Photocrosslinkable Sericin Hydrogel Injected into the Anterior Chamber of Mice with Chronic Ocular Hypertension Efficacy, Medication Sensitivity, and Material Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Welfare and Anesthesia
2.3. High Intraocular Pressure Induced in the Injection Process
2.4. Clinical Signs and Intraocular Pressure Measurement
2.5. Histopathological Observation
2.6. HE Staining
2.7. Toluidine Blue Staining
2.8. Immunofluorescent Staining
2.9. Effects of Two IOP Drugs on the COH Model
2.10. Toxic Effect
2.11. Statistical Analysis
3. Results
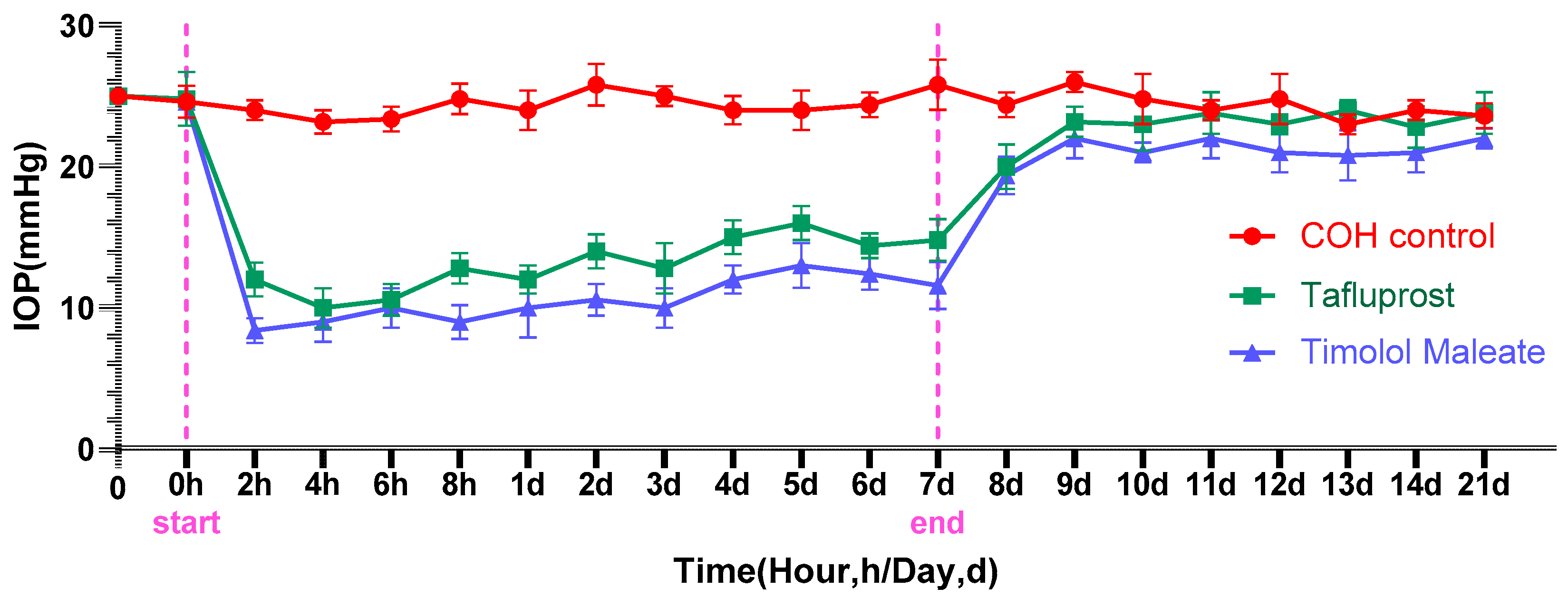
3.1. The Impact of Medication on Lowering Intraocular Pressure
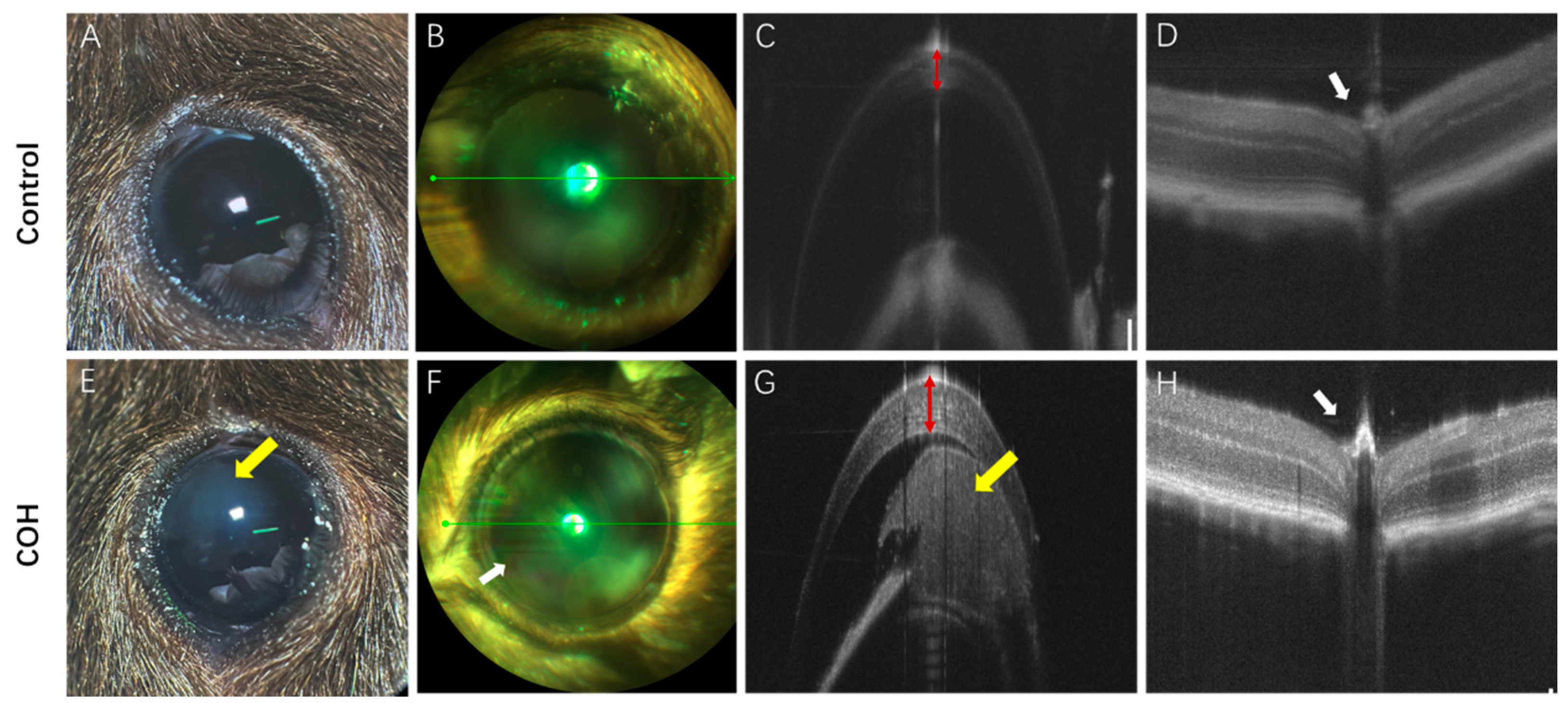
3.2. Clinical Characteristic Change
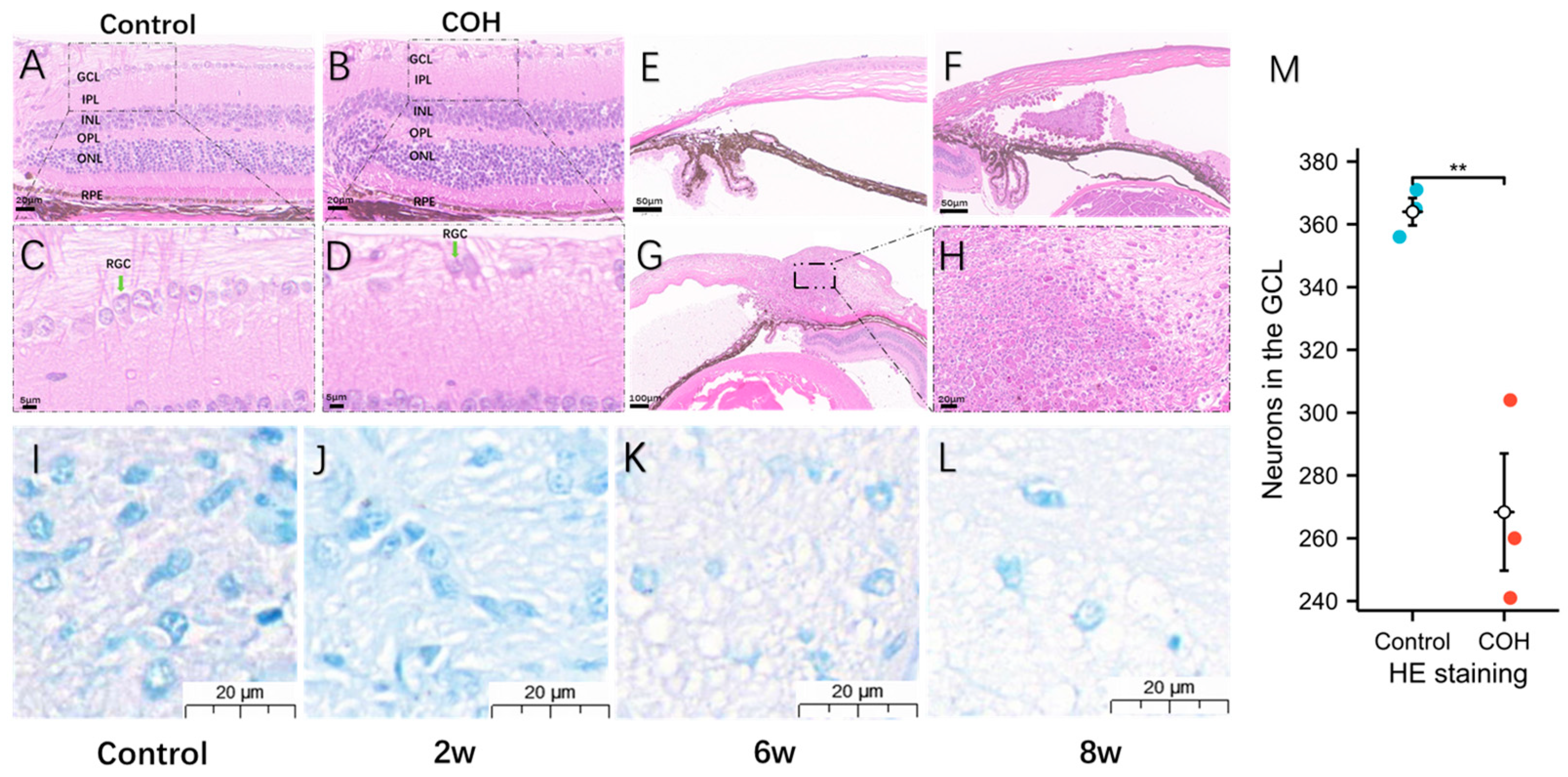
3.3. Morphological and Degenerative Changes in Ganglia after Increased Intraocular Pressure
3.4. The Hydrogel’s Non-Cytotoxicity for HTFs Was Demonstrated by the MTS Assay
4. Discussion
4.1. Analysis of the COH Model Created by Anterior Chamber Injection
4.2. The Mechanism of Causing COH by Photocrosslinkable Sericin Hydrogel into the Anterior Chamber
4.3. Comparison of the Efficacy of the COH Model
4.4. The Application of Hydrogels in Medicine
4.5. Complications of the COH Model
4.6. Medication Sensitivity Analysis of the COH Model
4.7. Material Safety Analysis of the COH Model
5. Advantages and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Calkins, D.J. Critical Pathogenic Events Underlying Progression of Neurodegeneration in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 702–719. [Google Scholar] [CrossRef]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal Models of Glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef] [PubMed]
- Cone, F.E.; Gelman, S.E.; Son, J.L.; Pease, M.E.; Quigley, H.A. Differential Susceptibility to Experimental Glaucoma among 3 Mouse Strains Using Bead and Viscoelastic Injection. Exp. Eye Res. 2010, 91, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Huang, H.; Fang, F.; Webber, H.C.; Zhuang, P.; Liu, L.; Dalal, R.; Tang, P.H.; Mahajan, V.B.; et al. Silicone Oil-Induced Ocular Hypertension and Glaucomatous Neurodegeneration in Mouse. eLife 2019, 8, e45881. [Google Scholar] [CrossRef]
