Pectin as a Biomaterial in Regenerative Endodontics—Assessing Biocompatibility and Antibacterial Efficacy against Common Endodontic Pathogens: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pectin Suspensions Preparation
2.2. Planktonic Bacteria Culture Conditions
2.3. Growth Assays of Planktonic Bacteria
2.4. Dual-Species Biofilm Model
2.5. DPSCs Culture and Cell Expansion
2.6. Pectin Preparation, Plate Coating, and Cell Seeding
2.7. Cytotoxicity Assays
2.8. Cell Proliferation Assay
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results
3.1. Antimicrobial Effect of Pectin
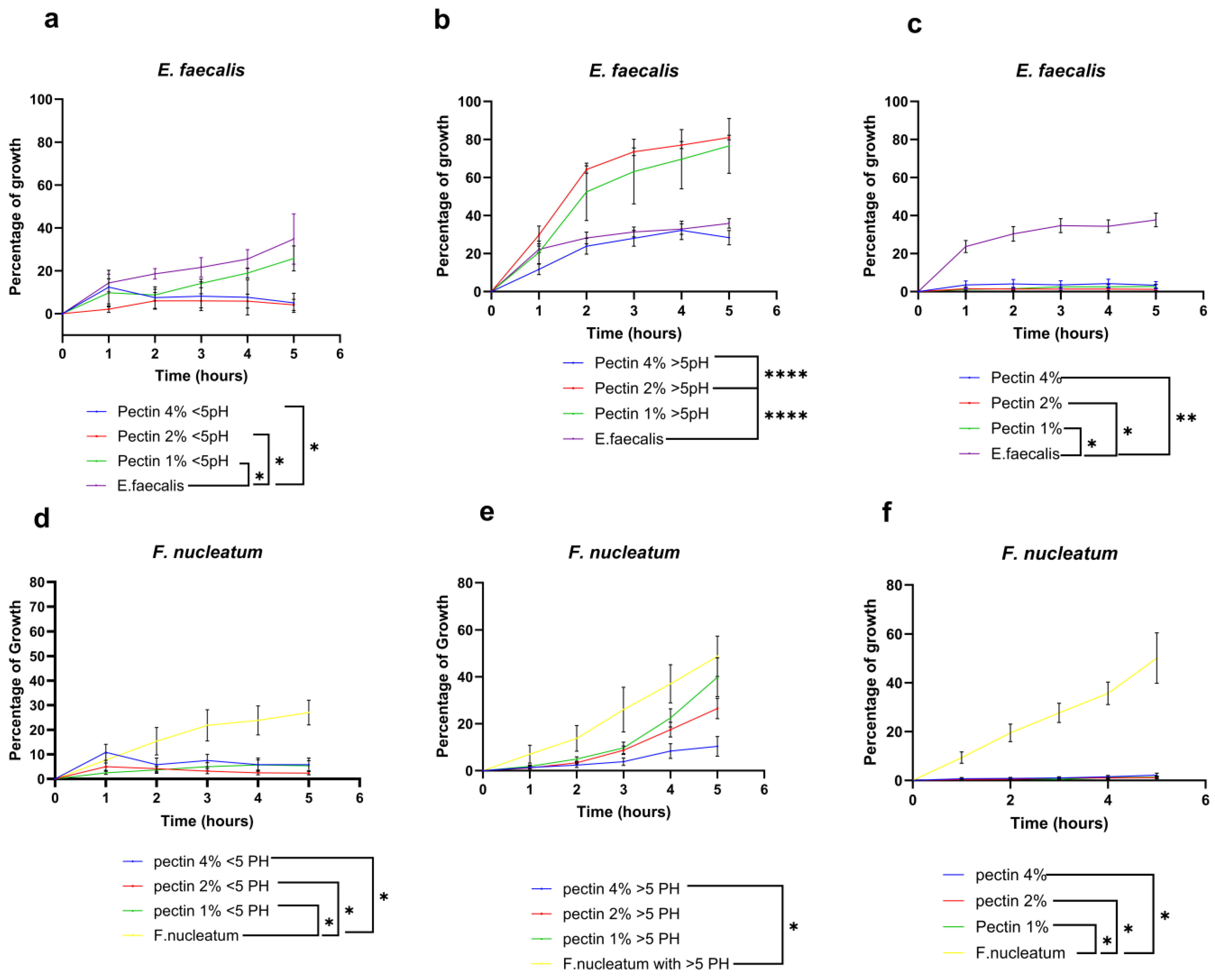
3.1.1. E. faecalis and F. nucleatum Bacterial Growth Curve in Response to Different Concentrations of Pectin Suspension
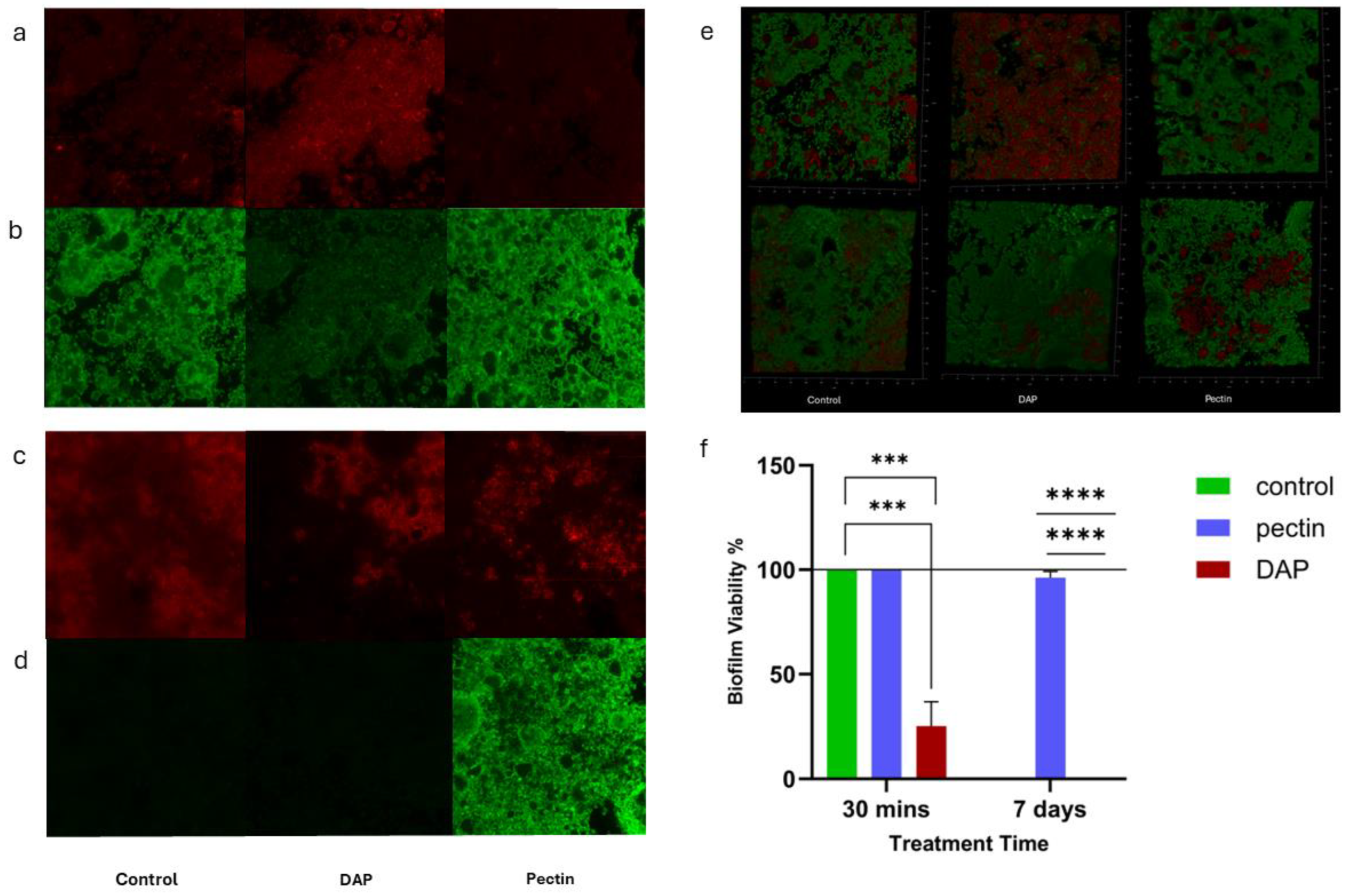
3.1.2. Antimicrobial Effect of Pectin against Dual-Species Biofilm
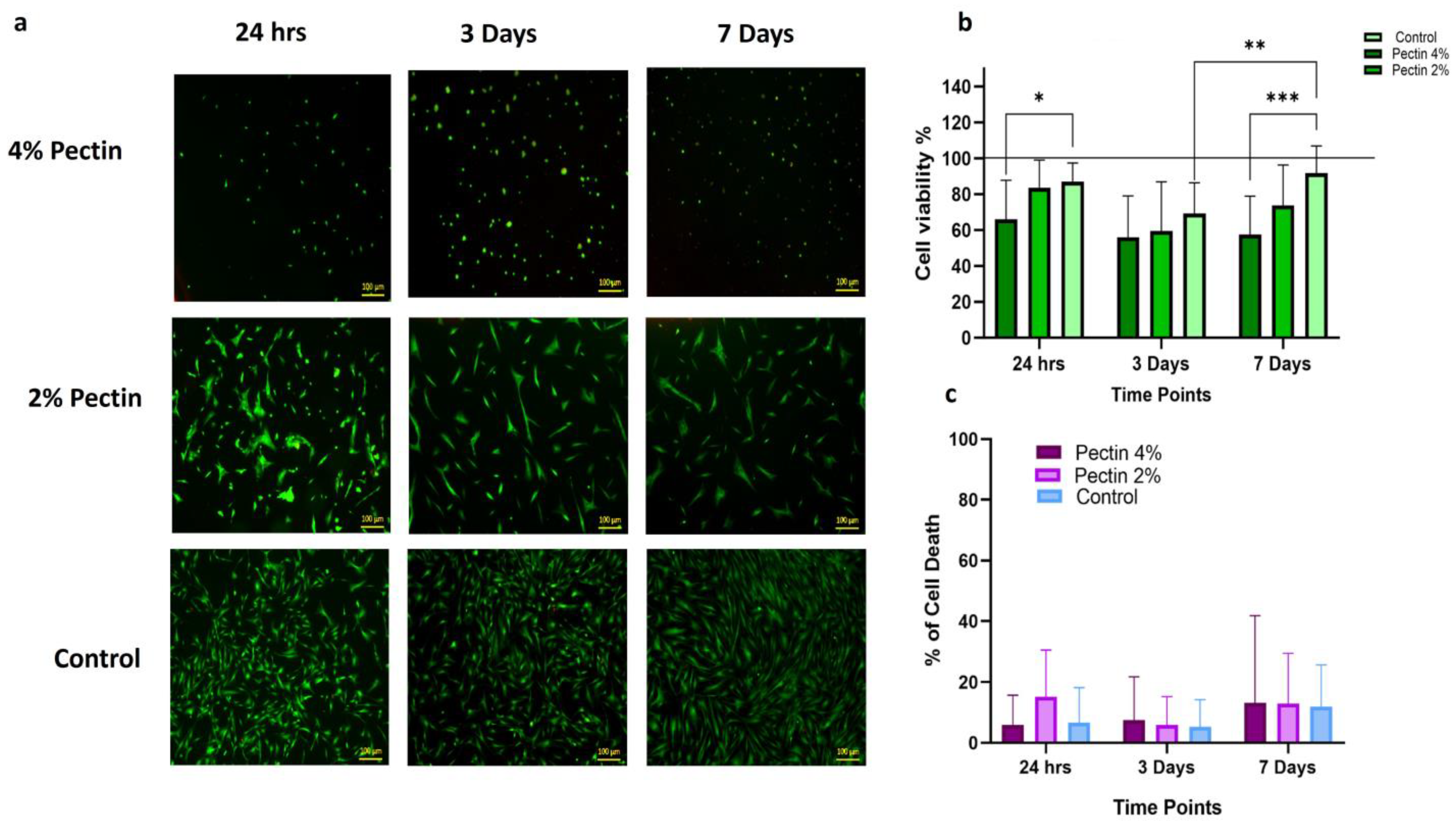
3.1.3. Biocompatibility of Different Concentrations of Pectin with Dental Pulp Stem Cells (DPSCs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ye, L.; Love, R.M.; Farges, J.C.; Yumoto, H. Inflammation of the Dental Pulp. Mediat. Inflamm. 2015, 2015, 980196. [Google Scholar] [CrossRef]
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological Aspects of Root Canal Infections and Disinfection Strategies: An Update Review on the Current Knowledge and Challenges. Front. Oral Health 2021, 2, 672887. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Moraes, T.; Leandro, L.F.; Santiago, M.B.; de Oliveira Silva, L.; Bianchi, T.C.; Veneziani, R.C.S.; Ambrósio, S.R.; Ramos, S.B.; Bastos, J.K.; Martins, C.H.G. Assessment of the antibacterial, antivirulence, and action mechanism of Copaifera pubiflora oleoresin and isolated compounds against oral bacteria. Biomed. Pharmacother. 2020, 129, 110467. [Google Scholar]
