Synergistic Cellular Responses Conferred by Concurrent Optical and Magnetic Stimulation Are Attenuated by Simultaneous Exposure to Streptomycin: An Antibiotic Dilemma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Studies and Analysis
2.1.1. Tissue Culture
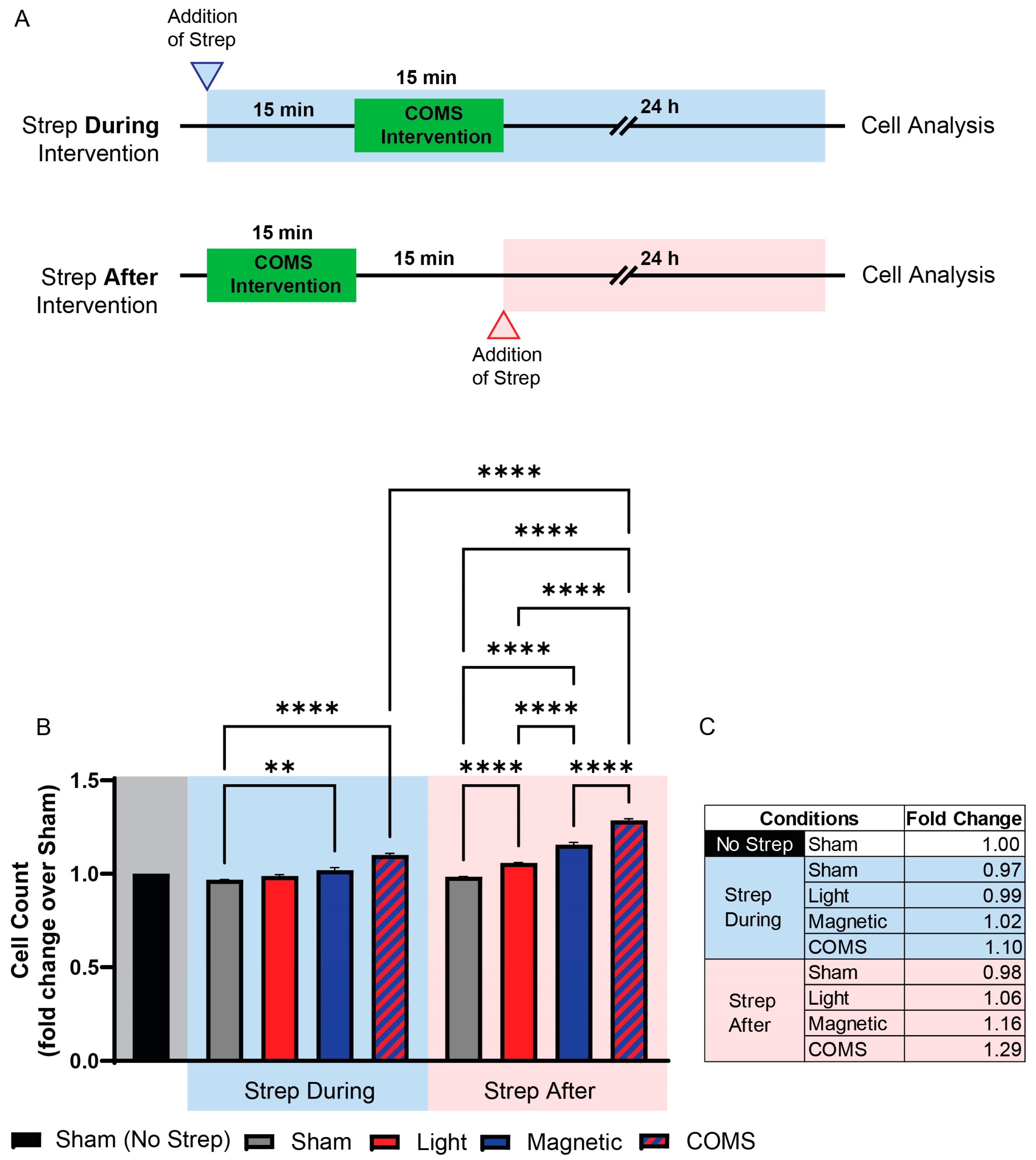
2.1.2. Streptomycin Treatments
2.1.3. Western Blot Analyses