- Fu, C.T.; Sretavan, D. Laser-Induced Ocular Hypertension in Albino CD-1 Mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 980–990. [Google Scholar] [CrossRef]
- Morrison, J.C. Elevated Intraocular Pressure and Optic Nerve Injury Models in the Rat. J. Glaucoma 2005, 14, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Urcola, H.; Bayon, A.; Sharma, S.C. Ocular Hypertension/Glaucoma in Minipigs: Episcleral Veins Cauterization and Microbead Occlusion Methods. Methods Mol. Biol. 2018, 1695, 41–48. [Google Scholar] [CrossRef]
- Pang, I.-H.; Clark, A.F. Inducible Rodent Models of Glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef]
- Razali, N.; Agarwal, R.; Agarwal, P.; Kapitonova, M.Y.; Kannan Kutty, M.; Smirnov, A.; Salmah Bakar, N.; Ismail, N.M. Anterior and Posterior Segment Changes in Rat Eyes with Chronic Steroid Administration and Their Responsiveness to Antiglaucoma Drugs. Eur. J. Pharmacol. 2015, 749, 73–80. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H. Optic Nerve Cupping Represents Neuronal Loss. Ophthalmology 2020, 127, S43–S44. [Google Scholar] [CrossRef]
- Lafuente, M.P.; Villegas-Pérez, M.P.; Sobrado-Calvo, P.; García-Avilés, A.; Miralles de Imperial, J.; Vidal-Sanz, M. Neuroprotective Effects of Alpha(2)-Selective Adrenergic Agonists against Ischemia-Induced Retinal Ganglion Cell Death. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2074–2084. [Google Scholar]
- Wamsley, S.; Gabelt, B.T.; Dahl, D.B.; Case, G.L.; Sherwood, R.W.; May, C.A.; Hernandez, M.R.; Kaufman, P.L. Vitreous Glutamate Concentration and Axon Loss in Monkeys with Experimental Glaucoma. Arch. Ophthalmol. 2005, 123, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, Z.; Huang, Y.; Liu, H.; He, N.; Zhu, X.; Han, X.; Zhou, D.; Duan, X.; Chen, X.; et al. A Versatile 3D-Printable Hydrogel for Antichondrosarcoma, Antibacterial, and Tissue Repair. J. Mater. Sci. Technol. 2023, 136, 200–211. [Google Scholar] [CrossRef]