- De Paz Villanueva, L.E.C. Fusobacterium nucleatum in endodontic flare-ups. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 93, 179–183. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef] [PubMed]
- Abdulwahab, M.A.; Almotairi, D.M.; Aldawish, B.F.; Alluqmani, S.R.; Dajam, A.A.; Alzahrani, A.A.; Alghamdi, M.S.; Almutairi, S.S.; Alzarea, A.S.; Azzeem, R.A.; et al. Persistence of bacteria and its role in endodontic treatment failure. Int. J. Community Med. Public Health 2021, 9, 14834. [Google Scholar] [CrossRef]
- Logan, W.H.; Kronfeld, R. Development of The Human Jaws and Surrounding Structures from Birth to the Age of Fifteen Years. J. Am. Dent. Assoc. 1993, 20, 379–428. [Google Scholar]
- Ribeiro, J.S.; Münchow, E.A.; Bordini, E.A.F.; da Rosa, W.L.D.O.; Bottino, M.C. Antimicrobial Therapeutics in Regenerative Endodontics: A Scoping Review. J. Endod. 2020, 46, S115–S127. [Google Scholar] [CrossRef]
- Meschi, N.; Palma, P.J.; Cabanillas-Balsera, D. Effectiveness of revitalization in treating apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2023, 56, 510–532. [Google Scholar] [CrossRef] [PubMed]
- Pulyodan, M.K.; Mohan, S.P.; Valsan, D.; Divakar, N.; Moyin, S.; Thayyil, S. Regenerative endodontics: A paradigm shift in clinical endodontics. J. Pharm. Bioallied Sci. 2020, 12, S20–S26. [Google Scholar] [PubMed]
- Jamshidi, D.; Ansari, M.; Gheibi, N. Cytotoxicity and Genotoxicity of Calcium Hydroxide and Two Antibiotic Pastes on Human Stem Cells of The Apical Papilla. Eur. Endod. J. 2021, 6, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Pallasch, T.J. Global Antibiotic Resistance and Its Impact on the Dental Community. J. Calif. Dent. Assoc. 