Mesenchymal Stromal Cells for the Enhancement of Surgical Flexor Tendon Repair in Animal Models: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Algorithm
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
- Study characteristics, including the study design, animal model, cohort size, tendon defect location, post-operative weight-bearing protocol and timing of sacrifice.
- The type of intervention, including MSC source, cell delivery method, composition of the delivery method, cell number and/or density, and method of surgical repair.
- Biomechanical properties including maximum load, surrogate measures of adhesion formation, maximum stress, maximum strain, elastic modulus, and energy absorption.
2.4. Data Analysis
2.5. Assessing Risk of Bias
- Bias arising from the randomization process
- Was the allocation system random?
- Was the allocation sequence concealed until participants were enrolled and assigned to interventions?
- Did baseline differences between intervention groups suggest a problem with the randomisation process?
- Bias due to deviations from the intended interventions
- Were participants aware of their assigned intervention during the trial?
- Were carers and people delivering the interventions aware of participants’ assigned intervention during the trial?
- If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the trial context?
- If Y/PY/NI to 2.3: Were these deviations likely to have affected the outcome?
- If Y/PY to 2.4: Were these deviations from intended intervention balanced between groups?
- Was an appropriate analysis used to estimate the effect of assignment to intervention?
- If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomised?
- Bias due to missing outcome data
- Were data for this outcome available for all, or nearly all, participants randomised?
- If N/PN/NI to 3.1: Is there evidence that the result was not biased by missing outcome data?
- If N/PN to 3.2: Could missingness in the outcome depend on its true value?
- If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value?
- Bias in measurement of the outcome
- Was the method of measuring the outcome inappropriate?
- Could measurement or ascertainment of the outcome have differed between intervention groups?
- If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants?
- If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received?
- If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received?
- Bias in selection of the reported result
- 1.
- Were the data that produced this result analysed in accordance with a prespecified analysis plan that was finalised before unblinded outcome data were available for analysis?
- Is the numerical result being assessed likely to have been selected, on the basis of the results, from the following:
- 2.
- Multiple eligible outcome measurements (e.g., scales, definitions, time points) within the outcome domain?
- 3.
- Multiple eligible analyses of the data?
3. Results
3.1. Search Results
3.2. Study Characteristics
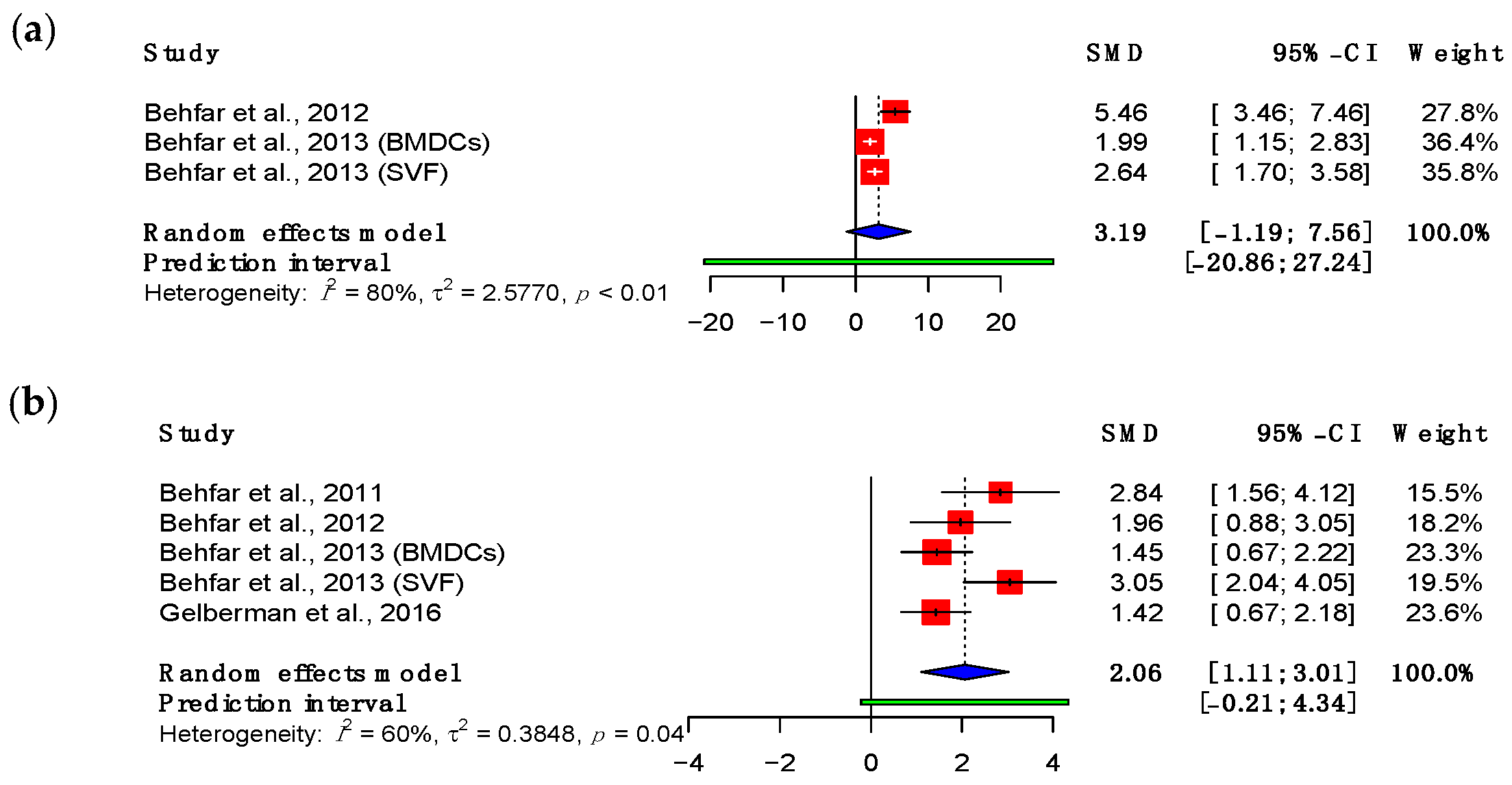
3.3. Mechanical Properties
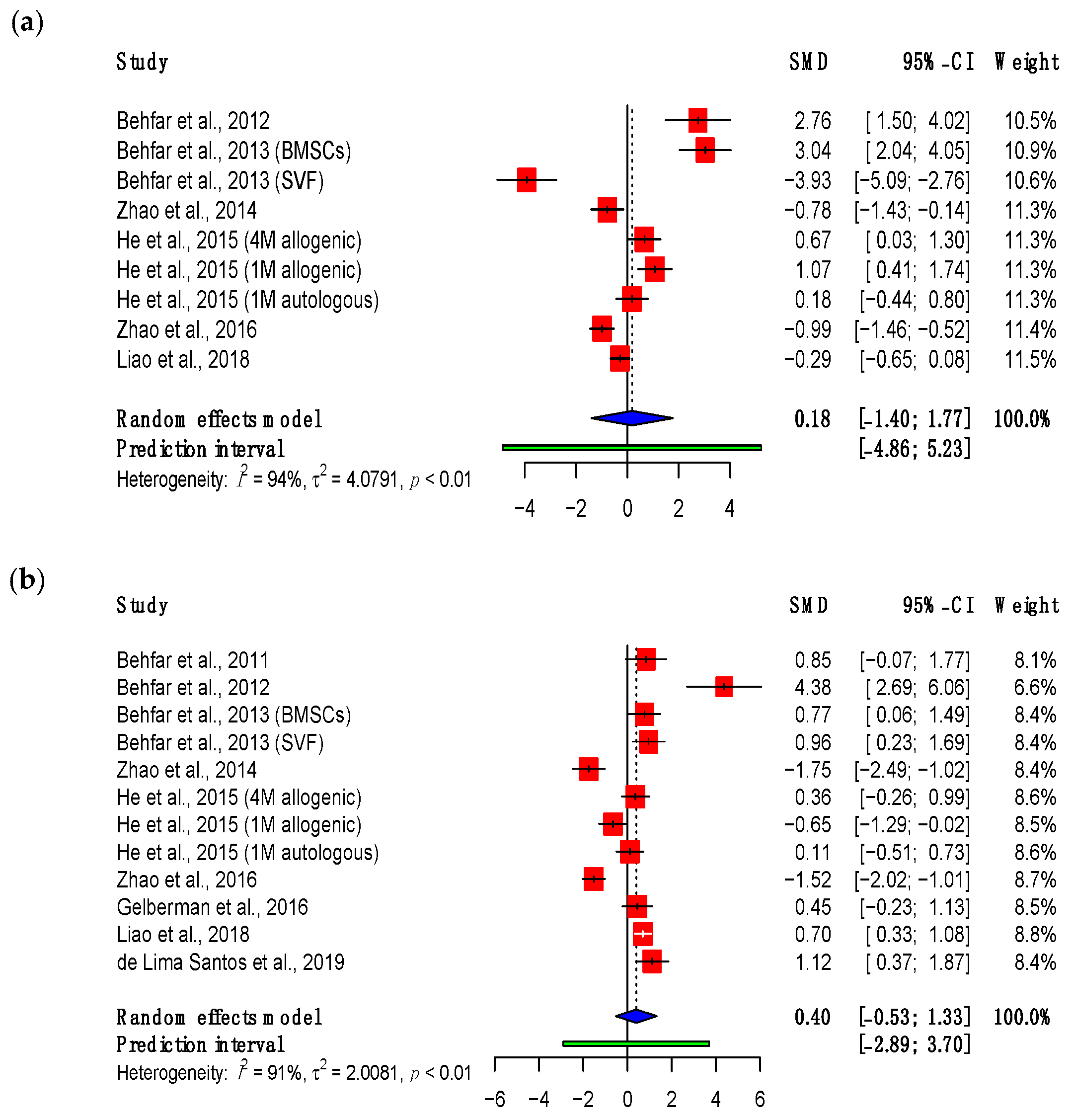
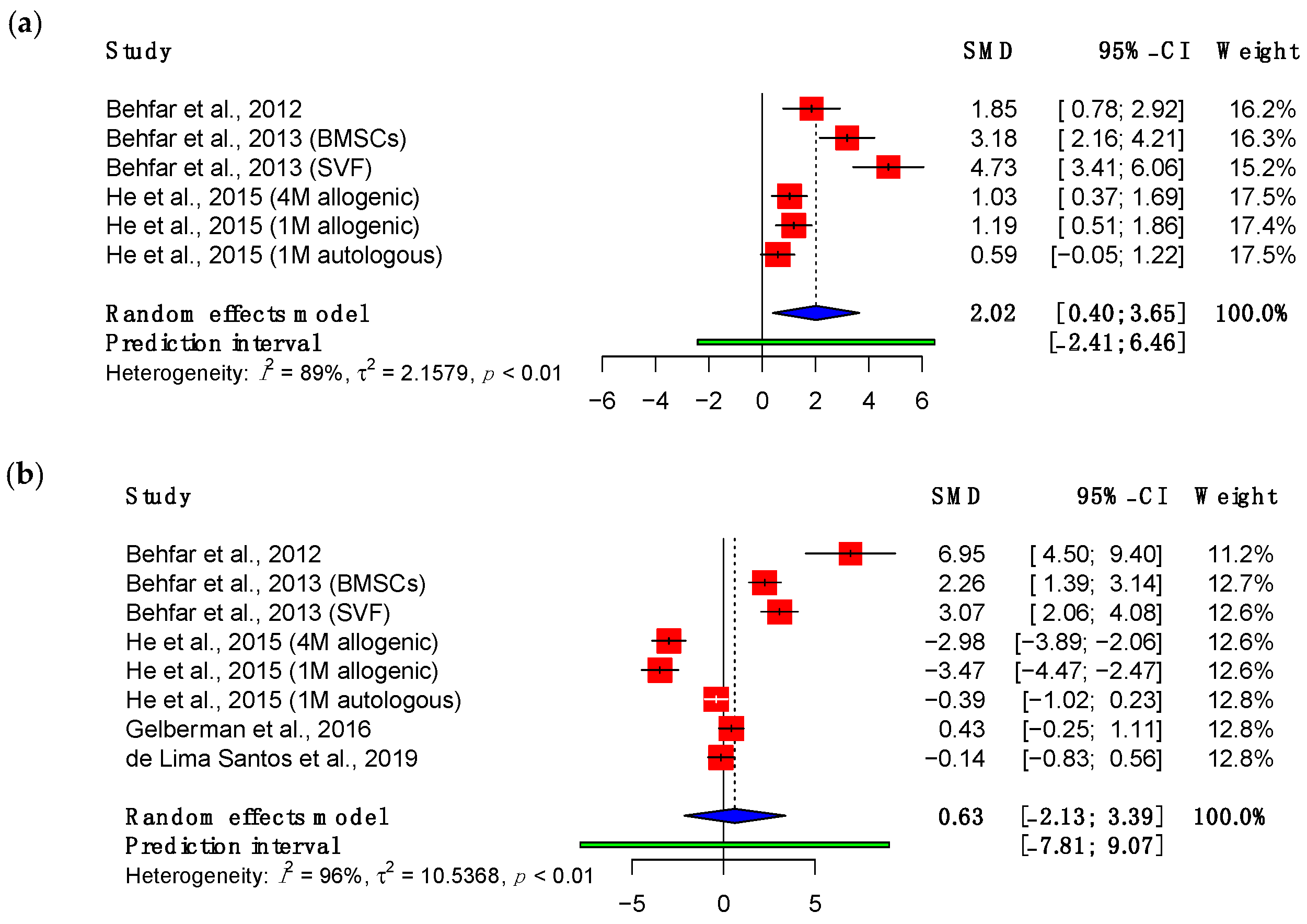
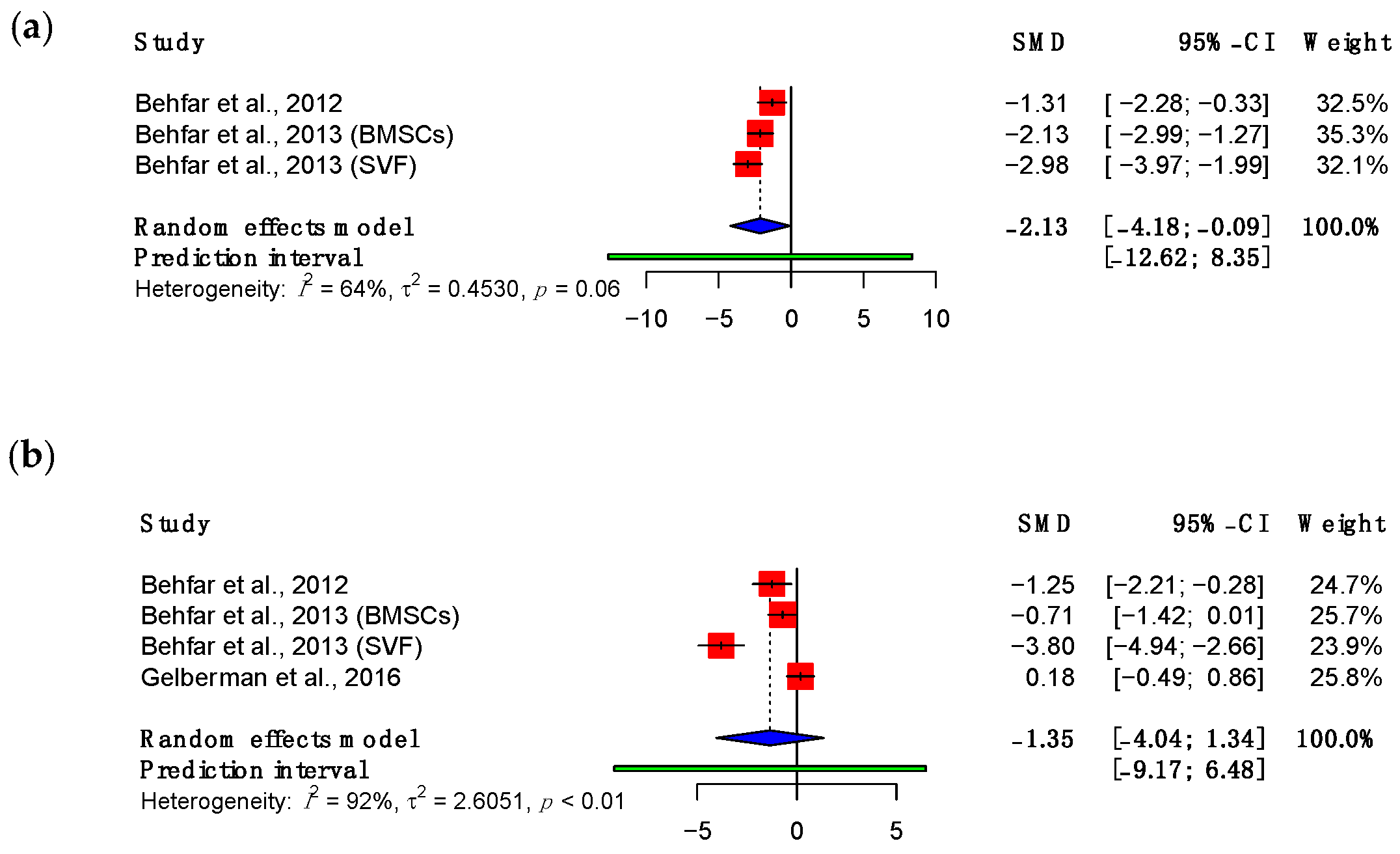
3.3.1. Maximum Load
3.3.2. Adhesions
3.3.3. Viscoelastic Properties
3.4. Subgroup Meta-Analyses
3.5. Risk of Bias Assessment
4. Discussion
4.1. Biomechanical Properties
4.1.1. Maximum Load and Adhesion Formation
4.1.2. Viscoelastic Behaviour
4.2. Challenges in Tendon Tissue Engineering and Considerations for Future Research
4.2.1. Mesenchymal Stromal Cells
4.2.2. Experimental Models
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, L.S.; Sarkies, M.; Brown, T.; O’Brien, L. Direct, indirect and intangible costs of acute hand and wrist injuries: A systematic review. Injury 2016, 47, 2614–2626. [Google Scholar] [CrossRef] [PubMed]
- de Putter, C.E.; Selles, R.W.; Polinder, S.; Panneman, M.J.; Hovius, S.E.; van Beeck, E.F. Economic impact of hand and wrist injuries: Health-care costs and productivity costs in a population-based study. J. Bone Jt. Surg. 2012, 94, e56. [Google Scholar] [CrossRef] [PubMed]