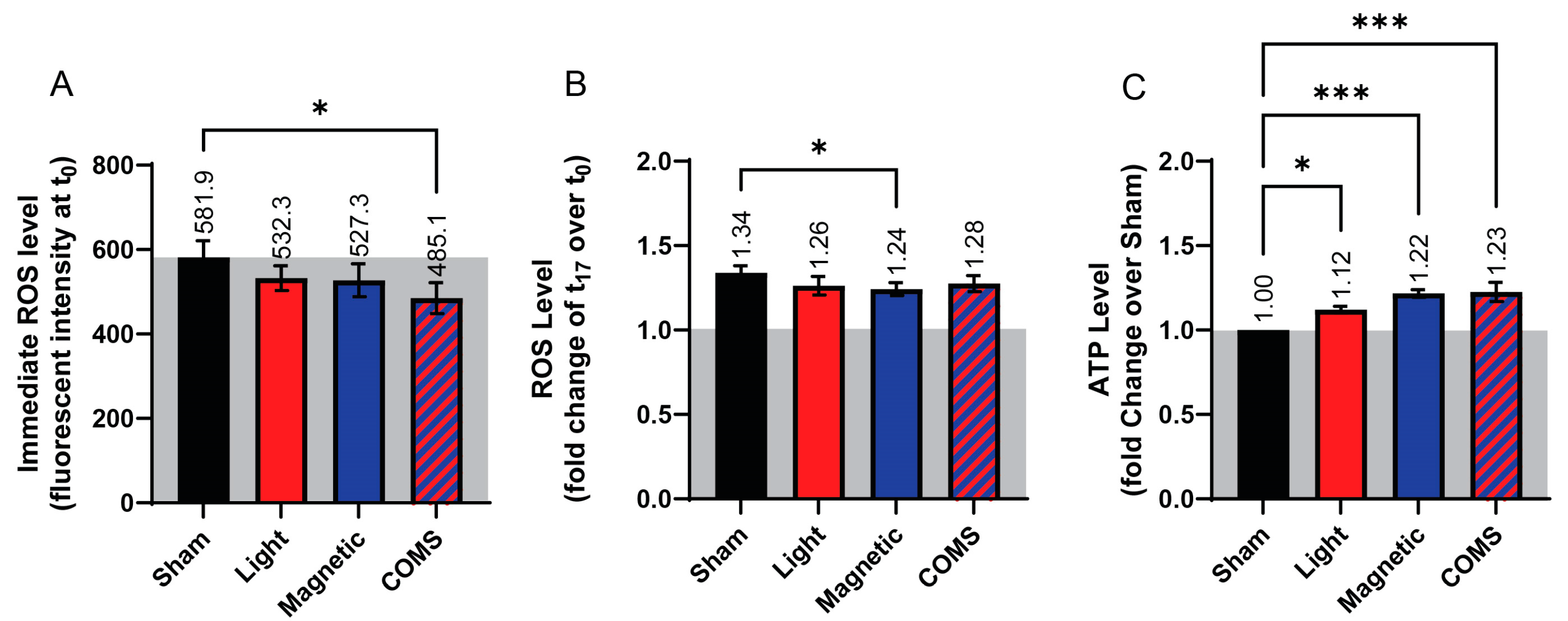
2.1.4. Reactive Oxygen Species Measurements
2.1.5. ATP Measurements
2.1.6. Statistical Analyses
2.2. Device
- An optically functional model in which the magnetic stimulation component was deactivated;
- A magnetically functional model with the optical stimulation turned off;
- A fully functional unit operating as per the manufacturer’s specifications.