2000, 28, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does apical papilla survive and develop in apical periodontitis presence after regenerative endodontic procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Nazzal, H.; Duggal, M.; El-Gendy, R. What the future holds for regenerative endodontics: Novel antimicrobials and regenerative strategies. Eur. Cells Mater. 2021, 41, 811–833. [Google Scholar] [CrossRef] [PubMed]
- Daoud, Z.; Sura, M.; Abdel-Massih, R.M. Pectin shows antibacterial activity against Helicobacter pylori. Adv. Biosci. Biotechnol. 2013, 4, 273–277. [Google Scholar] [CrossRef]
- Junmahasathien, T.; Panraksa, P.; Protiarn, P.; Hormdee, D.; Noisombut, R.; Kantrong, N.; Jantrawut, P. Preparation and evaluation of metronidazole-loaded pectin films for potentially targeting a microbial infection associated with perio-dontal disease. Polymers 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Haynes, E.; Tompkins, C.A.; Washburn, G.; Winters, M. Bactericidal Action of pectin. Proc. Soc. Exp. Biol. Med. 1937, 36, 839–840. [Google Scholar] [CrossRef]
- El–Nakeeb, M.A.; Yousef, R.T. Study of Antimicrobial Action of Pectin—I. Antibacterial and Antifungal Activities of Pectin. Planta Med. 1970, 18, 201–209. [Google Scholar] [CrossRef]
- Gomes, B.P.; Bronzato, J.D.; Almeida-Gomes, R.F.; Pinheiro, E.T.; Sousa, E.L.; Jacinto, R.C. Identification of Fusobacterium nucleatum in primary and secondary endodontic infections and its association with clinical features by using two different methods. Clin. Oral. Investig. 2021, 25, 6249–6258. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. NPJ Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Bozzini, S.; Visai, L.; Tanzi, M.C.; Petrini, P. Sterilization treatments on polysaccharides: Effects and side effects on pectin. Food Hydrocoll. 2013, 31, 74–84. [Google Scholar] [CrossRef]