- Polinder, S.; Iordens, G.I.; Panneman, M.J.; Eygendaal, D.; Patka, P.; Den Hartog, D.; Van Lieshout, E.M. Trends in incidence and costs of injuries to the shoulder, arm and wrist in The Netherlands between 1986 and 2008. BMC Public Health 2013, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Venkatramani, H.; Varadharajan, V.; Bhardwaj, P.; Vallurupalli, A.; Sabapathy, S.R. Flexor tendon injuries. J. Clin. Orthop. Trauma 2019, 10, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, R.; Mookerjee, V.; Schmidt, S.; Jehle, C.C.; Kiwanuka, E.; Liu, P.Y. The Economic Impact of Flexor Tendon Lacerations of the Hand in the United States. Ann. Plast. Surg. 2019, 83, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Grunert, B.K.; Matloub, H.S.; Sanger, J.R.; Yousif, N.J. Treatment of posttraumatic stress disorder after work-related hand trauma. J. Hand Surg. 1990, 15, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.A.; Caruso, J.C.; Fallahi, A.K.M.; Patiño, J.M. Flexor Tendon Lacerations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tang, J.B.; Lalonde, D.; Harhaus, L.; Sadek, A.F.; Moriya, K.; Pan, Z.J. Flexor tendon repair: Recent changes and current methods. J. Hand Surg. Eur. Vol. 2022, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Skirven, T.M.; DeTullio, L.M. Therapy after Flexor Tendon Repair. Hand Clin. 2023, 39, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Calfee, R.P. General Principles of Flexor Tendon Repair. Hand Clin. 2023, 39, 131–139. [Google Scholar] [CrossRef]
- Elliot, D.; Giesen, T. Treatment of unfavourable results of flexor tendon surgery: Ruptured repairs, tethered repairs and pulley incompetence. Indian J. Plast. Surg. 2013, 46, 458–471. [Google Scholar] [CrossRef]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon injury: From biology to tendon repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Citro, V.; Clerici, M.; Boccaccini, A.R.; Della Porta, G.; Maffulli, N.; Forsyth, N.R. Tendon tissue engineering: An overview of biologics to promote tendon healing and repair. J. Tissue Eng. 2023, 14, 20417314231196275. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. 2008, 33, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Screen, H.R.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Nerlich, M.; Pfeifer, C.G.; Docheva, D. Boosting tendon repair: Interplay of cells, growth factors and scaffold-free and gel-based carriers. J. Exp. Orthop. 2018, 5, 1. [Google Scholar] [CrossRef]
- Lee, C. Tendon physiology and repair. Orthop. Trauma 2021, 35, 274–281. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Amadio, P.C. Flexor Tendon Adhesion Formation: Current Concepts. Hand Clin. 2023, 39, 171–180. [Google Scholar] [CrossRef]
- Rawson, S.; Cartmell, S.; Wong, J. Suture techniques for tendon repair; a comparative review. Muscles Ligaments Tendons J. 2013, 3, 220–228. [Google Scholar] [CrossRef]
- Wieskötter, B.; Herbort, M.; Langer, M.; Raschke, M.J.; Wähnert, D. The impact of different peripheral suture techniques on the biomechanical stability in flexor tendon repair. Arch. Orthop. Trauma Surg. 2018, 138, 139–145. [Google Scholar] [CrossRef]
- Mishra, V.; Kuiper, J.H.; Kelly, C.P. Influence of core suture material and peripheral repair technique on the strength of Kessler flexor tendon repair. J. Hand Surg. 2003, 28, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lyras, D.N.; Kazakos, K.; Verettas, D.; Botaitis, S.; Agrogiannis, G.; Kokka, A.; Pitiakoudis, M.; Kotzakaris, A. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch. Orthop. Trauma Surg. 2009, 129, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, H.; Li, Y.; Yun, Z.; Zhang, Z.; Zhu, Q. Effectiveness of platelet-rich plasma injections for the treatment of acute Achilles tendon rupture: A systematic review and meta-analysis. Medicine 2021, 100, e27526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hashimoto, T.; Kirk, R.L.; Thoreson, A.R.; Jay, G.D.; Moran, S.L.; An, K.N.; Amadio, P.C. Resurfacing with chemically modified hyaluronic acid and lubricin for flexor tendon reconstruction. J. Orthop. Res. 2013, 31, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wei, Z.; Reisdorf, R.L.; Thoreson, A.R.; Jay, G.D.; Moran, S.L.; An, K.N.; Amadio, P.C. The effects of biological lubricating molecules on flexor tendon reconstruction in a canine allograft model in vivo. Plast. Reconstr. Surg. 2014, 133, 628e–637e. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, S.; Li, E.N.; Fritch, M.R.; Alexander, P.G.; Lin, H.; Tuan, R.S. Tissue Engineering for Musculoskeletal Regeneration and Disease Modeling. Handb. Exp. Pharmacol. 2021, 265, 235–268. [Google Scholar]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Xu, H.; Lin, Z.; Chang, H.; Liu, W.; Kong, L. Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J. Orthop. Transl. 2020, 24, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef]
