Validation of Pelvis and Trunk Range of Motion as Assessed Using Inertial Measurement Units
Abstract
:1. Introduction
2. Materials and Methods
3. Results
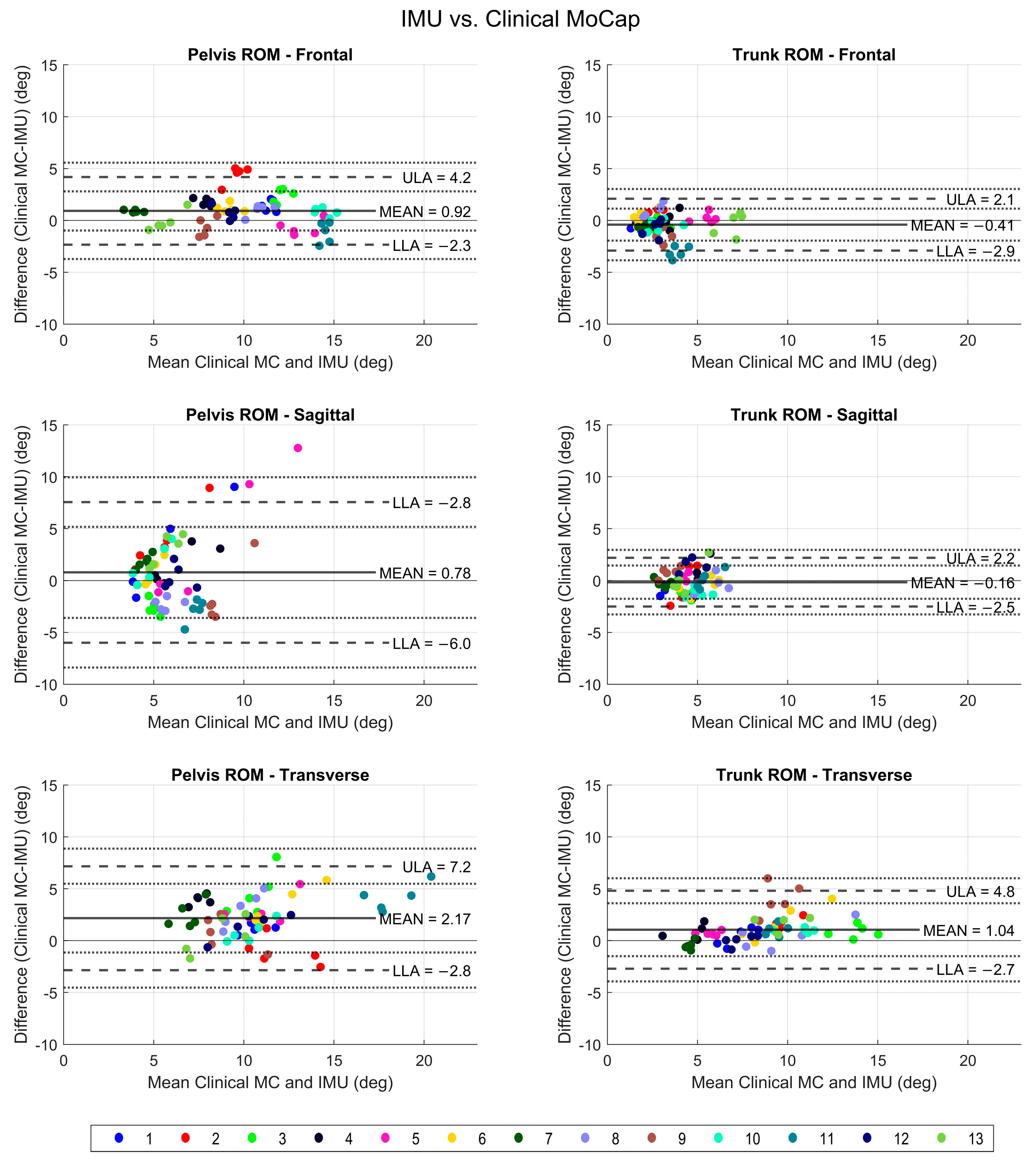
3.1. IMU versus Clinical Mocap
3.2. IMU versus Triad Mocap
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond Engl. 2018, 392, 1789–1858. [CrossRef]
- Komnik, I.; Weiss, S.; Fantini Pagani, C.H.; Potthast, W. Motion analysis of patients after knee arthroplasty during activities of daily living—A systematic review. Gait Posture 2015, 41, 370–377. [Google Scholar] [CrossRef]
- Hyodo, K.; Masuda, T.; Aizawa, J.; Jinno, T.; Morita, S. Hip, knee, and ankle kinematics during activities of daily living: A cross-sectional study. Braz. J. Phys. Ther. 2017, 21, 159–166. [Google Scholar] [CrossRef]
- Patino-Hernandez, D.; David-Pardo, D.G.; Borda, M.G.; Pérez-Zepeda, M.U.; Cano-Gutiérrez, C. Association of Fatigue With Sarcopenia and its Elements: A Secondary Analysis of SABE-Bogotá. Gerontol. Geriatr. Med. 2017, 3, 2333721417703734. [Google Scholar] [CrossRef]
- Perera, S.; Patel, K.V.; Rosano, C.; Rubin, S.M.; Satterfield, S.; Harris, T.; Ensrud, K.; Orwoll, E.; Lee, C.G.; Chandler, J.M.; et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 63–71. [Google Scholar] [CrossRef]
- Martin, K.L.; Blizzard, L.; Wood, A.G.; Srikanth, V.; Thomson, R.; Sanders, L.M.; Callisaya, M.L. Cognitive function, gait, and gait variability in older people: A population-based study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 726–732. [Google Scholar]
- Santos-Lozada, A.R. Trends in Deaths From Falls Among Adults Aged 65 Years or Older in the US, 1999–2020. JAMA 2023, 329, 1605–1607. [Google Scholar] [CrossRef]
- Buurke, T.J.W.; van de Venis, L.; Keijsers, N.; Nonnekes, J. The effect of walking with reduced trunk motion on dynamic stability in healthy adults. Gait Posture 2023, 103, 113–118. [Google Scholar] [CrossRef]
- Sandamas, P.; Gutierrez-Farewik, E.M.; Arndt, A. The relationships between pelvic range of motion, step width and performance during an athletic sprint start. J. Sports Sci. 2020, 38, 2200–2207. [Google Scholar] [CrossRef]