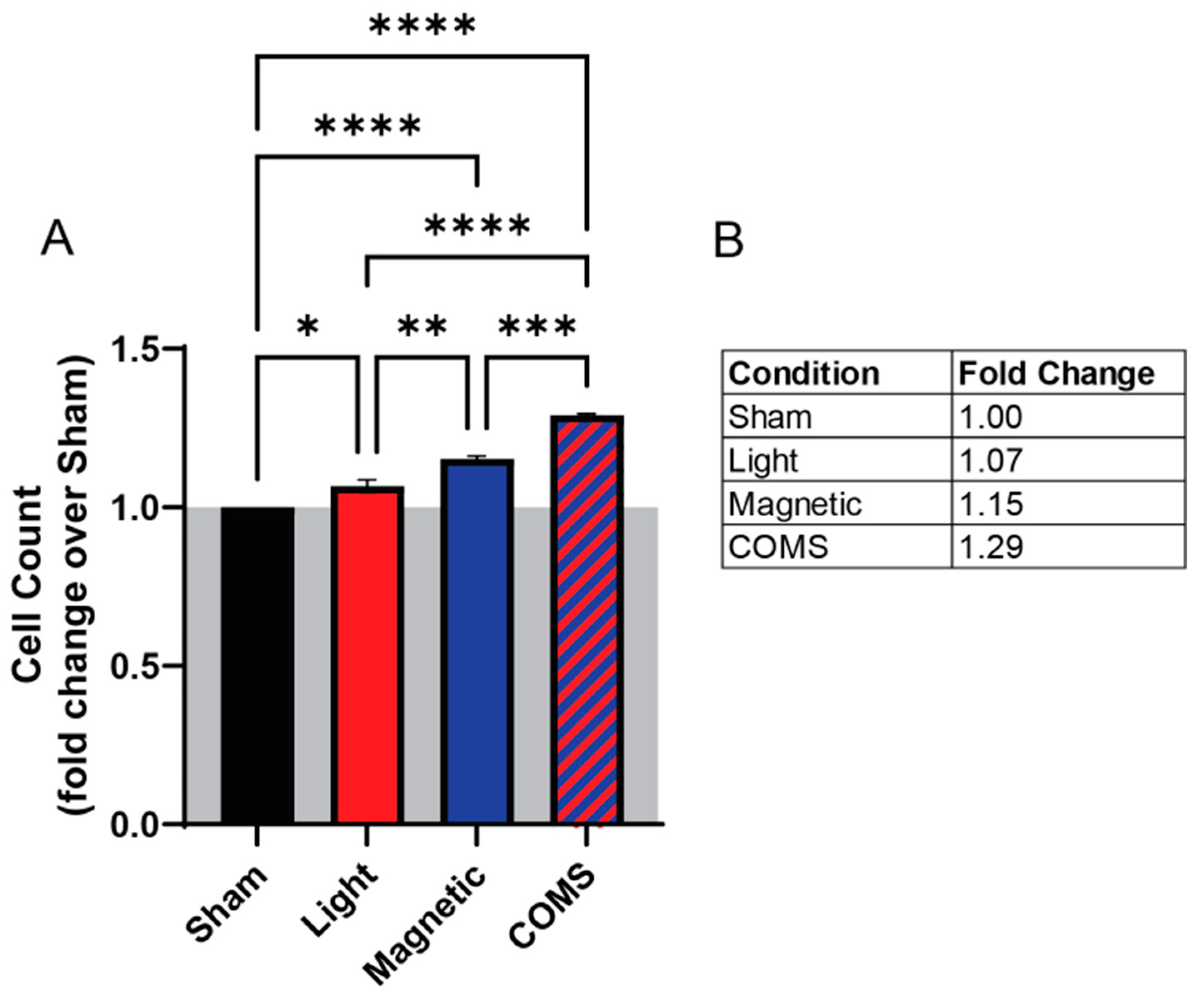
3. Results
4. Discussion
4.1. Aminoglycoside Antagonism of Other Calcium Channels
4.2. COMS Electromagnetic Transduction
4.3. Clinical Implications of Aminoglycoside Antibiotic Antagonism of Electromagnetic Therapies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinboldt-Jockenhofer, F.; Traber, J.; Liesch, G.; Bittner, C.; Benecke, U.; Dissemond, J. Concurrent optical and magnetic stimulation therapy in patients with lower extremity hard-to-heal wounds. J. Wound Care 2022, 31, S12–S21. [Google Scholar] [CrossRef] [PubMed]
- Traber, J.; Wild, T.; Marotz, J.; Berli, M.C.; Franco-Obregon, A. Concurrent Optical- and Magnetic-Stimulation-Induced Changes on Wound Healing Parameters, Analyzed by Hyperspectral Imaging: An Exploratory Case Series. Bioengineering 2023, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Franco-Obregon, A. Harmonizing Magnetic Mitohormetic Regenerative Strategies: Developmental Implications of a Calcium-Mitochondrial Axis Invoked by Magnetic Field Exposure. Bioengineering 2023, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.L.Y.; Tai, Y.K.; Fröhlich, J.; Fong, C.H.H.; Yin, J.N.; Foo, Z.L.; Ramanan, S.; Beyer, C.; Toh, S.J.; Casarosa, M.; et al. Ambient and supplemental magnetic fields promote myogenesis via a TRPC1-mitochondrial axis: Evidence of a magnetic mitohormetic mechanism. FASEB J. 2019, 33, 12853–12872. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Chen, C.; Deng, P.; Zhu, G.; Lin, M.; Zhang, L.; Xu, S.; He, M.; Lu, Y.; Duan, W.; et al. Extremely Low-Frequency Electromagnetic Fields Promote In Vitro Neuronal Differentiation and Neurite Outgrowth of Embryonic Neural Stem Cells via Up-Regulating TRPC1. PLoS ONE 2016, 11, e0150923. [Google Scholar] [CrossRef] [PubMed]
- Madanagopal, T.T.; Tai, Y.K.; Lim, S.H.; Fong, C.H.; Cao, T.; Rosa, V.; Franco-Obregón, A. Pulsed electromagnetic fields synergize with graphene to enhance dental pulp stem cell-derived neurogenesis by selectively targeting TRPC1 channels. Eur. Cell Mater. 2021, 41, 216–232. [Google Scholar] [CrossRef]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef]
- Ma, T.; Ding, Q.; Liu, C.; Wu, H. Electromagnetic fields regulate calcium-mediated cell fate of stem cells: Osteogenesis, chondrogenesis and apoptosis. Stem Cell Res. Ther. 2023, 14, 133. [Google Scholar] [CrossRef]
- Sendera, A.; Pikula, B.; Banas-Zabczyk, A. Preconditioning of Mesenchymal Stem Cells with Electromagnetic Fields and Its Impact on Biological Responses and “Fate”-Potential Use in Therapeutic Applications. Front. Biosci. 2023, 28, 285. [Google Scholar] [CrossRef]
- Kurth, F.; Tai, Y.K.; Parate, D.; van Oostrum, M.; Schmid, Y.R.F.; Toh, S.J.; Yap, J.L.Y.; Wollscheid, B.; Othman, A.; Dittrich, P.S.; et al. Cell-Derived Vesicles as TRPC1 Channel Delivery Systems for the Recovery of Cellular Respiratory and Proliferative Capacities. Adv. Biosyst. 2020, 4, e2000146. [Google Scholar] [CrossRef] [PubMed]