- Rynecki, N.D.; Pereira, D.S. The Role of Mesenchymal Stem Cells in Augmenting Rotator Cuff Repairs. Bull. Hosp. Jt. Dis. 2018, 76, 232–237. [Google Scholar]
- Cho, W.S.; Chung, S.G.; Kim, W.; Jo, C.H.; Lee, S.U.; Lee, S.Y. Mesenchymal Stem Cells Use in the Treatment of Tendon Disorders: A Systematic Review and Meta-Analysis of Prospective Clinical Studies. Ann. Rehabil. Med. 2021, 45, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Cai, X.; Bian, Y.; Wei, Z.; Zhu, W.; Zhao, X.; Weng, X. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering 2023, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bowles-Welch, A.C.; Jimenez, A.C.; Stevens, H.Y.; Frey Rubio, D.A.; Kippner, L.E.; Yeago, C.; Roy, K. Mesenchymal stromal cells for bone trauma, defects, and disease: Considerations for manufacturing, clinical translation, and effective treatments. Bone Rep. 2023, 18, 101656. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Salameh, J.P.; Bossuyt, P.M.; McGrath, T.A.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; Korevaar, D.A.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. Some Methods for Strengthening the Common χ2 Tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Behfar, M.; Sarrafzadeh-Rezaei, F.; Hobbenaghi, R.; Delirezh, N.; Dalir-Naghadeh, B. Adipose-derived stromal vascular fraction improves tendon healing in rabbits. Chin. J. Traumatol. 2011, 14, 329–335. [Google Scholar] [PubMed]
- Behfar, M.; Sarrafzadeh-Rezaei, F.; Hobbenaghi, R.; Delirezh, N.; Dalir-Naghadeh, B. Enhanced mechanical properties of rabbit flexor tendons in response to intratendinous injection of adipose derived stromal vascular fraction. Curr. Stem Cell Res. Ther. 2012, 7, 173–178. [Google Scholar] [CrossRef]
- Behfar, M.; Javanmardi, S.; Sarrafzadeh-Rezaei, F. Comparative study on functional effects of allotransplantation of bone marrow stromal cells and adipose derived stromal vascular fraction on tendon repair: A biomechanical study in rabbits. Cell J. 2014, 16, 263–270. [Google Scholar]
- Zhao, C.; Ozasa, Y.; Reisdorf, R.L.; Thoreson, A.R.; Jay, G.D.; An, K.N.; Amadio, P.C. CORR® ORS Richard A. Brand Award for Outstanding Orthopaedic Research: Engineering flexor tendon repair with lubricant, cells, and cytokines in a canine model. Clin. Orthop. Relat. Res. 2014, 472, 2569–2578. [Google Scholar] [CrossRef]
- He, M.; Gan, A.W.; Lim, A.Y.; Goh, J.C.; Hui, J.H.; Chong, A.K. Bone Marrow Derived Mesenchymal Stem Cell Augmentation of Rabbit Flexor Tendon Healing. Hand Surg. 2015, 20, 421–429. [Google Scholar] [CrossRef]
- Zhao, C.; Ozasa, Y.; Shimura, H.; Reisdorf, R.L.; Thoreson, A.R.; Jay, G.; Moran, S.L.; An, K.N.; Amadio, P.C. Effects of lubricant and autologous bone marrow stromal cell augmentation on immobilized flexor tendon repairs. J. Orthop. Res. 2016, 34, 154–160. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Shen, H.; Kormpakis, I.; Rothrauff, B.; Yang, G.; Tuan, R.S.; Xia, Y.; Sakiyama-Elbert, S.; Silva, M.J.; Thomopoulos, S. Effect of adipose-derived stromal cells and BMP12 on intrasynovial tendon repair: A biomechanical, biochemical, and proteomics study. J. Orthop. Res. 2016, 34, 630–640. [Google Scholar] [CrossRef]
- Liao, J.C.Y.; He, M.; Gan, A.W.T.; Wen, F.; Tan, L.P.; Chong, A.K.S. The effects of bi-functional anti-adhesion scaffolds on flexor tendon healing in a rabbit model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- de Lima Santos, A.; Silva, C.G.D.; de Sá Barretto, L.S.; Franciozi, C.; Tamaoki, M.J.S.; de Almeida, F.G.; Faloppa, F. Biomechanical evaluation of tendon regeneration with adipose-derived stem cell. J. Orthop. Res. 2019, 37, 1281–1286. [Google Scholar] [CrossRef]
- Gordon, K.; Brett, A.; Weber, J.F. Chapter 22–Uniaxial Biomechanical Testing of Ligaments and Tendons. In Experimental Methods in Orthopaedic Biomechanics; Zdero, R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 349–362. [Google Scholar]