- Voglar, M.; Kozinc, Ž.; Kingma, I.; van Dieën, J.H.; Šarabon, N. The Effects of Intermittent Trunk Flexion with and without Support on Sitting Balance in Young Adults. Front. Hum. Neurosci. 2022. Available online: https://www.frontiersin.org/articles/10.3389/fnhum.2022.868153 (accessed on 15 November 2023). [CrossRef]
- Troke, M.; Moore, A.P.; Maillardet, F.J.; Cheek, E. A normative database of lumbar spine ranges of motion. Man. Ther. 2005, 10, 198–206. [Google Scholar] [CrossRef]
- Cano-de-la-Cuerda, R.; Vela-Desojo, L.; Moreno-Verdú, M.; Ferreira-Sánchez, M.D.R.; Macías-Macías, Y.; Miangolarra-Page, J.C. Trunk Range of Motion Is Related to Axial Rigidity, Functional Mobility and Quality of Life in Parkinson’s Disease: An Exploratory Study. Sensors 2020, 20, 2482. [Google Scholar] [CrossRef]
- Storm, F.A.; Nair, K.P.S.; Clarke, A.J.; Meulen, J.M.V.; der Mazzà, C. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS ONE 2018, 13, e0196463. [Google Scholar] [CrossRef]
- Moore, J.; Stuart, S.; McMeekin, P.; Walker, R.; Celik, Y.; Pointon, M.; Godfrey, A. Enhancing Free-Living Fall Risk Assessment: Contextualizing Mobility Based IMU Data. Sensors 2023, 23, 891. [Google Scholar] [CrossRef]
- Robert-Lachaine, X.; Mecheri, H.; Muller, A.; Larue, C.; Plamondon, A. Validation of a low-cost inertial motion capture system for whole-body motion analysis. J. Biomech. 2020, 99, 109520. [Google Scholar] [CrossRef]
- Taylor, L.; Miller, E.; Kaufman, K.R. Static and dynamic validation of inertial measurement units. Gait Posture 2017, 57, 80–84. [Google Scholar] [CrossRef]
- Morrow, M.M.B.; Lowndes, B.; Fortune, E.; Kaufman, K.R.; Hallbeck, M.S. Validation of Inertial Measurement Units for Upper Body Kinematics. J. Appl. Biomech. 2017, 33, 227–232. [Google Scholar]
- Bauer, C.M.; Rast, F.M.; Ernst, M.J.; Kool, J.; Oetiker, S.; Rissanen, S.M.; Suni, J.H.; Kankaanpää, M. Concurrent validity and reliability of a novel wireless inertial measurement system to assess trunk movement. J. Electromyogr. Kinesiol. 2015, 25, 782–790. [Google Scholar] [CrossRef]
- Mikami, K.; Shiraishi, M.; Kawasaki, T.; Kamo, T. Forward flexion of trunk in Parkinson’s disease patients is affected by subjective vertical position. PLoS ONE 2017, 12, e0181210. [Google Scholar]
