Chitosan Scaffolds from Crustacean and Fungal Sources: A Comparative Study for Bone-Tissue-Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication and Sterilisation of Scaffolds
2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3. Zeta Potential
2.4. Scanning Electron Microscopy (SEM)
2.5. Scaffold Swelling and Degradation
2.6. Ethics, Sample Processing and Cell Culture
2.7. Sterility Testing
2.8. Contact Cytotoxicity Assay by Giemsa Staining
2.9. Indirect Toxicity—Cytotoxicity and Proliferation via XTT Assay
2.10. Fluorescence Actin and Nuclei Staining
2.11. Statistics
3. Results
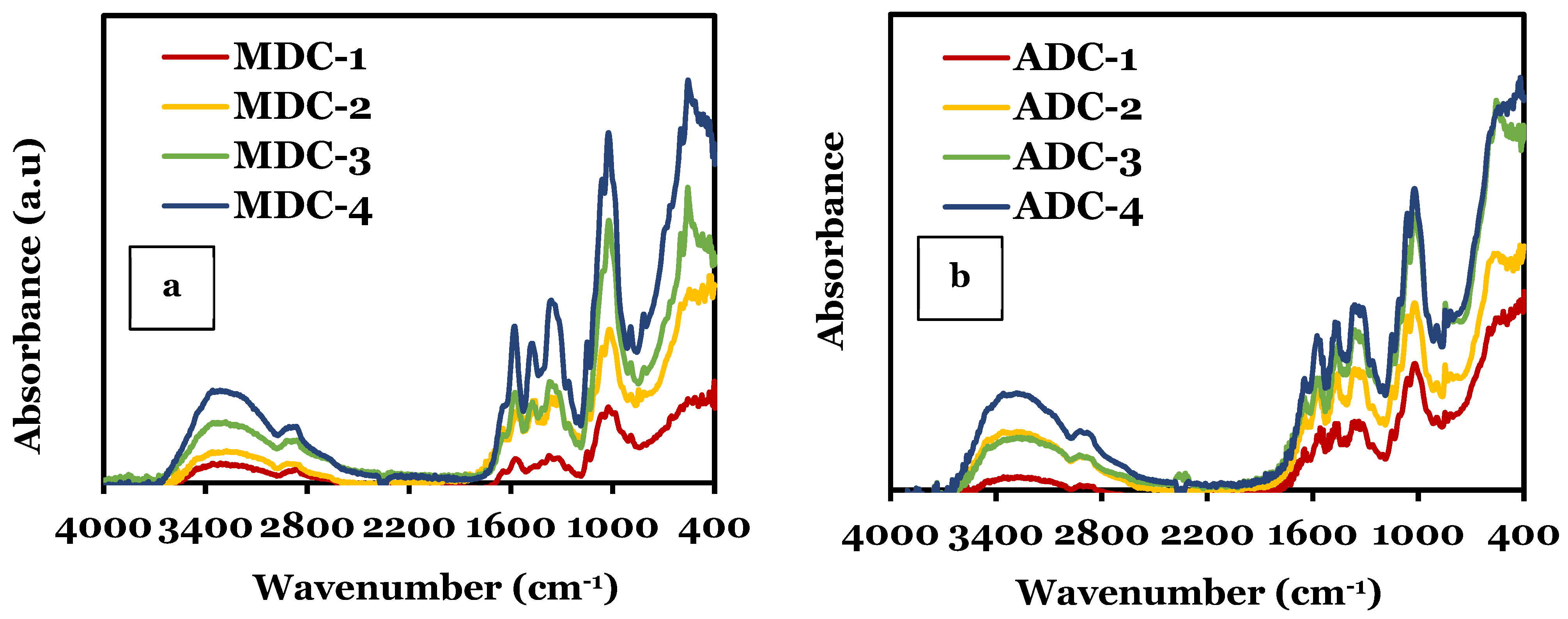
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
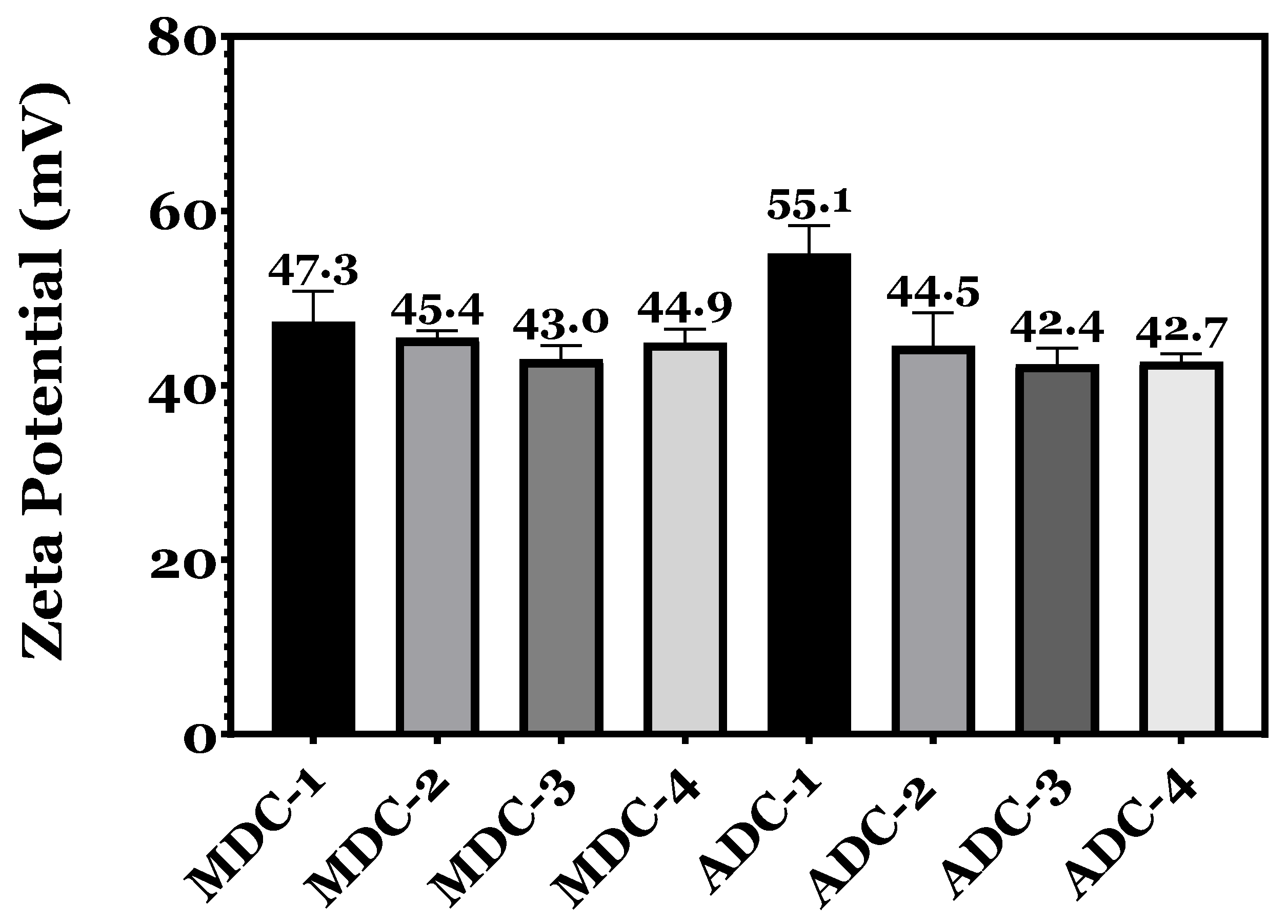
3.2. Zeta Potential
3.3. Scanning Electron Microscopy
3.4. Scaffold Swelling and Degradation
3.5. Sterility Testing and Direct Toxicity
3.6. Contact Cytotoxicity Assay by Giemsa Staining
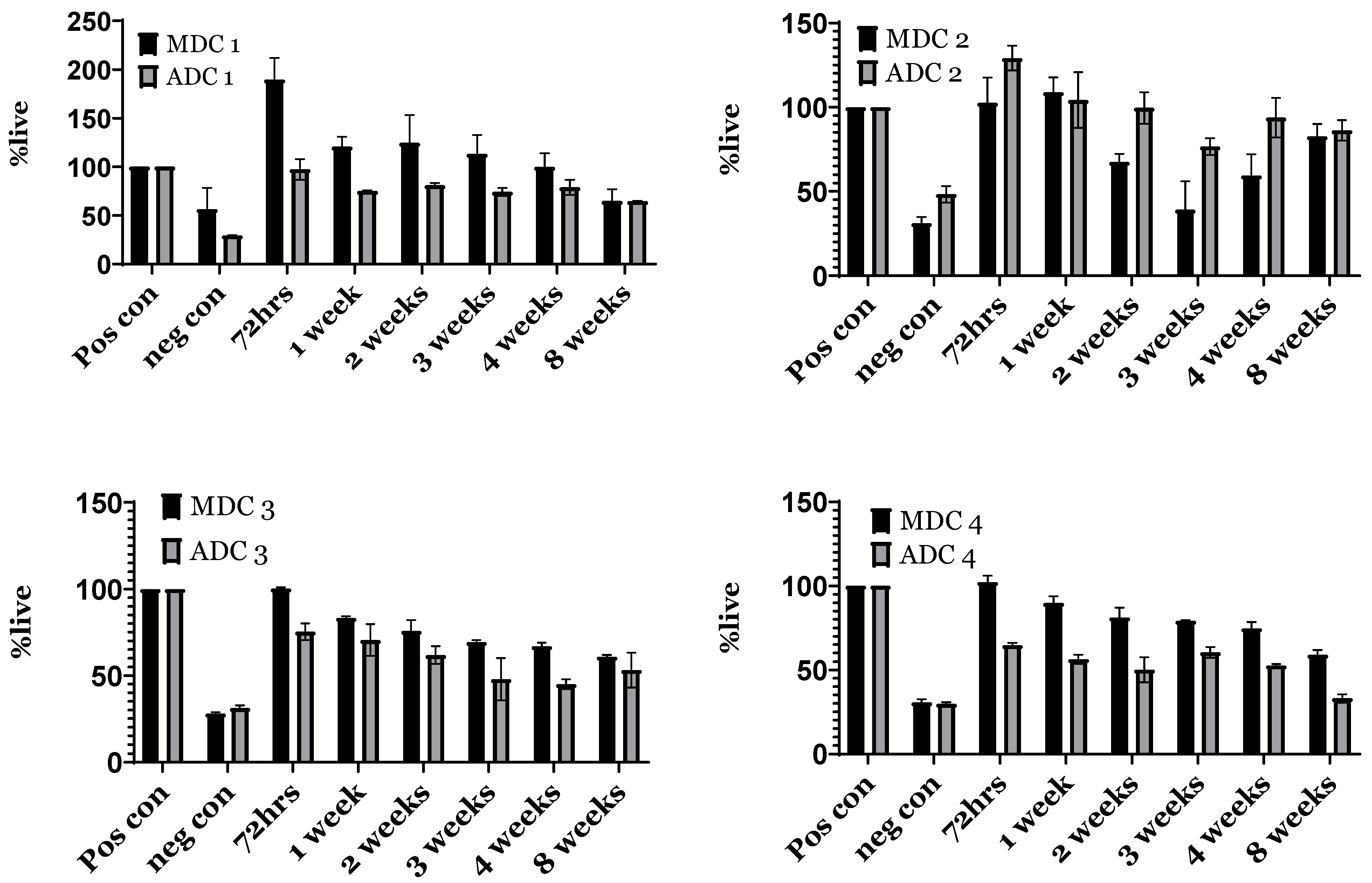
3.7. Indirect Toxicity—Cytotoxicity and Proliferation by XTT
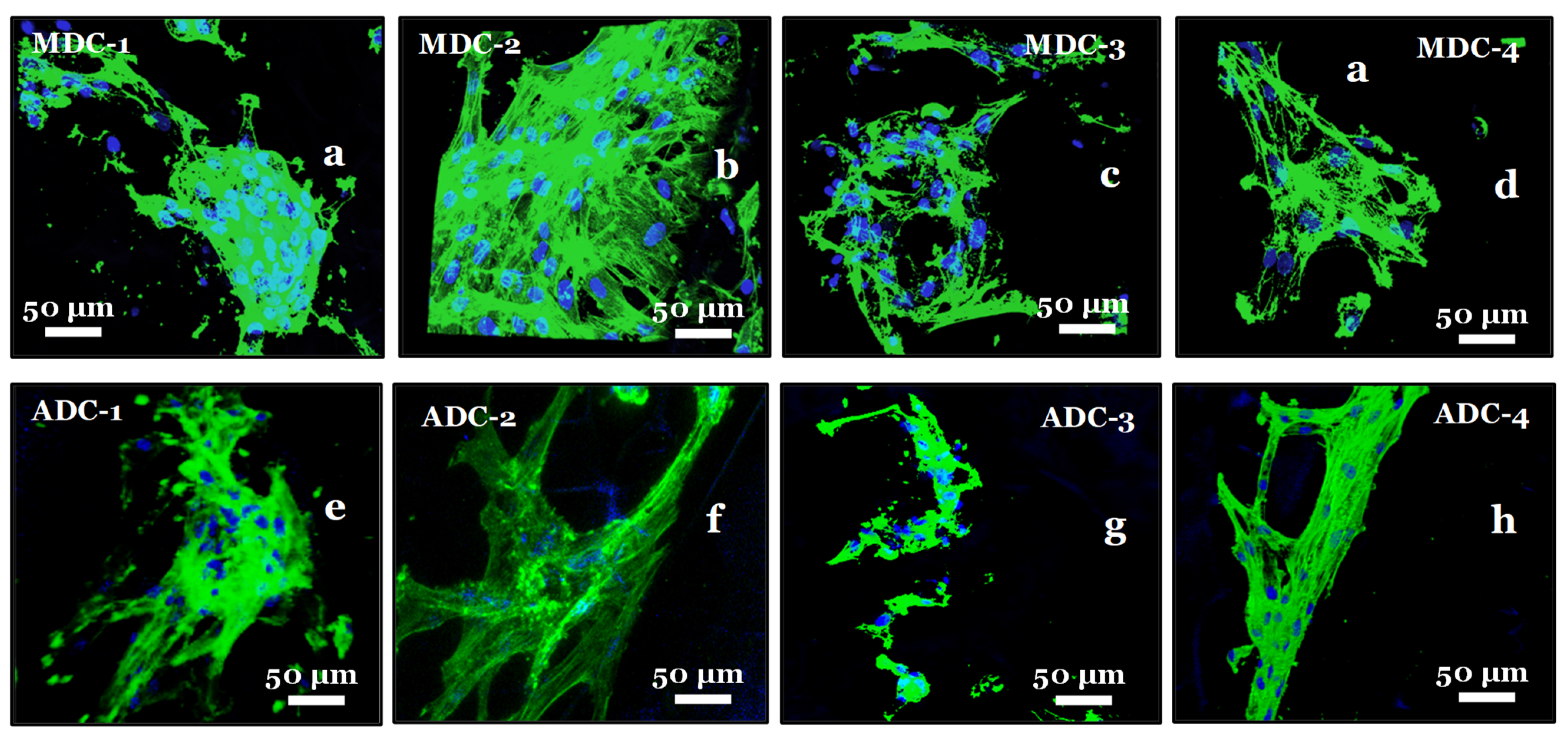
