Metered-Dose Inhaler Spacer with Integrated Spirometer for Home-Based Asthma Monitoring and Drug Uptake
Abstract
1. Introduction
2. Materials and Methods
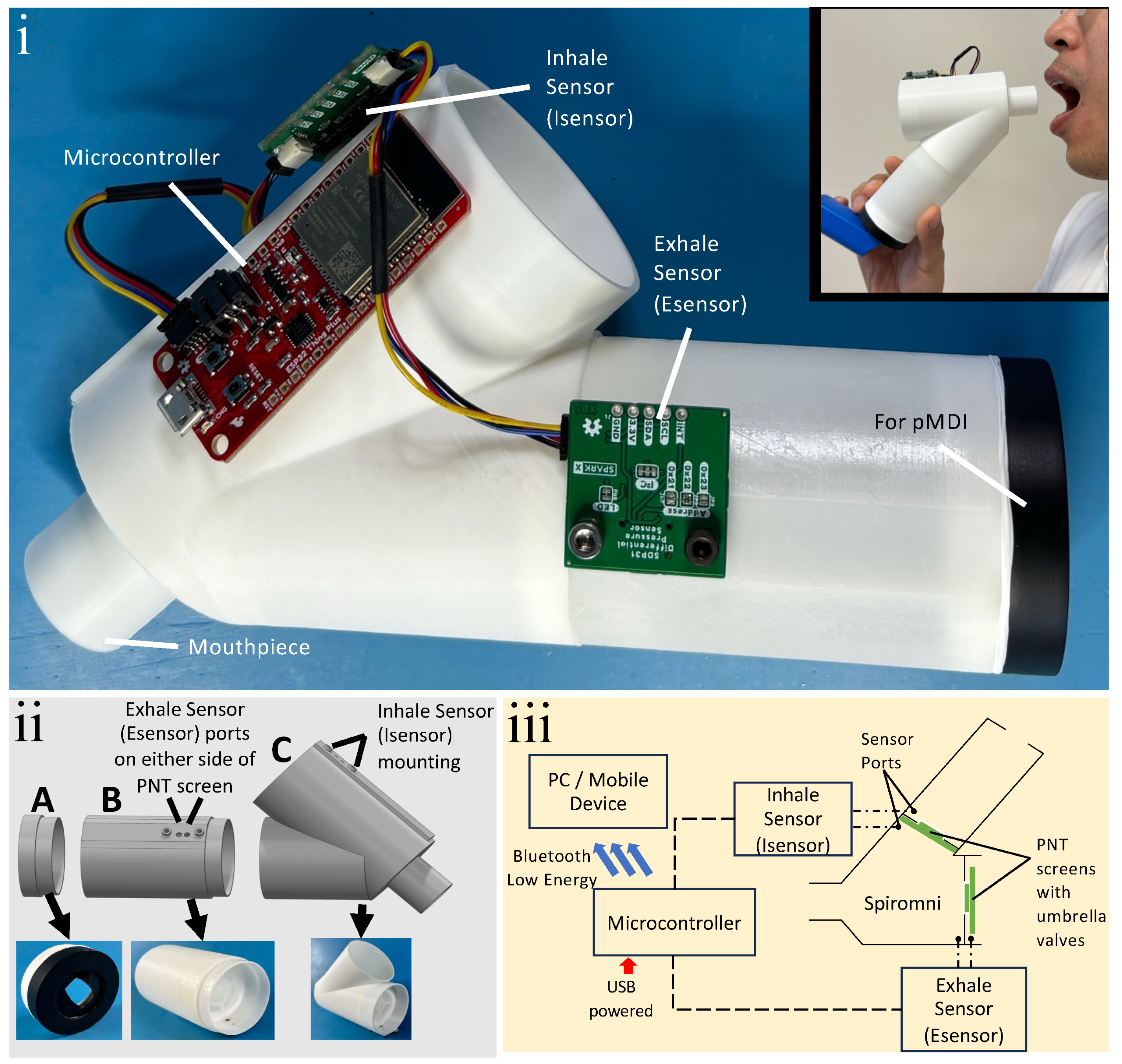
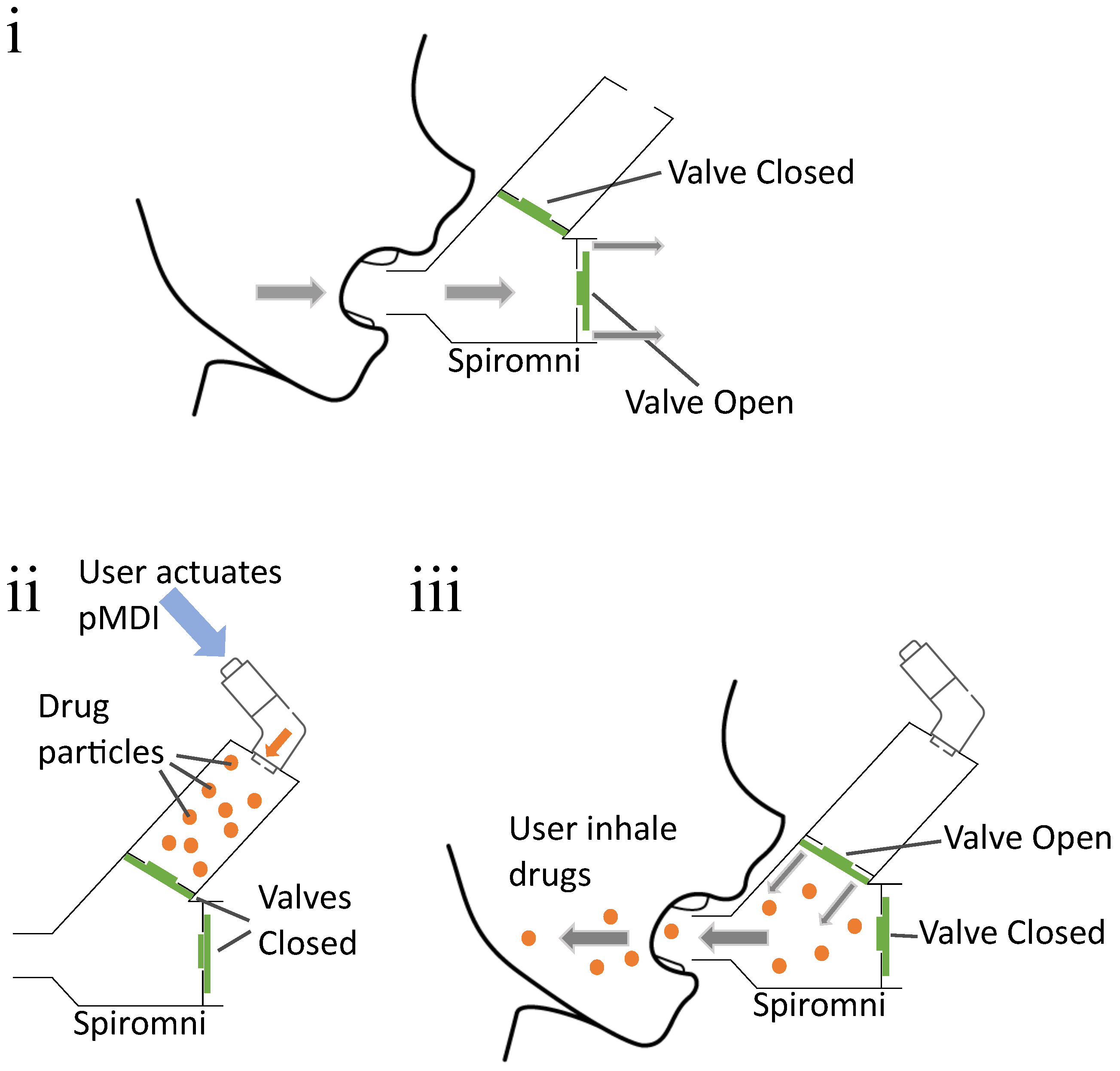
2.1. Spiromni
2.2. Simulation
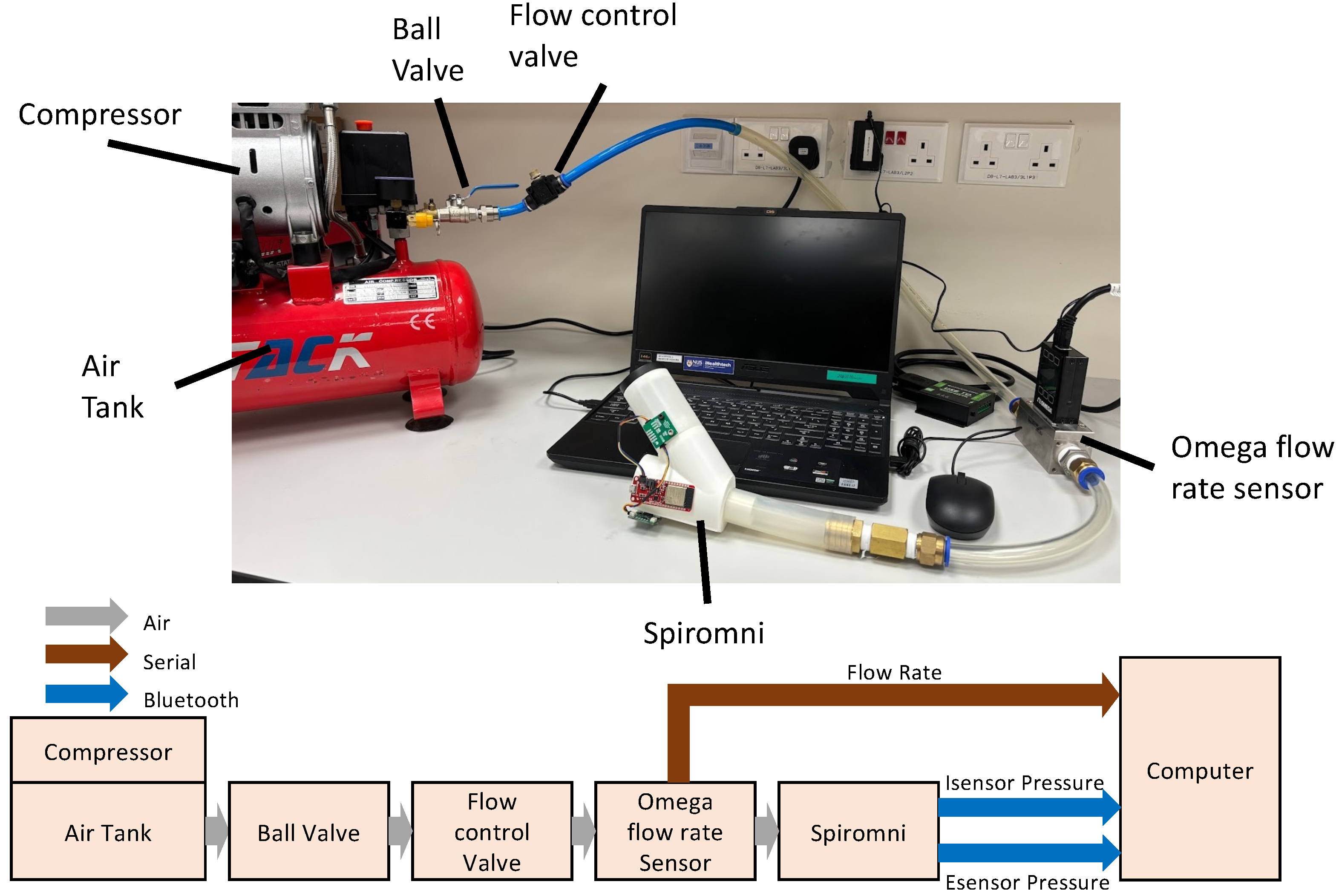
2.3. Sensor Calibration
2.3.1. Esensor Calibration
2.3.2. Isensor Calibration
3. Results
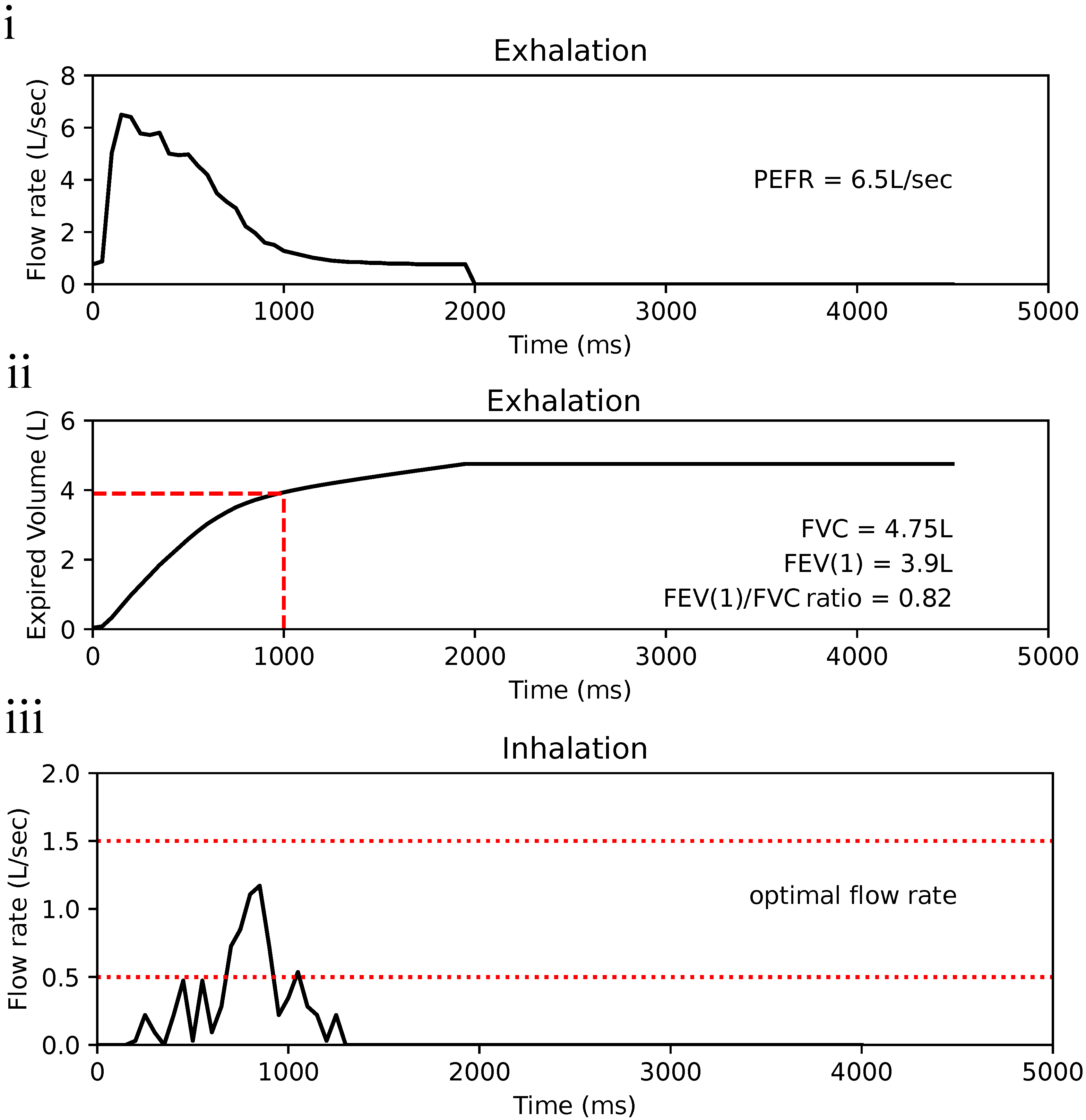
3.1. Flow Profile Simulation