- Mehyar, F.; Wilson, S.E.; Staggs, V.S.; Aoyagi, K.; Sharma, N.K. Quantifying Lumbar Mobilization With Inertial Measurement Unit. J. Manip. Physiol. Ther. 2020, 43, 114–122. [Google Scholar]
- Al-Amri, M.; Nicholas, K.; Button, K.; Sparkes, V.; Sheeran, L.; Davies, J.L. Inertial Measurement Units for Clinical Movement Analysis: Reliability and Concurrent Validity. Sensors 2018, 18, 719. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.; Sengupta, R.; Wade, L.; Cazzola, D. A novel IMU-based clinical assessment protocol for Axial Spondyloarthritis: A protocol validation study. PeerJ 2021, 9, e10623. [Google Scholar] [PubMed]
- Mancini, M.; Horak, F.B. Potential of APDM Mobility Lab for the monitoring of the progression of Parkinson’s disease. Expert Rev. Med. Devices 2016, 13, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Hübsch, T.; Brandt, A.U.; Pfueller, C.; Zange, L.; Seidel, A.; Kühn, A.A.; Paul, F.; Minnerop, M.; Doss, S. Accuracy and Repeatability of two methods of gait analysis—GaitRiteTM und Mobility LabTM—In subjects with cerebellar ataxia. Gait Posture 2016, 48, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Stuart, S.; McBarron, G.; Fino, P.C.; Mancini, M.; Curtze, C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol. Meas. 2019, 40, 095003. [Google Scholar] [PubMed]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar]
- Bland, J.M.; Altman, D.G. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [PubMed]
- Camp, C.L.; Loushin, S.; Nezlek, S.; Fiegen, A.P.; Christoffer, D.; Kaufman, K. Are Wearable Sensors Valid and Reliable for Studying the Baseball Pitching Motion? An Independent Comparison with Marker-Based Motion Capture. Am. J. Sports Med. 2021, 49, 3094–3101. [Google Scholar]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.S. Validity and Reliability of Wearable Sensors for Joint Angle Estimation: A Systematic Review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef]
- Chia, L.; Andersen, J.T.; McKay, M.J.; Sullivan, J.; Megalaa, T.; Pappas, E. Evaluating the validity and reliability of inertial measurement units for determining knee and trunk kinematics during athletic landing and cutting movements. J. Electromyogr. Kinesiol. 2021, 60, 102589. [Google Scholar] [PubMed]