3.8. Fluorescence Actin and Nuclei Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dash, M.; Federica, C.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry; Macmillan: London, UK, 1992. [Google Scholar]
- Iqbal, N.; Braxton, T.M.; Anastasiou, A.; Raif, E.M.; Chung, C.K.Y.; Kumar, S.; Giannoudis, P.; Jha, A. Dicalcium Phosphate Dihydrate Mineral Loaded Freeze-Dried Scaffolds for Potential Synthetic Bone Applications. Materials 2022, 15, 6245. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Zairy, E.M.R.E. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lim, H.; Chong, H.N.; Shim, W.S. Advances in Chitosan Material and its Hybrid Derivatives: A Review. Open Biomater. J. 2009, 1, 10–20. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Plapied, L.; Vandermeulen, G.; Vroman, B.; Préat, V.; Rieux, A. Bioadhesive nanoparticles of fungal chitosan for oral DNA delivery. Int. J. Pharm. 2010, 398, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Ospina Álvarez, S.P.; Ramírez Cadavid, D.A.; Escobar Sierra, D.M.; Ossa Orozco, C.P.; Rojas Vahos, D.F.; Ocampo, P.Z.; Atehortúa, L. Comparison of Extraction Methods of Chitin from Ganoderma lucidum Mushroom Obtained in Submerged Culture. BioMed Res. Int. 2014, 2014, 169071. [Google Scholar] [CrossRef] [PubMed]