- Tan, D.; Zhu, W.; Liu, L.; Pan, Y.; Xu, Y.; Huang, Q.; Li, L.; Rao, L. In Situ Formed Scaffold with Royal Jelly-Derived Extracellular Vesicles for Wound Healing. Theranostics 2023, 13, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Pantcheva, M.B.; Seibold, L.K.; Awadallah, N.S.; Kahook, M.Y. Tafluprost: A Novel Prostaglandin Analog for Treatment of Glaucoma. Adv. Ther. 2011, 28, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Metheetrairut, A.; Leumsamran, P.; Rojananin, S.; Kitnarong, N. A Comparison of 0.1% Timolol Eye Gel and 0.5% Timolol Eye Drop in Patients with Chronic Angle-Closure Glaucoma. J. Med. Assoc. Thail. 2012, 95 (Suppl. S4), S116–S122. [Google Scholar]
- Zhao, Y.; Zhang, F.; Pan, Z.; Luo, H.; Liu, K.; Duan, X. LncRNA NR_003923 Promotes Cell Proliferation, Migration, Fibrosis, and Autophagy via the miR-760/miR-215-3p/IL22RA1 Axis in Human Tenon’s Capsule Fibroblasts. Cell Death Dis. 2019, 10, 594. [Google Scholar] [CrossRef]
- Kim, H.-G.; Park, J.-W.; Park, S.-W. Experimental Chronic Ocular Hypertension by Anterior Chamber Injection of 0.3% Carbomer Solution in the Rat. Clin. Exp. Ophthalmol. 2013, 41, 404–412. [Google Scholar] [CrossRef]
- Aragón-Navas, A.; Rodrigo, M.J.; Garcia-Herranz, D.; Martinez, T.; Subias, M.; Mendez, S.; Ruberte, J.; Pampalona, J.; Bravo-Osuna, I.; Garcia-Feijoo, J.; et al. Mimicking Chronic Glaucoma over 6 Months with a Single Intracameral Injection of Dexamethasone/Fibronectin-Loaded PLGA Microspheres. Drug Deliv. 2022, 29, 2357–2374. [Google Scholar] [CrossRef]
- Zhong, N.; Scearce-Levie, K.; Ramaswamy, G.; Weisgraber, K.H. Apolipoprotein E4 Domain Interaction: Synaptic and Cognitive Deficits in Mice. Alzheimers Dement. J. Alzheimers Assoc. 2008, 4, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, N.; Yang, Y.-Y.; Xing, H.-Y.; Liu, X.-X.; Li, F.; La, G.-Y.; Huang, M.-J.; Zhou, M.-W. Lentivirus-Mediated Downregulation of α-Synuclein Reduces Neuroinflammation and Promotes Functional Recovery in Rats with Spinal Cord Injury. J. Neuroinflammation 2019, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia Metabolically Support Axons and Contribute to Neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Byrum, S.; Nishiyama, N.C.; Pecaut, M.J.; Sridharan, V.; Boerma, M.; Tackett, A.J.; Shiba, D.; Shirakawa, M.; Takahashi, S.; et al. Impact of Spaceflight and Artificial Gravity on the Mouse Retina: Biochemical and Proteomic Analysis. Int. J. Mol. Sci. 2018, 19, 2546. [Google Scholar] [CrossRef] [PubMed]