3.2. Sensor Calibration Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Jeyagurunathan, A.; Abdin, E.; Shafie, S.; Sambasivam, R.; Yunjue, Z.; Chua, B.Y.; Vaingankar, J.A.; Verma, S.; Ee, T.W.; Chong, S.A.; et al. Focus: Health Equity: Asthma Prevalence and its Risk Factors Among a Multi-Ethnic Adult Population. Yale J. Biol. Med. 2021, 94, 417. [Google Scholar]
- National Health Service (NHS). Asthma—Overview. 2017. Available online: https://www.nhs.uk/conditions/asthma/ (accessed on 27 September 2023).
- National Health Service (NHS). Asthma—Asthma Attacks. 2018. Available online: https://www.nhs.uk/conditions/asthma/asthma-attack/ (accessed on 27 September 2023).
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008, 31, 143–178. [Google Scholar] [CrossRef] [PubMed]
- GSK. What Triggers Asthma and How Do I Avoid Them?—ASTHMA SINGAPORE. 2023. Available online: https://asthmasingapore.com/about-asthma/what-triggers-asthma-and-how-do-i-avoid-them/ (accessed on 27 September 2023).
- Holmes, J.; Heaney, L.G. Measuring adherence to therapy in airways disease. Breathe 2021, 17, 210037. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Perrin, K.; Pritchard, A.; Williams, M.; Wijesinghe, M.; Weatherall, M.; Beasley, R. Accuracy of patient self-report as a measure of inhaled asthma medication use. Respirology 2013, 18, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Teva Respiratory, LLC. DIGIHALER®. 2023. Available online: https://www.digihaler.com/support (accessed on 27 September 2023).
- Adherium (NZ) Limited. Hailie® for Ventolin® HFA. 2023. Available online: https://www.hailie.com/products/hailie-for-ventolin-hfa (accessed on 27 September 2023).
