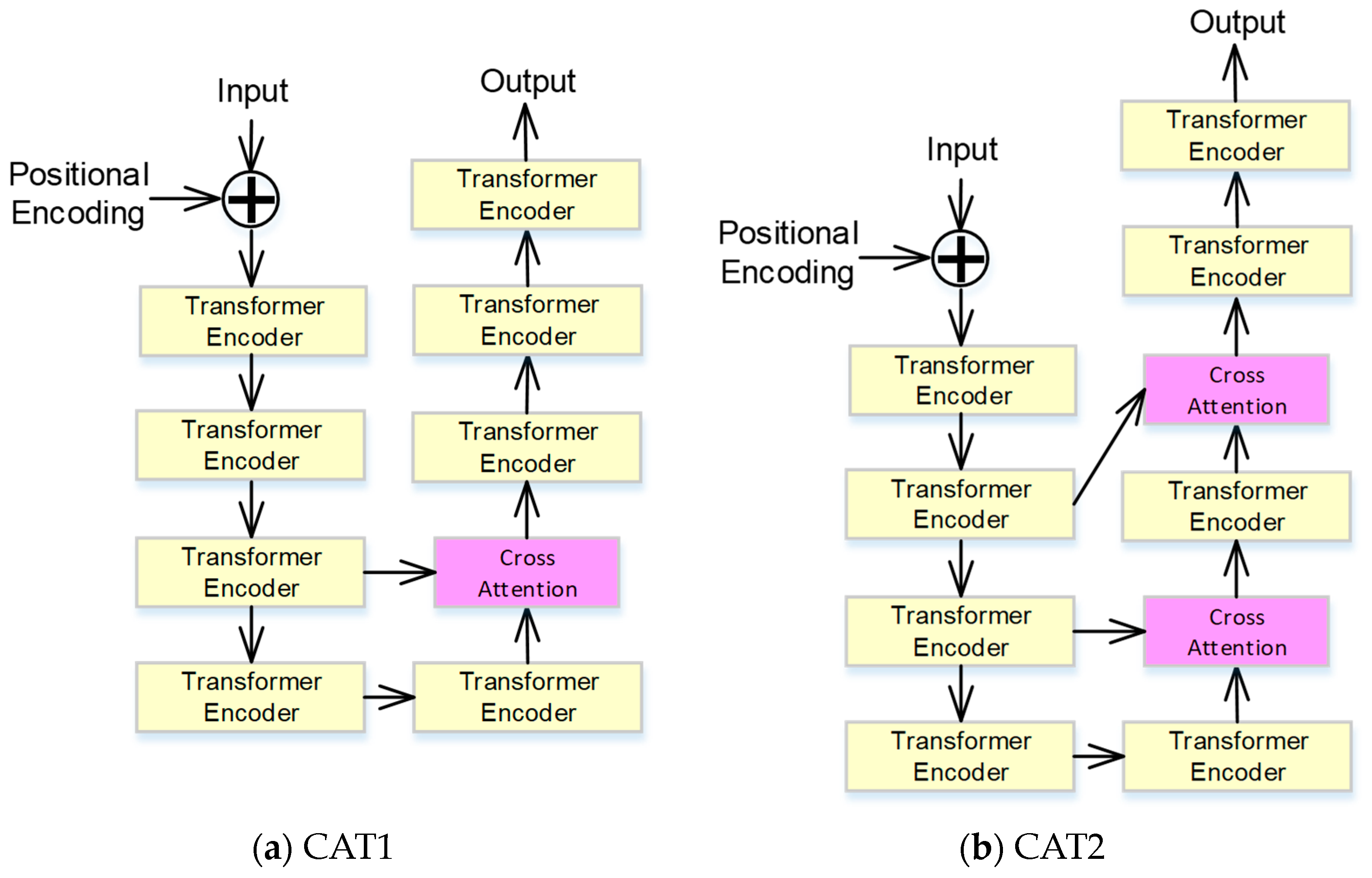
2.3. Cross-Attention-Based Transformer
3. The Experimental Results and Discussions
3.1. Dataset and Evaluation Metric
3.2. The Results of the Transformer with Cross-Attention
3.3. The Results of CSPNet
3.4. The Results Compared with Other Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bickerton, M.; Pooler, A. Misplaced ECG electrodes and the need for continuing training. Br. J. Cardiac Nurs. 2019, 14, 123–132. [Google Scholar] [CrossRef]
- Ye, C.; Coimbra, M.T.; Vijaya Kumar, B.V.K. Arrhythmia detection and classification using morphological and dynamic features of ECG signals. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar]
- World Health Organization. Available online: www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 5 May 2024).