- Skapek, S.X.; Rhee, J.; Spicer, D.B.; Lassar, A.B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 1995, 267, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, J.; Lei, Y.; Han, Z.; Rong, D.; Yu, Q.; Zhao, M.; Tian, J. Low frequency pulsed electromagnetic field promotes C2C12 myoblasts proliferation via activation of MAPK/ERK pathway. Biochem. Biophys. Res. Commun. 2016, 479, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Nobleman, A.P.; Robinson, P.R.; Schmidt, T.M. Melanopsin phototransduction: Beyond canonical cascades. J. Exp. Biol. 2021, 224, jeb226522. [Google Scholar] [CrossRef] [PubMed]
- Detwiler, P.B. Phototransduction in Retinal Ganglion Cells. Yale J. Biol. Med. 2018, 91, 49–52. [Google Scholar] [PubMed]
- Moraes, M.N.; de Assis, L.V.M.; Provencio, I.; Castrucci, A.M.L. Opsins outside the eye and the skin: A more complex scenario than originally thought for a classical light sensor. Cell Tissue Res. 2021, 385, 519–538. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, G.L.; Mizobuti, D.S.; da Silva, H.N.M.; Covatti, C.; de Lourenco, C.C.; Salvador, M.J.; Pereira, E.C.L.; Minatel, E. Multiple LEDT wavelengths modulate the Akt signaling pathways and attenuate pathological events in mdx dystrophic muscle cells. Photochem. Photobiol. Sci. 2022, 21, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Squecco, R.; Tani, A.; Idrizaj, E.; Nosi, D.; Giannelli, M.; Zecchi-Orlandini, S. Low intensity 635 nm diode laser irradiation inhibits fibroblast-myofibroblast transition reducing TRPC1 channel expression/activity: New perspectives for tissue fibrosis treatment. Lasers Surg. Med. 2016, 48, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Bellemer, A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature 2015, 2, 227–243. [Google Scholar] [CrossRef]
- Kiselyov, K.; Patterson, R.L. The integrative function of TRPC channels. Front. Biosci. 2009, 14, 45–58. [Google Scholar] [CrossRef]
- Goretzki, B.; Guhl, C.; Tebbe, F.; Harder, J.M.; Hellmich, U.A. Unstructural Biology of TRP Ion Channels: The Role of Intrinsically Disordered Regions in Channel Function and Regulation. J. Mol. Biol. 2021, 433, 166931. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, J.F.; Howard, M.J. Cryptochromes Mediate Intrinsic Photomechanical Transduction in Avian Iris and Somatic Striated Muscle. Front. Physiol. 2020, 11, 128. [Google Scholar] [CrossRef]
- Khodabukus, A.; Baar, K. Streptomycin decreases the functional shift to a slow phenotype induced by electrical stimulation in engineered muscle. Tissue Eng. Part A 2015, 21, 1003–1012. [Google Scholar] [CrossRef]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006, 1, 32. [Google Scholar] [CrossRef]
- Hwang, A.; Maity, A.; McKenna, W.G.; Muschel, R.J. Cell cycle-dependent regulation of the cyclin B1 promoter. J. Biol. Chem. 1995, 270, 28419–28424. [Google Scholar] [CrossRef]
- Benavides Damm, T.; Richard, S.; Tanner, S.; Wyss, F.; Egli, M.; Franco-Obregon, A. Calcium-dependent deceleration of the cell cycle in muscle cells by simulated microgravity. FASEB J. 2013, 27, 2045–2054. [Google Scholar] [CrossRef]