- Dhruve, H.; Jackson, D.J. Assessing adherence to inhaled therapies in asthma and the emergence of electronic monitoring devices. Eur. Respir. Rev. 2022, 31, 210271. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Izmailova, E.S.; Jackson, N.; Ellis, R.; Bhatia, G.; Ruddy, M.; Singh, D. Remote FEV1 Monitoring in Asthma Patients: A Pilot Study. Clin. Transl. Sci. 2021, 14, 529–535. [Google Scholar] [CrossRef] [PubMed]
- CMI Health. SpiroLink® Smart Spirometer at Home. 2023. Available online: https://www.cmihealth.com/products/cmi-healths-smart-spirometer-at-home-spirolink (accessed on 15 November 2023).
- MIR. Spirobank Smart App-Based Personal Spirometer. 2018. Available online: https://spirometry.com/en/products/spirobank-smart/ (accessed on 15 November 2023).
- Newman, S.P. Spacer Devices for Metered Dose Inhalers. Clin. Pharmacokinet. 2004, 43, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Bryant, L.; Bang, C.; Chew, C.; Baik, S.H.; Wiseman, D. Adequacy of inhaler technique used by people with asthma or chronic obstructive pulmonary disease. J. Prim. Health Care 2013, 5, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Broeders, M.E.; Molema, J.; Hop, W.C.; Folgering, H.T. Inhalation Profiles in Asthmatics and COPD Patients: Reproducibility and Effect of Instruction. J. Aerosol Med. 2003, 16, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.L.; Chia, K.M.; D’Cruz, J.; Gomez, C.A.; Muthusamy, N.; Saadon, N.S.; Jali, N.M.; Loh, L.C. Peak expiratory flow in the standing and sitting positions is equivalent in adults: A cross-over study. Fam. Pract. 2020, 37, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.; Goh, B. Peak expiratory flow rate in normal adult Chinese in Singapore. Singap. Med. J. 1973, 14, 511–514. [Google Scholar]
- Tang, Y.; Turner, M.J.; Yem, J.S.; Baker, A.B. Calibration of pneumotachographs using a calibrated syringe. J. Appl. Physiol. 2003, 95, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Muller, N.L.; Zamel, N. Pneumotachograph calibration for inspiratory and expiratory flows during HeO2 breathing. J. Appl. Physiol. 1981, 51, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Vincken, W.; Levy, M.L.; Scullion, J.; Usmani, O.S.; Dekhuijzen, P.R.; Corrigan, C.J. Spacer devices for inhaled therapy: Why use them, and how? ERJ Open Res. 2018, 4, 00065–2018. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, R.E.; Scanlon, P.D.; Nakamura, M. Interpretation of Pulmonary Function Tests; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Haughney, J.; Price, D.; Barnes, N.C.; Virchow, J.C.; Roche, N.; Chrystyn, H. Choosing inhaler devices for people with asthma: Current knowledge and outstanding research needs. Respir. Med. 2010, 104, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- ISO 23747:2015; ISO Central Secretary. Anaesthetic and respiratory equipment–Peak expiratory flow meters for the assessment of pulmonary function in spontaneously breathing humans. International Organization for Standardization: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/64926.html (accessed on 3 March 2024).
- ISO 20072:2009; ISO Central Secretary. Aerosol drug delivery device design verification – Requirements and test methods. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/41989.html (accessed on 3 March 2024).
- Chernev, A. Jack of All Trades or Master of One? Product Differentiation and Compensatory Reasoning in Consumer Choice. J. Consum. Res. 2007, 33, 430–444. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Au, C.-Y.; Koh, K.J.X.; Lim, H.F.; Bhagat, A.A.S. Metered-Dose Inhaler Spacer with Integrated Spirometer for Home-Based Asthma Monitoring and Drug Uptake. Bioengineering 2024, 11, 552. https://doi.org/10.3390/bioengineering11060552
Au C-Y, Koh KJX, Lim HF, Bhagat AAS. Metered-Dose Inhaler Spacer with Integrated Spirometer for Home-Based Asthma Monitoring and Drug Uptake. Bioengineering. 2024; 11(6):552. https://doi.org/10.3390/bioengineering11060552
Chicago/Turabian StyleAu, Cheuk-Yan, Kelleen J. X. Koh, Hui Fang Lim, and Ali Asgar Saleem Bhagat. 2024. "Metered-Dose Inhaler Spacer with Integrated Spirometer for Home-Based Asthma Monitoring and Drug Uptake" Bioengineering 11, no. 6: 552. https://doi.org/10.3390/bioengineering11060552
APA StyleAu, C.-Y., Koh, K. J. X., Lim, H. F., & Bhagat, A. A. S. (2024). Metered-Dose Inhaler Spacer with Integrated Spirometer for Home-Based Asthma Monitoring and Drug Uptake. Bioengineering, 11(6), 552. https://doi.org/10.3390/bioengineering11060552