- Ministry of Health and Welfare, Taiwan. Available online: https://www.mohw.gov.tw/cp-16-74869-1.html (accessed on 5 May 2024).
- McNamara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11. [Google Scholar]
- Broadwin, M.; Imarhia, F.; Oh, A.; Stone, C.R.; Sellke, F.W.; Bhowmick, S.; Abid, M.R. Exploring Electrospun Scaffold Innovations in Cardiovascular Therapy: A Review of Electrospinning in Cardiovascular Disease. Bioengineering 2024, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Tscheuschner, L.; Tzafriri, A.R. Cardiovascular Tissue Engineering Models for Atherosclerosis Treatment Development. Bioengineering 2023, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Xu, S.; Shen, E.; Ding, Z.; Zhao, R. Radiation Safety in Nuclear Power Plants: ResNet-Based Glove Image Classification. In Proceedings of the 2023 16th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Taizhou, China, 28–30 October 2023. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Reyna, M.A.; Perez Alday, E.A.; Gu, A.; Liu, C.; Seyedi, S.; Rad, A.B.; Elola, A.; Li, Q.; Sharma, A.; Clifford, G.D. Classification of 12-lead ECGs: The PhysioNet/Computing in Cardiology Challenge 2020. Comput. Cardiol. 2020, 41, 124003. [Google Scholar]
- Zhu, Z.; Wang, H.; Zhao, T.; Guo, Y.; Xu, Z.; Liu, Z.; Liu, S.; Lan, X.; Sun, X.; Feng, M. Classification of Cardiac Abnormalities from ECG Signals Using SE-ResNet. In Proceedings of the 2020 Computing in Cardiology, Rimini, Italy, 13–16 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Zhao, Z.; Fang, H.; Relton, S.D.; Yan, R.; Liu, Y.; Li, Z.; Qin, J.; Wong, D.C. Adaptive Lead Weighted ResNet Trained with Different Duration Signals for Classifying 12-lead ECGs. In Proceedings of the 2020 Computing in Cardiology, Rimini, Italy, 13–16 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Natarajan, A.; Chang, Y.; Mariani, S.; Rahman, A.; Boverman, G.; Vij, S.; Rubin, J. A Wide and Deep Transformer Neural Network for 12-Lead ECG Classification. In Proceedings of the 2020 Computing in Cardiology, Rimini, Italy, 13–16 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Chander Racha, S.; Deb, T.; Sahu, I.; Ukil, A.; Pal, A.; Khandelwal, S. Domain-principled Inference with ResNet-Transformer Model for 12-lead ECG Classification. In Proceedings of the 2022 International Joint Conference on Neural Networks (IJCNN), Padua, Italy, 18–23 July 2022. [Google Scholar]
- Wang, C.Y.; Liao, H.Y.M.; Wu, Y.H.; Chen, P.Y.; Hsieh, J.W.; Yeh, I.H. CSPNet: A New Backbone that can Enhance Learning Capability of CNN. In Proceedings of the 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Seattle, WA, USA, 14–19 June 2020. [Google Scholar]
- Ali, M.A.; Dhanaraj, R.K.; Sharma, A.K.; Balusamy, B.; Grover, G.V.; Grover, V. Multi-Module Deep Learning and IoT-Based Pest Detection System Using Sound Analytics in Large Agricultural Field. In Proceedings of the 2023 International Conference on Electrical, Electronics, Communication and Computers (ELEXCOM), Roorkee, India, 26–27 August 2023. [Google Scholar]
- Hao, Y.; Zhang, Y. A Lightweight Convolutional Neural Network for Ship Target Detection in SAR Images. IEEE Trans. Aerosp. Electron. Syst. 2024, 60, 1882–1898. [Google Scholar] [CrossRef]