- Christiansen, D.L.; Huang, E.K.; Silver, F.H. Assembly of type I collagen: Fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000, 19, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.R.; Evans, E.B.; Matuszewski, P.E.; Chen, Y.L.; Satchel, L.N.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Rockel, J.S.; Rabani, R.; Viswanathan, S. Anti-fibrotic mechanisms of exogenously-expanded mesenchymal stromal cells for fibrotic diseases. Semin. Cell Dev. Biol. 2020, 101, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yoneda, S.; Abu-Amer, Y.; Guilak, F.; Gelberman, R.H. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J. Orthop. Res. 2020, 38, 117–127. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, J.; Chen, Y.; Lyu, K.; Long, L.; Wang, X.; Liu, T.; Li, S. Mesenchymal stem cells: An efficient cell therapy for tendon repair (Review). Int. J. Mol. Med. 2023, 52, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chartier, C.; ElHawary, H.; Baradaran, A.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Tendon: Principles of Healing and Repair. Semin. Plast. Surg. 2021, 35, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Gott, M.; Ast, M.; Lane, L.B.; Schwartz, J.A.; Catanzano, A.; Razzano, P.; Grande, D.A. Tendon phenotype should dictate tissue engineering modality in tendon repair: A review. Discov. Med. 2011, 12, 75–84. [Google Scholar] [PubMed]
- Li, L.; Chen, X.; Wang, W.E.; Zeng, C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Stem Cells Int. 2016, 2016, 9682757. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.A.; Antunes, M.A.; Dos Santos, C.C.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467–479. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Nakayamada, S.; Tanaka, Y. Use of mesenchymal stem cells seeded on the scaffold in articular cartilage repair. Inflamm. Regen. 2018, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Frauz, K.; Teodoro, L.F.R.; Carneiro, G.D.; Cristina da Veiga, F.; Lopes Ferrucci, D.; Luis Bombeiro, A.; Waleska Simões, P.; Elvira Álvares, L.; Leite, R.d.O.A.; Pontes Vicente, C.; et al. Transected Tendon Treated with a New Fibrin Sealant Alone or Associated with Adipose-Derived Stem Cells. Cells 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Toledo, E.; Rose, M.; Agu, E.; Dahlenburg, H.; Yao, W.; Nolta, J.A.; Zhou, P. Enhancing Retention of Human Bone Marrow Mesenchymal Stem Cells with Prosurvival Factors Promotes Angiogenesis in a Mouse Model of Limb Ischemia. Stem Cells Dev. 2019, 28, 114–119. [Google Scholar] [CrossRef]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef]
- Bogaerts, S.; Desmet, H.; Slagmolen, P.; Peers, K. Strain mapping in the Achilles tendon—A systematic review. J. Biomech. 2016, 49, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, E.; Mahieu, N.; Roosen, P.; McNair, P. The role of stretching in tendon injuries. Br. J. Sports Med. 2007, 41, 224–226. [Google Scholar] [CrossRef]
- Seynnes, O.R.; Bojsen-Møller, J.; Albracht, K.; Arndt, A.; Cronin, N.J.; Finni, T.; Magnusson, S.P. Ultrasound-based testing of tendon mechanical properties: A critical evaluation. J. Appl. Physiol. 2015, 118, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Connizzo, B.K.; Yannascoli, S.M.; Soslowsky, L.J. Structure-function relationships of postnatal tendon development: A parallel to healing. Matrix Biol. 2013, 32, 106–116. [Google Scholar] [CrossRef]
- Chong, A.K.; Ang, A.D.; Goh, J.C.; Hui, J.H.; Lim, A.Y.; Lee, E.H.; Lim, B.H. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J. Bone Jt. Surg. 2007, 89, 74–81. [Google Scholar] [CrossRef]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. npj Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Asadi Rad, A.; Assadzadeh-Aghdaei, H.; Sharifian, A.; Zali, M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol. Hepatol. Bed Bench 2017, 10, 208–213. [Google Scholar] [PubMed]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- El-Badawy, A.; Amer, M.; Abdelbaset, R.; Sherif, S.N.; Abo-Elela, M.; Ghallab, Y.H.; Abdelhamid, H.; Ismail, Y.; El-Badri, N. Adipose Stem Cells Display Higher Regenerative Capacities and More Adaptable Electro-Kinetic Properties Compared to Bone Marrow-Derived Mesenchymal Stromal Cells. Sci. Rep. 2016, 6, 37801. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Hu, X.; Zhang, X.; Zhu, J.; Zhang, J.; Fu, X.; Duan, X.; Ao, Y.; Zhou, C. Different tenogenic differentiation capacities of different mesenchymal stem cells in the presence of BMP-12. J. Transl. Med. 2015, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Agaverdiev, M.; Shamsov, B.; Mirzoev, S.; Vardikyan, A.; Ramirez, M.E.; Nurmukhametov, R.; Beilerli, A.; Zhang, B.; Gareev, I.; Pavlov, V. MiRNA regulated therapeutic potential of the stromal vascular fraction: Current clinical applications—A systematic review. Non-Coding RNA Res. 2023, 8, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef] [PubMed]