- El–Nakeeb, M.A.; Yousef, R.T. Study of Antimicrobial Action of Pectin. II. Factors affecting the bactericidal activity of pectin. Planta Medica 1970, 18, 295–302. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of In-tegral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, O.; Khudyakov, A.; Sergushkina, M.; Solomina, O.; Polezhaeva, T. Pectins as a universal medicine. Fitoterapia 2020, 146, 104676. [Google Scholar] [CrossRef]
- Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Martino, D.C.; Alduina, R.; et al. A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics 2020, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.d.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Prickett, P.S.; Miller, N.J. Effect of Pectin on Bacterial Growth. Proc. Soc. Exp. Biol. Med. 1939, 40, 27–28. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.M.; Pagliaro, M. Pectin: A Long-Neglected Broad-Spectrum Antibacterial. ChemMedChem 2020, 15, 2228–2235. [Google Scholar] [CrossRef]
- Men’Shikov, D.D.; Lazareva, E.B.; Popova, T.S.; Shramko, L.U.; Tokaev, I.S.; Zalogueva, G.V.; Gaponova, I.N. Antimicrobial properties of pectins and their effects on antibiotics. Antibiot. I Khimioter. Antibiot. Chemoterapy 1997, 42, 10–15. [Google Scholar]
- Zaporozhets, T.S.; Besednova, N.N.; Liamkin, G.P.; Loenko, I.N.; Popov, A. Antibacterial and therapeutic effectiveness of a pectin from sea grass Zostera. Antibiot. Khimioter. 1991, 36, 24–26. [Google Scholar]
- Haile, S.; Ayele, A. Pectinase from Microorganisms and Its Industrial Applications. Sci. World J. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Gibson, G.R.; Rastall, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef]