- Benavides Damm, T.; Franco-Obregon, A.; Egli, M. Gravitational force modulates G2/M phase exit in mechanically unloaded myoblasts. Cell Cycle 2013, 12, 3001–3012. [Google Scholar] [CrossRef]
- Crocetti, S.; Beyer, C.; Unternahrer, S.; Benavides Damm, T.; Schade-Kampmann, G.; Hebeisen, M.; Di Berardino, M.; Frohlich, J.; Franco-Obregon, A. Impedance flow cytometry gauges proliferative capacity by detecting TRPC1 expression. Cytom. A 2014, 85, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, Y.; Kim, S.M.; Yang, Y.D.; Jung, J.; Oh, U. Quantitative analysis of TRP channel genes in mouse organs. Arch. Pharm. Res. 2012, 35, 1823–1830. [Google Scholar] [CrossRef]
- Matsumura, C.Y.; Taniguti, A.P.; Pertille, A.; Santo Neto, H.; Marques, M.J. Stretch-activated calcium channel protein TRPC1 is correlated with the different degrees of the dystrophic phenotype in mdx mice. Am. J. Physiol. Cell Physiol. 2011, 301, C1344–C1350. [Google Scholar] [CrossRef] [PubMed]
- Bielfeldt, M.; Rebl, H.; Peters, K.; Sridharan, K.; Staehlke, S.; Nebe, J.B. Sensing of Physical Factors by Cells: Electric Field, Mechanical Forces, Physical Plasma and Light—Importance for Tissue Regeneration. Biomed. Mater. Devices 2023, 1, 146–161. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, L.; Qin, Y.; Ouyang, Z.; Zhong, J.; Zeng, Y. Harnessing Mitochondrial Stress for Health and Disease: Opportunities and Challenges. Biology 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.V.; Guy, G.W.; Bell, J.D. The quantum mitochondrion and optimal health. Biochem. Soc. Trans. 2016, 44, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Diver, M.M.; Lin King, J.V.; Julius, D.; Cheng, Y. Sensory TRP Channels in Three Dimensions. Annu. Rev. Biochem. 2022, 91, 629–649. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, I.; Monteil, A.; Nargeot, J.; Lory, P. Properties and role of voltage-dependent calcium channels during mouse skeletal muscle differentiation. J. Muscle Res. Cell Motil. 2006, 27, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bijlenga, P.; Liu, J.H.; Espinos, E.; Haenggeli, C.A.; Fischer-Lougheed, J.; Bader, C.R.; Bernheim, L. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc. Natl. Acad. Sci. USA 2000, 97, 7627–7632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, Z.M.; Delbono, O. Charge movement and transcription regulation of L-type calcium channel alpha(1S) in skeletal muscle cells. J. Physiol. 2002, 540, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Shi, Y. TRPC Channels and Cell Proliferation. Adv. Exp. Med. Biol. 2017, 976, 149–155. [Google Scholar] [CrossRef]
- Suarez-Kurtz, G. Inhibition of membrane calcium activation by neomycin and streptomycin in crab muscle fibers. Pflug. Arch. 1974, 349, 337–349. [Google Scholar] [CrossRef]