- Shull, P.B.; Xu, J.; Yu, B.; Zhu, X. Magneto-Gyro Wearable Sensor Algorithm for Trunk Sway Estimation During Walking and Running Gait. IEEE Sens. J. 2017, 17, 480–486. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, H. Inertial sensors for motion detection of human upper limbs. Sens Rev. 2007, 27, 151–158. [Google Scholar] [CrossRef]
- Goodvin, C.; Park, E.J.; Huang, K.; Sakaki, K. Development of a real-time three-dimensional spinal motion measurement system for clinical practice. Med. Biol. Eng. Comput. 2006, 44, 1061–1075. [Google Scholar]
- Plamondon, A.; Delisle, A.; Larue, C.; Brouillette, D.; McFadden, D.; Desjardins, P.; Larivière, C. Evaluation of a hybrid system for three-dimensional measurement of trunk posture in motion. Appl. Ergon. 2007, 38, 697–712. [Google Scholar] [CrossRef]
- Jasiewicz, J.M.; Treleaven, J.; Condie, P.; Jull, G. Wireless orientation sensors: Their suitability to measure head movement for neck pain assessment. Man Ther. 2007, 12, 380–385. [Google Scholar] [PubMed]
- Schepers, H.M.; Roetenberg, D.; Veltink, P.H. Ambulatory human motion tracking by fusion of inertial and magnetic sensing with adaptive actuation. Med. Biol. Eng. Comput. 2010, 48, 27–37. [Google Scholar] [CrossRef]
- Roetenberg, D.; Slycke, P.J.; Veltink, P.H. Ambulatory position and orientation tracking fusing magnetic and inertial sensing. IEEE Trans. Biomed. Eng. 2007, 54, 883–890. [Google Scholar] [PubMed]
- Berner, K.; Cockcroft, J.; Morris, L.D.; Louw, Q. Concurrent validity and within-session reliability of gait kinematics measured using an inertial motion capture system with repeated calibration. J. Bodyw. Mov. Ther. 2020, 24, 251–260. [Google Scholar]
- Bolink, S.A.A.N.; Naisas, H.; Senden, R.; Essers, H.; Heyligers, I.C.; Meijer, K.; Grimm, B. Validity of an inertial measurement unit to assess pelvic orientation angles during gait, sit–stand transfers and step-up transfers: Comparison with an optoelectronic motion capture system*. Med. Eng. Phys. 2016, 38, 225–231. [Google Scholar] [CrossRef]
- Brouwer, N.P.; Yeung, T.; Bobbert, M.F.; Besier, T.F. 3D trunk orientation measured using inertial measurement units during anatomical and dynamic sports motions. Scand. J. Med. Sci. Sports 2021, 31, 358–370. [Google Scholar] [PubMed]