- Ju, C.; Guan, C. Graph Neural Networks on SPD Manifolds for Motor Imagery Classification: A Perspective from the Time–Frequency Analysis. IEEE Trans. Neural Netw. Learn. Syst. 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, K.; Chen, J.; Wang, S.; Lu, H.; Wen, D. Pilot Stress Detection Through Physiological Signals Using a Transformer-Based Deep Learning Model. IEEE Sens. J. 2023, 23, 11774–11784. [Google Scholar] [CrossRef]
- Qiu, J.; Zhu, J.; Xu, M.; Huang, P.; Rosenberg, M.; Weber, D.; Liu, E.; Zhao, D. Cardiac Disease Diagnosis on Imbalanced Electrocardiography Data Through Optimal Transport Augmentation. In Proceedings of the 2023 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP2023), Rhodes Island, Greece, 4–10 June 2023. [Google Scholar]
- Ribeiro, P.; Sá, J.; Paiva, D.; Rodrigues, P.M. Cardiovascular Diseases Diagnosis Using an ECG Multi-Band Non-Linear Machine Learning Framework Analysis. Bioengineering 2024, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Chui, K.T.; Gupta, B.B.; Zhao, M.; Malibari, A.; Arya, V.; Alhalabi, W.; Ruiz, M.T. Enhancing Electrocardiogram Classification with Multiple Datasets and Distant Transfer Learning. Bioengineering 2022, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, X.; Huang, L.; Huang, C.; Wei, Y.; Liu, W. CCNet: Criss-Cross Attention for Semantic Segmentation. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision (ICCV), Seoul, Republic of Korea, 27 October–2 November 2019. [Google Scholar]
- Chen, C.F.R.; Fan, Q.; Panda, R. CrossViT: Cross-Attention Multi-Scale Vision Transformer for Image Classification. In Proceedings of the 2021 IEEE/CVF International Conference on Computer Vision (ICCV), Montreal, QC, Canada, 10–17 October 2021. [Google Scholar]
- Yijun, L.; Jianmei, S.; Haoping, S. An Improved Siamese Tracking Network Based on Self-Attention and Cross-Attention. In Proceedings of the 2023 35th Chinese Control and Decision Conference (CCDC), Yichang, China, 20–22 May 2023. [Google Scholar]
- Yu, M.; Wu, D.; Rao, W.; Cheng, L.; Li, R.; Li, Y. Automated Road Crack Detection Method based on Visual Transformer with Multi-Head Cross-Attention. In Proceedings of the 2022 IEEE International Conference on Sensing, Diagnostics, Prognostics, and Control (SDPC), Chongqing, China, 5–7 August 2022. [Google Scholar]
- Lin, H.; Cheng, X.; Wu, X.; Shen, D. CAT: Cross Attention in Vision Transformer. In Proceedings of the 2022 IEEE International Conference on Multimedia and Expo (ICME), Taipei, Taiwan, 18–22 July 2022. [Google Scholar]
- Huo, Z.; Hu, S. Blind Face Restoration via Multi-head Cross-attention and Generative Priors. In Proceedings of the 2023 16th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Taizhou, China, 28–30 October 2023. [Google Scholar]
- Liu, F.; Liu, C.; Zhao, L.; Zhang, X.; Wu, X.; Xu, X.; Liu, Y.; Ma, C.; Wei, S.; He, Z.; et al. An Open Access Database for Evaluating the Algorithms of Electrocardiogram Rhythm and Morphology Abnormality Detection. J. Med. Imaging Health Inf. 2018, 8, 1368–1373. [Google Scholar] [CrossRef]