- Torimiro, N.; Okonji, E.R. A comparative study of pectinolytic enzyme production by Bacillus species. Afr. J. Biotechnol. 2013, 12, 6498–6503. [Google Scholar] [CrossRef]
- Larsen, N.; de Souza, C.B.; Krych, L.; Cahú, T.B.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Thunyakipisal, P.; Saladyanant, T.; Hongprasong, N.; Pongsamart, S.; Apinhasmit, W. Antibacterial activity of poly-saccharide gel extract from fruit rinds of Durio zibethinus Murr. against oral pathogenic bacteria. J. Investig. Clin. Dent. 2010, 1, 120–125. [Google Scholar] [CrossRef]
- Wooldridge, W.E. Effects of uronic acids, pectins and pectinates on the enteric flora, alone and in combination with antibiotics: I. In vitro studies. Am. J. Surg. 1949, 78, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Cahú, T.B.; Saad, S.M.I.; Blennow, A.; Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef]
- Rahmawati, I.S.R.I.S.; Kusumaningrum, H.D.K.H.D.; Yuliana, N.D.Y.N.D. Effect of Sterilization on the Degree of Esterification, FTIR Analysis, and Antibacterial Activity of Durian-Rind Pectin. Sains Malays. 2022, 51, 3677–3688. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.; Morck, D.; Storey, D.; Read, R.; Buret, A.; Olson, B. [25] The MBEC assay system: Multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 2001, 337, 377–385. [Google Scholar] [PubMed]
- Douglas, T.E.; Dziadek, M.; Schietse, J.; Boone, M.; Declercq, H.A.; Coenye, T.; Vanhoorne, V.; Vervaet, C.; Balcaen, L.; Buchweitz, M.; et al. Pectin-bioactive glass self-gelling, injectable composites with high antibacterial activity. Carbohydr. Polym. 2018, 205, 427–436. [Google Scholar] [CrossRef]
- American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedures. 2021. Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2021/08/ClinicalConsiderationsApprovedByREC062921.pdf (accessed on 7 July 2023).