- Ishara, J.; Buzera, A.; Mushagalusa, G.N.; Hammam, A.; Munga, J.; Karanja, P.; Kinyuru, J. Nutraceutical potential of mushroom bioactive metabolites and their food functionality. J. Food Biochem. 2022, 46, e14025. [Google Scholar] [CrossRef] [PubMed]
- Poverenov, E.; Arnon-Rips, H.; Zaitsev, Y.; Bar, V.; Danay, O.; Horev, B.; Bilbao-Sainz, C.; McHugh, T.; Rodov, V. Potential of chitosan from mushroom waste to enhance quality and storability of fresh-cut melons. Food Chem. 2018, 268, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Suneetha, M.; Park, G.T.; Babu, A.G.; Han, S.S. Hemostatic, biocompatible, and antibacterial non-animal fungal mushroom-based carboxymethyl chitosan-ZnO nanocomposite for wound-healing applications. Int. J. Biol. Macromol. 2020, 155, 71–80. [Google Scholar] [CrossRef]
- Paiva, W.S.; Queiroz, M.F.; Araujo Sabry, D.; Santiago, A.L.C.M.A.; Sassaki, G.L.; Batista, A.C.L.; Rocha, H.A.O. Preparation, Structural Characterisation, and Property Investigation of Gallic Acid-Grafted Fungal Chitosan Conjugate. J. Fungi 2021, 7, 812. [Google Scholar] [CrossRef]