- Hast, M.W.; Zuskov, A.; Soslowsky, L.J. The role of animal models in tendon research. Bone Jt. Res. 2014, 3, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.P.; Kelley, B.P.; Waljee, J.F.; Chung, K.C. Effect of Time to Hand Therapy following Zone II Flexor Tendon Repair. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3278. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.E.; Jha, B.; Ross, M. Rehabilitation following surgery for flexor tendon injuries of the hand. Cochrane Database Syst. Rev. 2021, 1, CD012479. [Google Scholar] [CrossRef]
- Little, D.; Amadio, P.C.; Awad, H.A.; Cone, S.G.; Dyment, N.A.; Fisher, M.B.; Huang, A.H.; Koch, D.W.; Kuntz, A.F.; Madi, R.; et al. Preclinical tendon and ligament models: Beyond the 3Rs (replacement, reduction, and refinement) to 5W1H (why, who, what, where, when, how). J. Orthop. Res. 2023, 41, 2133–2162. [Google Scholar] [CrossRef]
- Ozturk, M.B.; Basat, S.O.; Kayadibi, T.; Karahangil, M.; Akan, I.M. Atraumatic Flexor tendon retrieval- a simple method. Ann. Surg. Innov. Res. 2013, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.R.; Sala, M.; Nikkhah, D. Flexor tendon retrieval in zone 2 using the push-pull technique. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 685–710. [Google Scholar] [CrossRef] [PubMed]
- Kadar, A.; Gur, S.; Schermann, H.; Iordache, S.D. Techniques for Retrieval of Lacerated Flexor Tendons: A Scoping Review. Plast. Surg. 2024, 32, 127–137. [Google Scholar] [CrossRef] [PubMed]

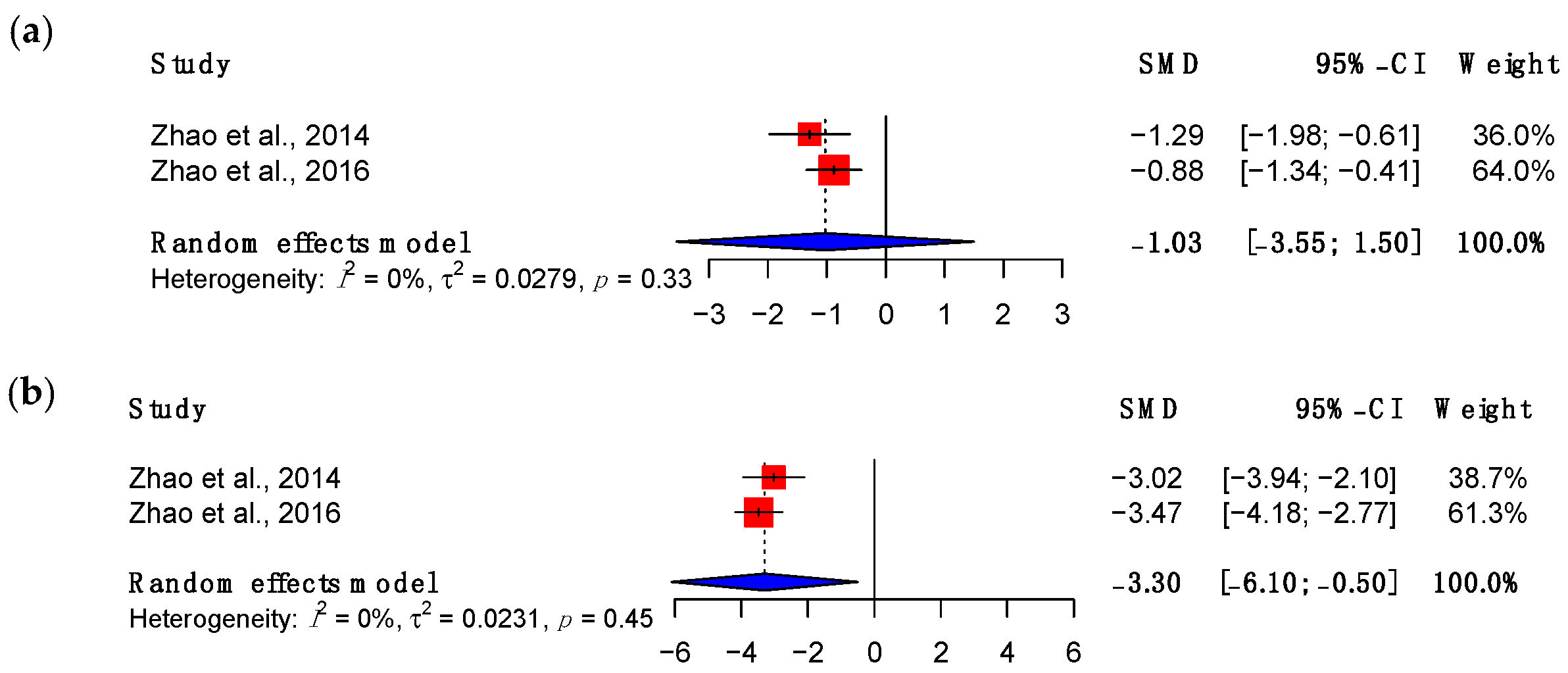


| Domain | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Any animals with completely transected digital flexor tendons. | Studies involving human or cadaveric subjects. Studies investigating repair of other tendons or the healing of tendons which are not completely transected. Ex vivo, in vitro, or in silico studies. |
| Intervention | Studies investigating allogenic and/or autologous MSC delivery to the injury site in addition to surgical repair. Studies using any cell delivery method, including intratendinous injection, gel droplets and scaffold implants. | Studies involving only cell-free therapies without comparison with MSCs or cell therapies which are not MSCs. |
| Comparison | Studies that compare the use of MSCs to other tissue engineering techniques or cell-free therapy. | None |
| Outcome | Studies that provide quantitative outcomes from mechanical testing of flexor tendons after surgery. | Studies which provide only qualitative outcome data. |
| Study type | Controlled trials, case series, articles published in English with full-text available. | Case reports and review articles. |
| Author, Year | Design | Animal | Cohort Size | Defect Location | Intervention | MSC Source | Cell Delivery Method | Cell Dosage | Repair Method | Post-Operative Weight Bearing | Timing of Sacrifice |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Behfar et al., 2011 [47] | RCT | Adult male New Zealand white rabbits, 2.5–3 kg | 25 (5 adipose tissue donors, 10 treated, 10 control) | Deep digital flexor tendon, central one third | Fresh stromal vascular fraction from enzymatic digestion of adipose tissue | Allogeneic adipose-derived stromal vascular fraction, obtained from inguinal fat pad | Intratendinous injection in both tendon stumps and the repair site | 4 × 106 nucleated cells in 0.2 mL PBS | 3-0 monofilament nylon, modified Kessler technique | Immobilization in below-stifle plaster cast for 2 weeks. | 8 weeks |
| Control: Suture + PBS injection | 0.2 mL PBS alone | ||||||||||
| Behfar et al., 2012 [48] | RCT | Adult male New Zealand white rabbits, 2.5–3 kg | 25 (5 adipose tissue donors, 10 treated, 10 control) | Deep digital flexor tendon, central one third | Allogeneic stromal vascular fraction | Allogeneic adipose-derived stromal vascular fraction, obtained from inguinal fat pad | Intratendinous injection into the suture site | 4 × 106 nucleated cells in 0.2 mL PBS | 3-0 monofilament nylon, modified Kessler technique | Immobilization in below-stifle plaster cast for 2 weeks. | 3 and 8 weeks |
| Control: Suture + PBS injection | 0.2 mL PBS alone | ||||||||||