- Haws, C.M.; Winegar, B.D.; Lansman, J.B. Block of single L-type Ca2+ channels in skeletal muscle fibers by aminoglycoside antibiotics. J. Gen. Physiol. 1996, 107, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Langton, P.D. Streptomycin inhibition of myogenic tone, K+-induced force and block of L-type calcium current in rat cerebral arteries. J. Physiol. 1998, 508 Pt 3, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.P.; Streamer, M.; Lusambili, L.I.; Sachs, F.; Allen, D.G. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul. Disord. 2006, 16, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Audesirk, G.; Audesirk, T.; Ferguson, C.; Lomme, M.; Shugarts, D.; Rosack, J.; Caracciolo, P.; Gisi, T.; Nichols, P. L-type calcium channels may regulate neurite initiation in cultured chick embryo brain neurons and N1E-115 neuroblastoma cells. Brain Res. Dev. Brain Res. 1990, 55, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Winegar, B.D.; Haws, C.M.; Lansman, J.B. Subconductance block of single mechanosensitive ion channels in skeletal muscle fibers by aminoglycoside antibiotics. J. Gen. Physiol. 1996, 107, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; McBride, D.W., Jr. The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev. 1996, 48, 231–252. [Google Scholar] [PubMed]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef] [PubMed]
- Usselman, R.J.; Hill, I.; Singel, D.J.; Martino, C.F. Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS ONE 2014, 9, e93065. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, F.; Soni, S.S.; Gonzalez-Lima, F.; Liu, H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016, 6, 30540. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Sidders, A.E.; Lu, K.Y.; Velez, A.Z.; Durham, P.G.; Bui, D.T.; Angeles-Solano, M.; Dayton, P.A.; Rowe, S.E. Overcoming biological barriers to improve treatment of a Staphylococcus aureus wound infection. Cell Chem. Biol. 2023, 30, 513–526.e5. [Google Scholar] [CrossRef] [PubMed]

| Protein Target | Vendor, Country | Dilution Factor |
|---|---|---|
| Cyclin D1 (CD1) | Santa Cruz Biotechnology, Dallas, TX, USA | 1:300 |
| Transient receptor potential canonical 1 (TRPC1) | Santa Cruz Biotechnology, Dallas, TX, USA | 1:500 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Proteintech Group, Inc., Rosemont, IL, USA | 1:10,000 |
| Phosphorylated ERK and Total ERK | Santa Cruz Biotechnology, Dallas, TX, USA | 1:300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iversen, J.N.; Fröhlich, J.; Tai, Y.K.; Franco-Obregón, A. Synergistic Cellular Responses Conferred by Concurrent Optical and Magnetic Stimulation Are Attenuated by Simultaneous Exposure to Streptomycin: An Antibiotic Dilemma. Bioengineering 2024, 11, 637. https://doi.org/10.3390/bioengineering11070637
Iversen JN, Fröhlich J, Tai YK, Franco-Obregón A. Synergistic Cellular Responses Conferred by Concurrent Optical and Magnetic Stimulation Are Attenuated by Simultaneous Exposure to Streptomycin: An Antibiotic Dilemma. Bioengineering. 2024; 11(7):637. https://doi.org/10.3390/bioengineering11070637
Chicago/Turabian StyleIversen, Jan Nikolas, Jürg Fröhlich, Yee Kit Tai, and Alfredo Franco-Obregón. 2024. "Synergistic Cellular Responses Conferred by Concurrent Optical and Magnetic Stimulation Are Attenuated by Simultaneous Exposure to Streptomycin: An Antibiotic Dilemma" Bioengineering 11, no. 7: 637. https://doi.org/10.3390/bioengineering11070637