- Fourie, J.; Taute, F.; du Preez, L.; De Beer, D. Chitosan Composite Biomaterials for Bone Tissue Engineering—A Review. Regen. Eng. Transl. Med. 2022, 8, 1–21. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Qasim, S.B.; Husain, S.; Huang, Y.; Pogorielov, M.; Deineka, V.; Lyndin, M.; Rawlinson, A.; Rehman, I.U. In-vitro and in-vivo degradation studies of freeze gelated porous chitosan composite scaffolds for tissue engineering applications. Polym. Degrad. Stab. 2017, 136, 31–38. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. BioMed Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Tsuchida, H.; Nagao, N. N-acetylation in chitosan and the rate of its enzymic hydrolysis. Biomaterials 1989, 10, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Aryaei, A.; Liu, J.; Jayatissa, A.H.; Jayasuriya, A.C. Cross-linked chitosan improves the mechanical properties of calcium phosphate-chitosan cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.F. Structure of Chitin and Chitosan. In Chitin Chemistry; Roberts, G.A.F., Ed.; Macmillan Education: London, UK, 1992; pp. 1–53. [Google Scholar]
- Aranaz, I.; Martínez-Campos, E.; Moreno-Vicente, C.; Civantos, A.; García-Arguelles, S.; Del Monte, F. Macroporous Calcium Phosphate/Chitosan Composites Prepared via Unidirectional Ice Segregation and Subsequent Freeze-Drying. Materials 2017, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Yildizbakan, L.; Iqbal, N.; Giannoudis, P.V.; Jha, A. Synthesis of Chitosan and Ferric-Ion (Fe3+)-Doped Brushite Mineral Cancellous Bone Scaffolds. Biomimetics 2024, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45lowCD271+ Phenotype. Stem Cells Int. 2019, 2019, 5197983. [Google Scholar] [CrossRef] [PubMed]
- Fragkakis, E.; El-Jawhari, J.J.; Dunsmuir, R.A.; Millner, P.A.; Rao, A.S.; Henshaw, K.T.; Pountos, I.; Jones, E.; Giannoudis, P.V. Vertebral body versus iliac crest bone marrow as a source of multipotential stromal cells: Comparison of processing techniques, tri-lineage differentiation and application on a scaffold for spine fusion. PLoS ONE 2018, 13, e0197969. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html/ (accessed on 17 May 2024).