| Behfar et al., 2013 [49] | RCT | Adult male New Zealand white rabbits | 48 (12 donors, 24 treated, 12 control) | Deep digital flexor tendon, central one third | Fresh allogeneic stromal vascular fraction | Allogeneic adipose-derived stromal vascular fraction, obtained from inguinal fat pad | Intratendinous injection at the suture site | 4 × 106 nucleated cells of freshly isolated SVF | 3/0 monofilament nylon, modified Kessler technique | Immobilization with a below-stifle plaster cast for two weeks. | 3 and 8 weeks |
| Cultured allogeneic BMSCs | Iliac crest bone marrow from allogeneic donors | 4 × 106 cultured BMSCs in 0.2 mL PBS | |||||||||
| Control: Suture + PBS injection | 0.2 mL PBS alone | ||||||||||
| Zhao et al., 2014 [50] | RCT | Female mixed-breed dogs, approximately 1 year old, approximately 20 kg | 60 dogs, 120 paws | Second and fifth FDP from one forepaw, Zone II-D level | Carbodiimide-derivatized hyaluronic acid, gelatin, and lubricin plus autologous BMSCs stimulated with growth and differentiation factor 5 | Tibial bone marrow | “Cell patch” (1 mm-diameter gel droplet composed of collagen/MEM solution MSC and GDF5-5) placed between lacerated tendon ends followed by cd-HA- lubricin surface treatment | 8 × 105 (four gel droplets) | 4-0 FiberWire1 suture (Arthrex Inc, Naples, FL, USA), modified Pennington technique, reinforced with running suture: 6-0 ProleneTM (Ethicon Inc., Somerville, NJ, USA) | Radial neurectomy was performed after treatment so that dogs could not bear weight and the treated paw was held with a sling in front of the chest for five days; synergistic motion rehabilitation was performed daily from day six until euthanasia. | 10 days, 21 days, 42 days |
| Suture repair only | |||||||||||
| Normal (uninjured) | |||||||||||
| He at al., 2015 [51] | RCT | Female New Zealand White rabbits, 2.5–3 kg | 40 rabbits | Rear paws index and ring fingers, FDP, middle of Zone II | Repair + four million allogeneic BMSCs + fibrin glue | Iliac crest bone marrow | Pipetted around the repair site | 106 MSCs per tendon | Modified Kessler’s technique | After surgery rabbits were allowed to move liberally. | 3 and 8 weeks |
| Repair + one million allogeneic BMSCs + fibrin glue | |||||||||||
| Repair + one million autologous BMSCs + fibrin glue | |||||||||||
| Repair + fibrin glue only | |||||||||||
| Zhao et al., 2016 [52] | RCT | Mixed-breed dogs | 39 dogs, 78 tendons | Second and fifth digit, FDP, Zone II-D level | Repair + cd-HA-lubricin + interpositional graft of 8 × 105 BMSCs and GDF-5 | Tibial bone marrow | “Cell patch” (1 mm-diameter gel droplet composed of collagen/MEM solution MSC and GDF5) placed between lacerated tendon ends followed by cd-HA-lubricin surface treatment | 8 × 105 cells (four gel droplets) | 4-0 FiberWire1 suture (Arthrex Inc., Naples, FL, USA), modified Pennington technique, reinforced with running suture: 6-0 ProleneTM (Ethicon Inc., Somerville, NJ, USA) | Radial neurectomy performed to paralyze the elbow and wrist extensors and prevent weight-bearing. Wrist immobilization in 90° of flexion achieved with a threaded, 1.6 mm diameter K-wire passing from distal radius to the proximal third of the metacarpal bone. Custom jackets immobilized the operated paw in front of the chest. Dogs living after day 21 underwent K-wire removal and started wrist and digit synergistic therapy. | 21 and 42 days |
| Repair only | |||||||||||
| Gelberman et al., 2016 [53] | RCT | Adult mongrel dogs, 20–30 kg | 17 dogs, 34 tendons | Second and fifth digits of the right forelimb, FDP, Zone 2 | Repair + Heparin/fibrin-based delivery system/nanofiber scaffold + BMP12 + ASC | Subcutaneous adipose tissue | Longitudinally oriented horizontal slits in the centre of each tendon stump followed by insertion of scaffold which was secured with core suture and epitenon suture | 7.5 μg BMP12 and 1 × 106 autologous ASCs | Core suture: 8-strand suture of 4-0 multifilament nylon (168; grant) (4-0 Supramid, S. Jackson, Alexandria, Virginia); Epitendinous suture: 6-0 nylon running epitenon suture | Controlled passive motion exercise until euthanasia. | 28 days |
| Repair + acellular scaffold | |||||||||||
| Repair only | |||||||||||
| Uninjured | |||||||||||