- Naguib, S.; Backstrom, J.R.; Gil, M.; Calkins, D.J.; Rex, T.S. Retinal Oxidative Stress Activates the NRF2/ARE Pathway: An Early Endogenous Protective Response to Ocular Hypertension. Redox Biol. 2021, 42, 101883. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Libby, R.T.; Jakobs, T.C.; Smith, R.S.; Phalan, F.C.; Barter, J.W.; Barbay, J.M.; Marchant, J.K.; Mahesh, N.; Porciatti, V.; et al. Axons of Retinal Ganglion Cells Are Insulted in the Optic Nerve Early in DBA/2J Glaucoma. J. Cell Biol. 2007, 179, 1523–1537. [Google Scholar] [CrossRef]
- Lin, B.; Wang, S.W.; Masland, R.H. Retinal Ganglion Cell Type, Size, and Spacing Can Be Specified Independent of Homotypic Dendritic Contacts. Neuron 2004, 43, 475–485. [Google Scholar] [CrossRef]
- Tribble, J.R.; Otmani, A.; Kokkali, E.; Lardner, E.; Morgan, J.E.; Williams, P.A. Retinal Ganglion Cell Degeneration in a Rat Magnetic Bead Model of Ocular Hypertensive Glaucoma. Transl. Vis. Sci. Technol. 2021, 10, 21. [Google Scholar] [CrossRef]
- Chung, K.; Wallace, J.; Kim, S.-Y.; Kalyanasundaram, S.; Andalman, A.S.; Davidson, T.J.; Mirzabekov, J.J.; Zalocusky, K.A.; Mattis, J.; Denisin, A.K.; et al. Structural and Molecular Interrogation of Intact Biological Systems. Nature 2013, 497, 332–337. [Google Scholar] [CrossRef]
- Chen, H.; Wei, X.; Cho, K.-S.; Chen, G.; Sappington, R.; Calkins, D.J.; Chen, D.F. Optic Neuropathy Due to Microbead-Induced Elevated Intraocular Pressure in the Mouse. Investig. Ophthalmol. Vis. Sci. 2011, 52, 36–44. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, H.; Chen, J.; Sun, J.; Huang, P.; Xu, X.; Huang, S.; Zhong, Y. Efficacy, Drug Sensitivity, and Safety of a Chronic Ocular Hypertension Rat Model Established Using a Single Intracameral Injection of Hydrogel into the Anterior Chamber. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e925852. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Galindo-Romero, C.; Lucas-Ruiz, F.; Marsh-Amstrong, N.; Li, W.; Vidal-Sanz, M.; Agudo-Barriuso, M. Pan-Retinal Ganglion Cell Markers in Mice, Rats, and Rhesus Macaques. Zool. Res. 2023, 44, 226–248. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Sobrado-Calvo, P.; Jiménez-López, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Long-Term Effect of Optic Nerve Axotomy on the Retinal Ganglion Cell Layer. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6095. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Shi, M.; Liu, H.-H.; Ge, S.; Ou, Y.; Flanagan, J.G.; Chen, L. Establishment and Characterization of an Acute Model of Ocular Hypertension by Laser-Induced Occlusion of Episcleral Veins. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3879–3886. [Google Scholar] [CrossRef]
- Patil, S.V.; Kasetti, R.B.; Millar, J.C.; Zode, G.S. A Novel Mouse Model of TGFβ2-Induced Ocular Hypertension Using Lentiviral Gene Delivery. Int. J. Mol. Sci. 2022, 23, 6883. [Google Scholar] [CrossRef]
- Zahavi, A.; Friedman Gohas, M.; Sternfeld, A.; Daoud Zreiq, N.; Muhsinoglu, O.; Ofri, R.; BarKana, Y.; Goldenberg-Cohen, N. Histological and Molecular Characterization of Glaucoma Model Induced by One or Two Injections of Microbeads to the Anterior Chamber of Mice. Int. Ophthalmol. 2022, 42, 3763–3775. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, X.; Liu, R.; Zhao, Y.; Sun, L. ECM-Inspired Hydrogels with ADSCs Encapsulation for Rheumatoid Arthritis Treatment. Adv. Sci. 2023, 10, e2206253. [Google Scholar] [CrossRef]
- Jian, X.; Feng, X.; Luo, Y.; Li, F.; Tan, J.; Yin, Y.; Liu, Y. Development, Preparation, and Biomedical Applications of DNA-Based Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 661409. [Google Scholar] [CrossRef]
- Ma, S.; Gu, S.; Zhang, J.; Qi, W.; Lin, Z.; Zhai, W.; Zhan, J.; Li, Q.; Cai, Y.; Lu, Y. Robust Drug Bioavailability and Safety for Rheumatoid Arthritis Therapy Using D-Amino Acids-Based Supramolecular Hydrogels. Mater. Today Bio 2022, 15, 100296. [Google Scholar] [CrossRef]
- Wu, K.Y.; Ashkar, S.; Jain, S.; Marchand, M.; Tran, S.D. Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma. Polymers 2023, 15, 1373. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, L.; Yang, R.; Hu, R.; Zheng, Q.; Zan, X. Combined Delivery of Small Molecule and Protein Drugs as Synergistic Therapeutics for Treating Corneal Neovascularization by a One-Pot Coassembly Strategy. Mater. Today Bio 2022, 17, 100456. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.; Kook, M.S. Efficacy, Safety and Patient-Reported Outcomes with Preservative-Free (PF) Tafluprost or PF-Dorzolamide/Timolol Compared with Preserved Latanoprost: A Prospective Multicenter Study in Korean Glaucoma Patients with Ocular Surface Disease. Pharmaceuticals 2022, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cho, K.-S.; Chen, H.; Yu, D.; Wang, W.-H.; Luo, G.; Pang, I.-H.; Guo, W.; Chen, D.F. Microbead-Induced Ocular Hypertensive Mouse Model for Screening and Testing of Aqueous Production Suppressants for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Poursamar, S.A.; Lehner, A.N.; Azami, M.; Ebrahimi-Barough, S.; Samadikuchaksaraei, A.; Antunes, A.P.M. The Effects of Crosslinkers on Physical, Mechanical, and Cytotoxic Properties of Gelatin Sponge Prepared via in-Situ Gas Foaming Method as a Tissue Engineering Scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 1–9. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.; Zhu, W.; Liu, H.; Wu, P.; Zhang, X.; Zhou, X.; Xu, J.; Zhao, Y.; Duan, X. Photocrosslinkable Sericin Hydrogel Injected into the Anterior Chamber of Mice with Chronic Ocular Hypertension Efficacy, Medication Sensitivity, and Material Safety. Bioengineering 2024, 11, 607. https://doi.org/10.3390/bioengineering11060607
Liao L, Zhu W, Liu H, Wu P, Zhang X, Zhou X, Xu J, Zhao Y, Duan X. Photocrosslinkable Sericin Hydrogel Injected into the Anterior Chamber of Mice with Chronic Ocular Hypertension Efficacy, Medication Sensitivity, and Material Safety. Bioengineering. 2024; 11(6):607. https://doi.org/10.3390/bioengineering11060607
Chicago/Turabian StyleLiao, Li, Wenxiang Zhu, Hairong Liu, Ping Wu, Xinyue Zhang, Xiaoyu Zhou, Jiahao Xu, Yang Zhao, and Xuanchu Duan. 2024. "Photocrosslinkable Sericin Hydrogel Injected into the Anterior Chamber of Mice with Chronic Ocular Hypertension Efficacy, Medication Sensitivity, and Material Safety" Bioengineering 11, no. 6: 607. https://doi.org/10.3390/bioengineering11060607
APA StyleLiao, L., Zhu, W., Liu, H., Wu, P., Zhang, X., Zhou, X., Xu, J., Zhao, Y., & Duan, X. (2024). Photocrosslinkable Sericin Hydrogel Injected into the Anterior Chamber of Mice with Chronic Ocular Hypertension Efficacy, Medication Sensitivity, and Material Safety. Bioengineering, 11(6), 607. https://doi.org/10.3390/bioengineering11060607