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-rot095497. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Amiri-Rigi, A.; Abdoun-Ouallouche, K.; Aberkane, L.; Djefal-Kerrar, A.; Mahomoodally, M.F.; Emmambux, M.N. Assessing the bioactivity, cytotoxicity, and rheological properties of pectin recovered from citrus peels. Food Biosci. 2022, 46, 101550. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional Antioxidant, Non-Cytotoxic Activity of Integral Lemon Pectin from Hydrodynamic Cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Gurzawska, K.; Svava, R.; Syberg, S.; Yihua, Y.; Haugshøj, K.B.; Damager, I.; Ulvskov, P.; Christensen, L.H.; Gotfredsen, K.; Jørgensen, N.R. Effect of nanocoating with rham-nogalacturonan-I on surface properties and osteoblasts response. J. Biomed. Mater. Res. A 2012, 100, 654–664. [Google Scholar] [CrossRef]
- Folkert, J.; Mieszkowska, A.; Gaber, T.; Miksch, K.; Dirscherl, K.; Gurzawska, K. Surface Nanocoating with Plant-Derived Pectins Improves Fibroblast Response In Vitro. Starch Starke 2018, 71, 162. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Zhang, R.; Li, R.; Yin, Y.; Wang, H.; Liu, Y.; Yao, F.; Yao, K. Modulation of mesenchymal stem cells behaviors by chitosan/gelatin/pectin network films. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 308–319. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rheological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Lee, Y.L.; Lin, F.H.; Hasirci, V.; Tezcaner, A. Injectable methacrylated gelatin/thiolated pectin hy-drogels carrying melatonin/tideglusib-loaded core/shell PMMA/silk fibroin electrospun fibers for vital pulp re-generation. Colloids Surf. B Biointerfaces 2023, 222, 113078. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelgawad, R.M.; Damé-Teixeira, N.; Gurzawska-Comis, K.; Alghamdi, A.; Mahran, A.H.; Elbackly, R.; Do, T.; El-Gendy, R. Pectin as a Biomaterial in Regenerative Endodontics—Assessing Biocompatibility and Antibacterial Efficacy against Common Endodontic Pathogens: An In Vitro Study. Bioengineering 2024, 11, 653. https://doi.org/10.3390/bioengineering11070653
Abdelgawad RM, Damé-Teixeira N, Gurzawska-Comis K, Alghamdi A, Mahran AH, Elbackly R, Do T, El-Gendy R. Pectin as a Biomaterial in Regenerative Endodontics—Assessing Biocompatibility and Antibacterial Efficacy against Common Endodontic Pathogens: An In Vitro Study. Bioengineering. 2024; 11(7):653. https://doi.org/10.3390/bioengineering11070653
Chicago/Turabian StyleAbdelgawad, Raghda Magdy, Nailê Damé-Teixeira, Katarzyna Gurzawska-Comis, Arwa Alghamdi, Abeer H. Mahran, Rania Elbackly, Thuy Do, and Reem El-Gendy. 2024. "Pectin as a Biomaterial in Regenerative Endodontics—Assessing Biocompatibility and Antibacterial Efficacy against Common Endodontic Pathogens: An In Vitro Study" Bioengineering 11, no. 7: 653. https://doi.org/10.3390/bioengineering11070653