| Liao et al., 2018 [54] | RCT | Female New Zealand white rabbits | 29 rabbits, 116 tendons | Index and ring digits of the hind paws, FDP, level of proximal phalanx | Scaffold + BMSC | Iliac crest bone marrow | L-lactide and ℇ -caprolactone (PLCL) (Purac Biomaterials, Lincolnshire, IL)—Hyaluronic acid (HA) scaffold | 105 MSCs per scaffold | Core suture: modified-Kessler technique, 5/0 prolene (Ethicon, Somerville, NJ). Epitendinous suture: none, due to the small size of the tendons. Scaffolds were wrapped around the repair site and tagged with prolene 6/0 interrupted sutures. | Flexor tendons were divided at the MCPJ to unload the repair. Animals were allowed to move freely without splinting post-operatively. | 3 and 8 weeks |
| Scaffold | |||||||||||
| Repair only | |||||||||||
| de Lima Santos et al., 2019 [55] | RCT | Male New Zealand rabbits, 2–2.5 kg | 16 rabbits, 32 tendons | Hind leg, FDS, 1–2 cm from the distal part of the calcaneus | Repair + ASC | Inguinal fat pad | Injection (composition not specified) | 1–2 × 106 per injection | Core suture: modified-Kessler technique, Nylon 2/0 (Nylon 2-0; Shalon, Alto da Boa Vista, GO, Brazil). Epitendinous suture: polyglycolic acid 4–0 (Polyglycolic Acid 2-0; Brasuture, Sao Sebastiao da Grama, SP, Brazil) | Free movement without postoperative cast immobilisation. | 4 weeks |
| Repair only | |||||||||||
| No suture | |||||||||||
| Uninjured |
| Author, Year | Intervention | Cohort Size | Max. Load, N | Energy Absorption, N·mm | Max. Stress, N/mm2 | Max. Strain, % | Elastic Modulus, MPa | Range of Motion/Gliding Resistance/Friction | Significance (If Any) |
|---|---|---|---|---|---|---|---|---|---|
| Behfar et al., 2011 [47] | Stromal vascular fraction | 5 (8 weeks) | 34.67 ± 3.17 | 49.12 ± 17.66 | p < 0.05 for all parameters | ||||
| Suture + PBS injection | 5 (8 weeks) | 8.64 ± 3.85 | 13.01 ± 3.40 | ||||||
| Behfar et al., 2012 [48] | Stromal vascular fraction | 5 (3 weeks); 5 (8 weeks) | 13.30 ± 3.98 (3 weeks); 53.10 ± 10.17 (8 weeks) | 29.74 ± 3.17 (3 weeks); 96.34 ± 47.84 (8 weeks) | 4.43 ± 1.32 (3 weeks); 18.92 ± 1.49 (8 weeks) | 12.60 ± 2.04 (3 weeks); 11.01 ± 1.52 (8 weeks) | p < 0.05 for maximum load, energy absorption and maximum stress at 3 and 8 weeks | ||
| Suture + PBS injection | 5 (3 weeks); 5 (8 weeks) | 5.07 ± 1.40 (3 weeks); 14.10 ± 7.44 (8 weeks) | 9.07 ± 4.31 (3 weeks); 26.01 ± 8.05 (8 weeks) | 2.18 ± 1.10 (3 weeks); 4.7 ± 2.48 (8 weeks) | 19.61 ± 7.30 3 weeks); 15.49 ± 4.85 (8 weeks) | ||||
| Behfar et al., 2013 [49] | Stromal vascular fraction | 6 (3 weeks); 6 (8 weeks) | 10 (3 weeks); 35 (8 weeks) | 16 (3 weeks); 49 (8 weeks) | 20 (3 weeks); 38 (8 weeks) | 2 (3 weeks); 1 (8 weeks) | Treatment groups vs. control: p < 0.05 for maximum load, energy absorption, and stress at 3 and 8 weeks. SVF vs. BMSC: p < 0.05 for energy absorption and stress at 8 weeks. | ||
| BMSCs | 6 (3 weeks); 6 (8 weeks) | 13 (3 weeks); 34 (8 weeks) | 11 (3 weeks); 31 (8 weeks) | 13 (3 weeks); 33 (8 weeks) | 2 (3 weeks); 2.5 (8 weeks) | ||||
| Suture + PBS injection | 6 (3 weeks); 6 (8 weeks) | 4 (3 weeks); 27 (8 weeks) | 6 (3 weeks); 21 (8 weeks) | 6 (3 weeks); 25 (8 weeks) | 3 (3 weeks); 3 (8 weeks) | ||||
| Zhao et al., 2014 [50] | cd-HA-lubricin + interpositional graft of BMSCs and GDF-5 | 18 (10 days), 18 (21 days), 16 (42 days) | 42 (10 days); 35 (21 days); 44.7 ± 8.5 (42 days) | Work of flexion in N/mm/degree (10 digits per group): 0.28 ± 0.08 (10 days), 0.29 ± 0.19 (21 days), and 0.32 ± 0.22 (42 days) Friction: 0.55 ± 0.15 N (10 days), 0.52 ± 0.2 (21 days); 0.36 ± 0.12 (42 days) | p < 0.05 for work of flexion and friction in favour of MSC at 10, 21 and 42 days. p < 0.05 for maximum load in favour of suture repair alone at 42 days. | ||||
| Suture repair only | 16 (10 days), 17 (21 days), 16 (42 days) | 38 (10 days); 43 (21 days); 70.2 ± 18.77 (42 days) | 0.46 ± 0.19 (10 days), 0.77 ± 0.49 (21 days), 1.17 ± 0.82 (42 days) 0.93 ± 0.3 (10 days), 0.98 ± 0.46 (21 days), 0.62 ± 0.02 (42 days) | ||||||
| Normal (uninjured) | 10 (0 days) | 47 (day 0) | Contralateral, non-operated paw (no incision): approx. 0.2 at all time points (bar-chart estimate) Contralateral, non-operated paw (no incision): approx. 0.05, 0.08, 0.08 (bar-chart estimate) | ||||||
| He at al., 2015 [51] | four million allogeneic BMSCs + fibrin glue | 9 (3 weeks); 9 (8 weeks) | 12.5 (3 weeks), 27 (8 weeks) | 4.5 (3 weeks), 38 (8 weeks) | 60 (3 weeks), 750 (8 weeks) | Post-operative degrees of flexion: 50 (3 weeks), 41 (8 weeks) | p < 0.05 for ROM in favour of 4 M allogeneic cells at 3 weeks but not 8 weeks. | ||