- Bousseljot, R.; Kreiseler, D.; Schnabel, A. Nutzung der EKG-Signaldatenbank CARDIODAT der PTB über das Internet. Biomed. Eng. Biomed. Tech. 1995, 40, 317–318. [Google Scholar] [CrossRef]

| Model | Sub-Model | Hyperparameters | Value |
|---|---|---|---|
| CSPNet 1 | CBM 1 CBM 2 CBM 3 CBM 4 ResUnit x 1 CBM 5, 6 Dropout | CS, KS, S, P CS, KS, S, P CS, KS, S, P CS, KS, S, P CS, KS, S, P CS, KS, S, P R | 32,15,1,7 32,14,2,6 64,10,2,4 32,10,2,4 32,11,1,5 64,11,1,5 0.2 |
| CSPNet 4 | CBM 1 CBM 2 CBM 3 CBM 4 ResUnit x 1 CBM 5 CBM 6 Dropout | CS, KS, S, P CS, KS, S, P CS, KS, S, P CS, KS, S, P CS, KS, S, P CS, KS, S, P CS, KS, S, P R | 64,15,3,6 32,10,2,4 32,11,1,5 64,11,1,5 64,11,1,5 32,11,1,5 32,14,3,0 0.2 |
| CBM | CBM | CS, KS, S, P | 64,15,1,7 |
| Cross- attention- based transformer | Embedding size Head Layers Feedforward Dropout | dm Nh NL KS R | 64 4 8 2048 0.1 |
| Fully connected layer | FC1 Dropout FC2 | KS R KS | 64 0.2 27 |
| Fold | Proposed Approach | Baseline | CAT1 | CAT2 |
|---|---|---|---|---|
| 0 | 0.6272 | 0.6190 | 0.5896 | 0.5959 |
| 1 | 0.6322 | 0.6175 | 0.6271 | 0.6223 |
| 2 | 0.6199 | 0.6076 | 0.6121 | 0.6280 |
| 3 | 0.6324 | 0.6101 | 0.6333 | 0.6324 |
| 4 | 0.6304 | 0.6194 | 0.6217 | 0.6242 |
| 5 | 0.6065 | 0.6103 | 0.6207 | 0.6064 |
| 6 | 0.5919 | 0.6103 | 0.5886 | 0.5935 |
| 7 | 0.6024 | 0.5925 | 0.6114 | 0.6028 |
| 8 | 0.5922 | 0.5742 | 0.5745 | 0.5879 |
| 9 | 0.5765 | 0.5740 | 0.5737 | 0.5805 |
| Avg C.M | 0.6112 ± 0.0201 | 0.6035 ± 0.0173 | 0.6053 ± 0.0219 | 0.6074 ± 0.0183 |
| Fold | CSP_S | CSP_P1 | CSP_P2 |
|---|---|---|---|
| 0 | 0.6052 | 0.6064 | 0.6379 |
| 1 | 0.6102 | 0.6283 | 0.5995 |
| 2 | 0.6126 | 0.6078 | 0.6086 |
| 3 | 0.6129 | 0.6195 | 0.6213 |
| 4 | 0.5743 | 0.6196 | 0.6216 |
| 5 | 0.5835 | 0.6209 | 0.6202 |
| 6 | 0.6000 | 0.5869 | 0.5827 |
| 7 | 0.5796 | 0.5881 | 0.5920 |
| 8 | 0.5463 | 0.5838 | 0.5859 |
| 9 | 0.5683 | 0.5416 | 0.5737 |
| Avg C.M | 0.5893 ± 0.0225 | 0.6000 ± 0.0258 | 0.6043 ± 0.0208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Chuang, C.-C.; Yeng, C.-H.; So, E.-C.; Chen, Y.-J. A Cross-Stage Partial Network and a Cross-Attention-Based Transformer for an Electrocardiogram-Based Cardiovascular Disease Decision System. Bioengineering 2024, 11, 549. https://doi.org/10.3390/bioengineering11060549
Lee C-C, Chuang C-C, Yeng C-H, So E-C, Chen Y-J. A Cross-Stage Partial Network and a Cross-Attention-Based Transformer for an Electrocardiogram-Based Cardiovascular Disease Decision System. Bioengineering. 2024; 11(6):549. https://doi.org/10.3390/bioengineering11060549
Chicago/Turabian StyleLee, Chien-Ching, Chia-Chun Chuang, Chia-Hong Yeng, Edmund-Cheung So, and Yeou-Jiunn Chen. 2024. "A Cross-Stage Partial Network and a Cross-Attention-Based Transformer for an Electrocardiogram-Based Cardiovascular Disease Decision System" Bioengineering 11, no. 6: 549. https://doi.org/10.3390/bioengineering11060549
APA StyleLee, C.-C., Chuang, C.-C., Yeng, C.-H., So, E.-C., & Chen, Y.-J. (2024). A Cross-Stage Partial Network and a Cross-Attention-Based Transformer for an Electrocardiogram-Based Cardiovascular Disease Decision System. Bioengineering, 11(6), 549. https://doi.org/10.3390/bioengineering11060549