- Wang, X.; Qureshi, A.; Vepa, A.; Rahman, U.; Palit, A.; Williams, M.A.; King, R.; Elliott, M.T. A Sensor-Based Screening Tool for Identifying High Pelvic Mobility in Patients Due to Undergo Total Hip Arthroplasty. Sensors 2020, 20, 6182. [Google Scholar] [CrossRef] [PubMed]
- Catelli, D.S.; Cotter, B.; Lamontagne, M.; Grammatopoulos, G. Spine, Pelvis and Hip Kinematics—Characterizing the Axial Plane in Healthy and Osteoarthritic Hips. Appl. Sci. 2021, 11, 9921. [Google Scholar] [CrossRef]
- Vazirian, M.; Shojaei, I.; Bazrgari, B. Lumbopelvic Kinematics in the Primary and Secondary Planes of Motion During Lateral Bending and Axial Twisting: Age-Related Differences. IISE Trans. Occup. Ergon. Hum. Factors 2019, 7, 1–11. [Google Scholar]
- Ford, K.R.; Taylor-Haas, J.A.; Genthe, K.; Hugentobler, J. Relationship between hip strength and trunk motion in college cross-country runners. Med. Sci. Sports Exerc. 2013, 45, 1125–1130. [Google Scholar] [PubMed]
- Taniguchi, M.; Tateuchi, H.; Ibuki, S.; Ichihashi, N. Relative mobility of the pelvis and spine during trunk axial rotation in chronic low back pain patients: A case-control study. PLoS ONE 2017, 12, e0186369. [Google Scholar] [CrossRef] [PubMed]
- Staszkiewicz, R.; Chwała, W.; Forczek-Karkosz, W.; Laska, J. Three-dimensional analysis of the pelvic and hip mobility during gait on a treadmill and on the ground. Acta Bioeng. Biomech. Wroc. Univ. Technol. 2012, 14, 83–89. [Google Scholar]
- Cerfoglio, S.; Capodaglio, P.; Rossi, P.; Conforti, I.; D’Angeli, V.; Milani, E.; Galli, M.; Cimolin, V. Evaluation of Upper Body and Lower Limbs Kinematics through an IMU-Based Medical System: A Comparative Study with the Optoelectronic System. Sensors 2023, 23, 6156. [Google Scholar] [CrossRef] [PubMed]
- Rattanakoch, J.; Samala, M.; Limroongreungrat, W.; Guerra, G.; Tharawadeepimuk, K.; Nanbancha, A.; Niamsang, W.; Kerdsomnuek, P.; Suwanmana, S. Validity and Reliability of Inertial Measurement Unit (IMU)-Derived 3D Joint Kinematics in Persons Wearing Transtibial Prosthesis. Sensors 2023, 23, 1738. [Google Scholar] [CrossRef]
- Bent, M.A.; Ciccodicola, E.M.; Rethlefsen, S.A.; Wren, T.A.L. Increased Asymmetry of Trunk, Pelvis, and Hip Motion during Gait in Ambulatory Children with Spina Bifida. Symmetry 2021, 13, 1595. [Google Scholar] [CrossRef]