| one million allogeneic BMSCs + fibrin glue | 11 (3 weeks); 11 (8 weeks) | 14 (3 weeks), 19 (8 weeks) | 4.5 (3 weeks), 37 (8 weeks) | 70 (3 weeks), 500 (8 weeks) | 36 (3 weeks), 45 (8 weeks) | ||||
| one million autologous BMSCs + fibrin glue | 9 (3 weeks); 11 (8 weeks) | 11 (3 weeks); 25 (8 weeks) | 3.5 (3 weeks), 48 (8 weeks) | 50 (3 weeks), 650 (8 weeks) | 38 (3 weeks), 44 (8 weeks) | ||||
| Fibrin glue only | 7 (3 weeks); 12 (8 weeks) | 10.5 (3 weeks); 24 (8 weeks) | 3 (3 weeks); 50 (8 weeks) | 40 (3 weeks), 800 (8 weeks) | 30 (3 weeks), 46 (8 weeks) | ||||
| Zhao et al., 2016 [52] | Repair + cd-HA-lubricin + BMSC + GDF-5 | 19 (21 days); 20 (42 days) | 30 (21 days); 38 (42 days) | Work of flexion in N/mm/degree: 0.25 (21 days); 0.3 (42 days) Friction in N: 0.45 (21 days); 0.5 (42 days) | p < 0.05 for work of flexion and gliding resistance in favour of MSC at 21 and 42 days. p < 0.05 for failure strength in favour of surgical repair alone at 21 and 42 days. | ||||
| Repair | 19 (21 days); 20 (42 days) | 41 (21 days); 62 (42 days) | 0.5 (21 days); 0.9 (42 days) 0.7 (21 days); 0.9 (42 days) | ||||||
| Contralateral, non-operated paw cut and sutured immediately post-mortem | 8 (0 days) | 37 (0 days) | |||||||
| Gelberman et al., 2016 [53] | Repair + scaffold + BMP12 + ASC | 10 (4 weeks) | 85 | 2.6 | 14 | 3.3 ± 1.1 | PIP + DIP degrees of motion: 35.7 | p < 0.05 for range of motion in favour of uninjured control. | |
| Repair + acellular scaffold | 15 (4 weeks) | 77 | 2.2 | 13 | 3.8 ± 1.4 | 35.2 | |||
| Repair only | 8 (4 weeks) | 83 | 1.7 | 15 | 3.1 ± 1.1 | 41.3 | |||
| Normal (uninjured) tendon from opposite limb | 25 (4 weeks) | 55 | |||||||
| Liao et al., 2018 [54] | PLCL-HA scaffold + BMSC | 15 (3 weeks); 8 (8 weeks) | 14 (3 weeks); 28 (8 weeks) | ROM at PIPJ and DIPJ: 43 (3 weeks); 40 (8 weeks) | p < 0.05 for maximum load in favour of suture repair alone | ||||
| PLCL-HA scaffold | 14 (3 weeks); 8 (8 weeks) | 15 (3 weeks); 22 (8 weeks) | 52 (3 weeks); 48 (8 weeks) | ||||||
| Repair only | 19 (3 weeks); 8 (8 weeks) | 17.5 (3 weeks); 37 (8 weeks) | 40 (3 weeks); 48 (8 weeks) | ||||||
| de Lima Santos et al., 2019 [55] | Suture + ASC | 9 (4 weeks) | 96.56 (21.27) | 11.04 (3.17) | 6.25 (2.61) | p < 0.001 for all tests relative to uninjured control (ANOVA); p < 0.05 for maximum load in favour of ASC. | |||
| Suture alone | 10 (4 weeks) | 70.82 (24.66) | 11.53 (3.88) | 12.02 (4.04) | |||||
| No suture | 0 (4 weeks) | ||||||||
| Control (uninjured) | 9 (4 weeks) | 132.69 (17.48) | 44.42 (12.13) | 57.80 (33.48) |
| MSC Source | Number of Cohorts | SMD | 95% Confidence Interval | psubgroup |
|---|---|---|---|---|
| Maximum load | ||||
| 3 weeks | ||||
| Adipose | 2 | −0.5882 | −43.0557, 41.8792 | 0.7758 |
| Bone marrow | 7 | 0.3746 | −0.8715, 1.6207 | |
| 8 weeks | ||||
| Adipose | 4 | 1.5782 | −1.1612, 4.3176 | 0.0693 |
| Bone marrow | 8 | −0.1256 | −1.0072, 0.7561 | |
| Stress | ||||
| 3 weeks | ||||
| Adipose | 2 | 3.2620 | −15.0288, 21.5528 | 0.2390 |
| Bone marrow | 4 | 1.4436 | −0.3370, 3.2242 | |
| 8 weeks | ||||
| Adipose | 4 | 2.4274 | −2.5892, 7.4439 | 0.0831 |
| Bone marrow | 4 | −1.1350 | −5.3330, 3.0629 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epanomeritakis, I.E.; Eleftheriou, A.; Economou, A.; Lu, V.; Khan, W. Mesenchymal Stromal Cells for the Enhancement of Surgical Flexor Tendon Repair in Animal Models: A Systematic Review and Meta-Analysis. Bioengineering 2024, 11, 656. https://doi.org/10.3390/bioengineering11070656
Epanomeritakis IE, Eleftheriou A, Economou A, Lu V, Khan W. Mesenchymal Stromal Cells for the Enhancement of Surgical Flexor Tendon Repair in Animal Models: A Systematic Review and Meta-Analysis. Bioengineering. 2024; 11(7):656. https://doi.org/10.3390/bioengineering11070656
Chicago/Turabian StyleEpanomeritakis, Ilias Ektor, Andreas Eleftheriou, Anna Economou, Victor Lu, and Wasim Khan. 2024. "Mesenchymal Stromal Cells for the Enhancement of Surgical Flexor Tendon Repair in Animal Models: A Systematic Review and Meta-Analysis" Bioengineering 11, no. 7: 656. https://doi.org/10.3390/bioengineering11070656
APA StyleEpanomeritakis, I. E., Eleftheriou, A., Economou, A., Lu, V., & Khan, W. (2024). Mesenchymal Stromal Cells for the Enhancement of Surgical Flexor Tendon Repair in Animal Models: A Systematic Review and Meta-Analysis. Bioengineering, 11(7), 656. https://doi.org/10.3390/bioengineering11070656