- Duffy, C.M.; Hill, A.E.; Cosgrove, A.P.; Corry, I.S.; Mollan, R.A.; Graham, H.K. Three-dimensional gait analysis in spina bifida. J. Pediatr. Orthop. 1996, 16, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Vayalapra, S.; Wang, X.; Qureshi, A.; Vepa, A.; Rahman, U.; Palit, A.; Williams, M.A.; King, R.; Elliott, M.T. Repeatability of Inertial Measurement Units for Measuring Pelvic Mobility in Patients Undergoing Total Hip Arthroplasty. Sensors 2022, 23, 377. [Google Scholar] [CrossRef] [PubMed]
- Rekant, J.; Rothenberger, S.; Chambers, A. Obesity-Specific Considerations for Assessing Gait with Inertial Measurement Unit-Based vs. Optokinetic Motion Capture. Sensors 2024, 24, 1232. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.H.; Silburn, P.A.; Wood, J.M.; Worringham, C.J.; Kerr, G.K. Falls in Parkinson’s disease: Kinematic evidence for impaired head and trunk control. Mov. Disord. 2010, 25, 2369–2378. [Google Scholar] [PubMed]
- Jehu, D.; Nantel, J. Fallers with Parkinson’s disease exhibit restrictive trunk control during walking. Gait Posture 2018, 65, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Wang, B.; Xu, H.; Lin, S.; Zhang, H. Post-surgical functional recovery, lumbar lordosis, and range of motion associated with MR-detectable redundant nerve roots in lumbar spinal stenosis. Clin. Neurol. Neurosurg. 2016, 140, 79–84. [Google Scholar] [PubMed]
- Igawa, T.; Katsuhira, J.; Hosaka, A.; Uchikoshi, K.; Ishihara, S.; Matsudaira, K. Kinetic and kinematic variables affecting trunk flexion during level walking in patients with lumbar spinal stenosis. PLoS ONE 2018, 13, e0197228. [Google Scholar]
- Sotirakis, C.; Conway, N.; Su, Z.; Villarroel, M.; Tarassenko, L.; FitzGerald, J.J.; Antoniades, C.A. Longitudinal Monitoring of Progressive Supranuclear Palsy using Body-Worn Movement Sensors. Mov. Disord. 2022, 37, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Lebleu, J.; Gosseye, T.; Detrembleur, C.; Mahaudens, P.; Cartiaux, O.; Penta, M. Lower Limb Kinematics Using Inertial Sensors during Locomotion: Accuracy and Reproducibility of Joint Angle Calculations with Different Sensor-to-Segment Calibrations. Sensors 2020, 20, 715. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Galán-Mercant, A.; Williams, J.M. The use of inertial sensors system for human motion analysis. Phys. Ther. Rev. 2010, 15, 462–473. [Google Scholar] [CrossRef]


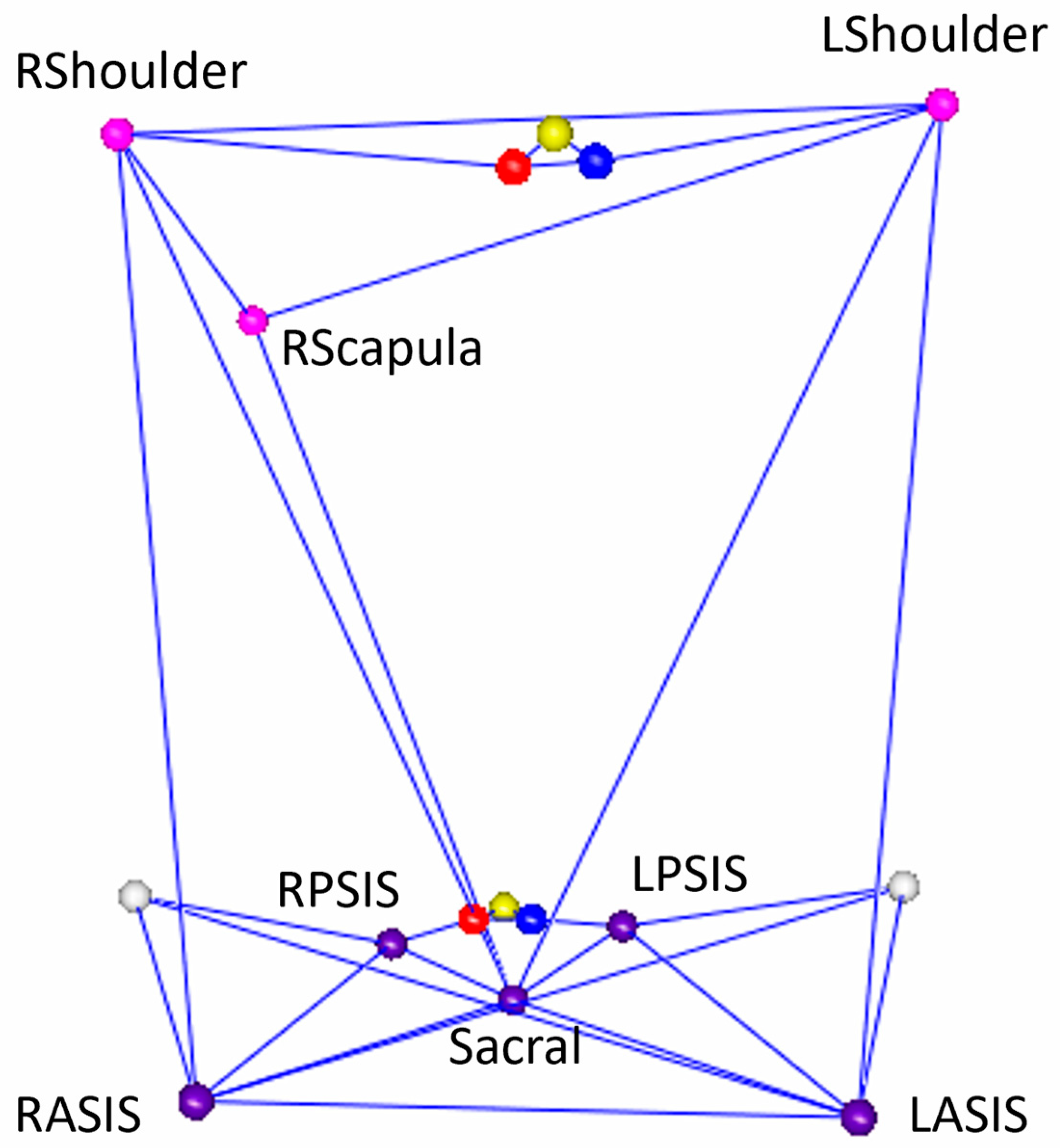

| IMU | Mocap | Triad | |||||
|---|---|---|---|---|---|---|---|
| Range | Median (QI–Q3) | Range | Median (QI–Q3) | Range | Median (QI–Q3) | ||
| Pelvis | Frontal | 2.8–15.8 | 9.3 (7.3–11.4) | 3.8–15.5 | 11.3 (8.7–12.6) | 5.8–18.1 | 11.9 (7.4–15.6) |
| Sagittal | 3.0–10.2 | 5.1 (4.0–6.8) | 3.2–19.4 | 5.7 (4.5–7.1) | 2.3–15.8 | 6.2 (5.2–7.8) | |
| Transverse | 5.0–17.3 | 9.1 (7.7–10.5) | 6.2–23.5 | 11.3 (9.9–12.7) | 4.5–25.1 | 8.8 (6.7–14.0) | |
| Trunk | Frontal | 1.3–8.1 | 2.9 (2.3–4.4) | 0.9–7.7 | 25.5 (1.9–3.6) | 2.6–10.1 | 5.3 (3.6–7.5) |
| Sagittal | 2.4–7.1 | 4.6 (3.9–5.3) | 2.2–7.2 | 4.2 (3.6–5.2) | 3.3–11.6 | 5.4 (4.3–6.7) | |
| Transverse | 2.8–14.7 | 8.1 (6.2–9.3) | 3.3–15.3 | 9.4 (6.5–11.5) | 5.3–13.7 | 9.6 (8.5–10.9) | |
| RMSE Range | ||
|---|---|---|
| Mocap | IMU | |
| Pelvis Frontal ROM | 0.34–1.26 | 0.26–0.88 |
| Pelvis Sagittal ROM | 0.27–6.65 | 0.08–0.98 |
| Pelvis Transverse ROM | 0.64–2.61 | 0.26–2.27 |
| Trunk Frontal ROM | 0.37–1.04 | 0.26–0.75 |
| Trunk Sagittal ROM | 0.34–1.49 | 0.29–0.78 |
| Trunk Transverse ROM | 0.37–3.13 | 0.17–2.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.; Hogen, C.A.; Miller, E.J.; Kaufman, K.R. Validation of Pelvis and Trunk Range of Motion as Assessed Using Inertial Measurement Units. Bioengineering 2024, 11, 659. https://doi.org/10.3390/bioengineering11070659
Ali F, Hogen CA, Miller EJ, Kaufman KR. Validation of Pelvis and Trunk Range of Motion as Assessed Using Inertial Measurement Units. Bioengineering. 2024; 11(7):659. https://doi.org/10.3390/bioengineering11070659
Chicago/Turabian StyleAli, Farwa, Cecilia A. Hogen, Emily J. Miller, and Kenton R. Kaufman. 2024. "Validation of Pelvis and Trunk Range of Motion as Assessed Using Inertial Measurement Units" Bioengineering 11, no. 7: 659. https://doi.org/10.3390/bioengineering11070659
APA StyleAli, F., Hogen, C. A., Miller, E. J., & Kaufman, K. R. (2024). Validation of Pelvis and Trunk Range of Motion as Assessed Using Inertial Measurement Units. Bioengineering, 11(7), 659. https://doi.org/10.3390/bioengineering11070659