- ISO 10993-12:2021; Biological Evaluation of Medical Devices Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/75769.html (accessed on 17 May 2024).
- Legan, L.; Leskovar, T.; Črešnar, M.; Cavalli, F.; Innocenti, D.; Ropret, P. Non-invasive reflection FTIR characterisation of archaeological burnt bones: Reference database and case studies. J. Cult. Herit. 2020, 41, 13–26. [Google Scholar] [CrossRef]
- Kumar, V.; Vora, S.D.; Asodiya, F.A.; Kumar, N.; Gangwar, A.K. Fourier Transform Infrared Spectroscopy of the Animal Tissues. In Real Perspective of Fourier Transforms and Current Developments in Superconductivity; Arcos, J.M.V., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 4; pp. 39–48. [Google Scholar]
- Rey, C.; Renugopalakrishman, V.; Collins, B.; Glimcher, M.J. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif. Tissue Int. 1991, 49, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Anastasiou, A.; Aslam, Z.; Raif, E.M.; Do, T.; Giannoudis, P.V.; Jha, A. Interrelationships between the structural, spectroscopic, and antibacterial properties of nanoscale (<50 nm) cerium oxides. Sci. Rep. 2021, 11, 20875. [Google Scholar]
- Hassan, N.; Ahmad, T.; Zain, N.M.; Awang, S.R. Identification of bovine, porcine and fish gelatin signatures using chemometrics fuzzy graph method. Sci. Rep. 2021, 11, 9793. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Veis, A. Fourier transform IR spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolym. Orig. Res. Biomol. 1988, 27, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dimzon, I.K.; Knepper, T. Degree of Deacetylation of Chitosan by Infrared Spectroscopy and Partial Least Squares. Int. J. Biol. Macromol. 2014, 72, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Bierhalz, A.C.K.; Westin, C.B.; Moraes, Â.M. Comparison of the properties of membranes produced with alginate and chitosan from mushroom and from shrimp. Int. J. Biol. Macromol. 2016, 91, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Maji, K.; Mondal, S. Calcium Phosphate Biomaterials for Bone Tissue Engineering: Properties and Relevance in Bone Repair. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Springer International: Cham, Switzerland, 2020; pp. 535–555. [Google Scholar]
- Lowe, B.; Ottensmeyer, M.P.; Xu, C.; He, Y.; Ye, Q.; Troulis, M.J. The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective. J. Funct. Biomater. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Sujon, M.K.; Mohd Noor, S.N.F.; Zabidi, M.A.; Shariff, K.A. Combined sol–gel bioactive glass and β-tricalcium phosphate for potential dental tissue engineering: A preliminary study. J. Aust. Ceram. Soc. 2023, 59, 415–424. [Google Scholar] [CrossRef]
- Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. 3D printed tricalcium phosphate bone tissue engineering scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci. 2013, 1, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ye, F.; Cui, J.; Zhang, F.; Li, X.; Yao, K. Preparation and characterisation of macroporous chitosan-gelatin/beta-tricalcium phosphate composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2003, 67, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Takayama, K.; Machida, Y.; Nagai, T. Characteristics of polyion complexes of chitosan with sodium alginate and sodium polyacrylate. Int. J. Pharm. 1990, 61, 35–41. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jiang, H.L.; Jere, D.; Park, I.K.; Cho, M.H.; Nah, J.W.; Choi, Y.J.; Akaike, T.; Cho, C.S. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog. Polym. Sci. 2007, 32, 726–753. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N. Chitosan in Nanostructured Thin Films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.P.; Freitas, S.R.M.; Tanaka, C.B.; Delechiave, G.; Kikuchi, L.N.T.; Braga, R.R.; Kruzic, J.J.; Moreira, M.S.; Boaro, L.C.C.; Catalani, L.H.; et al. Synthesis of Submicrometric Chitosan Particles Loaded with Calcium Phosphate for Biomedical Applications. AAPS PharmSciTech 2023, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qian, Y.; Gong, J.; Zhu, Z.; Yin, J.; Ma, L.; Yu, M.; Wang, H. Biofabrication of aligned structures that guide cell orientation and applications in tissue engineering. Bio-Des. Manuf. 2021, 4, 258–277. [Google Scholar] [CrossRef]
- Liuyun, J.; Yubao, L.; Chengdong, X. A novel composite membrane of chitosan-carboxymethyl cellulose polyelectrolyte complex membrane filled with nano-hydroxyapatite I. Preparation and properties. J. Mater. Sci. Mater. Med. 2009, 20, 1645–1652. [Google Scholar] [CrossRef]
- Farag, R.K.; Mohamed, R.R. Synthesis and characterisation of carboxymethyl chitosan nanogels for swelling studies and antimicrobial activity. Molecules 2012, 18, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Alahmadi, T.E.; Jayasuriya, A.C. Chitosan microparticles based polyelectrolyte complex scaffolds for bone tissue engineering in vitro and effect of calcium phosphate. Carbohydr. Polym. 2018, 199, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Rong Huei, C.; Hwa, H.-D. Effect of molecular weight of chitosan with the same degree of deacetylation on the thermal, mechanical, and permeability properties of the prepared membrane. Carbohydr. Polym. 1996, 29, 353–358. [Google Scholar] [CrossRef]
- Jennings, J.A. 7—Controlling chitosan degradation properties in vitro and in vivo. In Chitosan Based Biomaterials; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 1, pp. 159–182. [Google Scholar]
- Zeng, L.; Qin, C.; Wang, W.; Chi, W.; Li, W. Absorption and distribution of chitosan in mice after oral administration. Carbohydr. Polym. 2008, 71, 435–440. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Wright, L.; Haggard, W.O.; Bumgardner, J.D. Mechanical property, degradation rate, and bone cell growth of chitosan coated titanium influenced by degree of deacetylation of chitosan. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 245–252. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Utturkar, G.; Haggard, W.O.; Yang, Y.; Ong, J.L.; Bumgardner, J.D. The effect of cross-linking of chitosan microspheres with genipin on protein release. Carbohydr. Polym. 2007, 68, 561–567. [Google Scholar] [CrossRef]
- Pangburn, S.H.; Trescony, P.V.; Heller, J. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials 1982, 3, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Shanmugasundaram, N.; Ravichandran, P.; Reddy, P.N.; Ramamurty, N.; Pal, S.; Rao, K.P. Collagen–chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials 2001, 22, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic synthesis of chitosan derivatives and their potential applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.S.; Wu, Y.B.; Lee, S.T.; Shyong, J.Y.; Huang, R.N. Fabrication and characterisation of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, W.M.F.W.; Jones, M.P.; Kontturi, E.; Mautner, A.; Bismarck, A. Plastic to elastic: Fungi-derived composite nanopapers with tunable tensile properties. Compos. Sci. Technol. 2020, 198, 108327. [Google Scholar] [CrossRef]
- Mohamed, K.R.; El-Rashidy, Z.M.; Salama, A.A. In vitro properties of nano-hydroxyapatite/chitosan biocomposites. Ceram. Int. 2011, 37, 3265–3271. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Wu, Q.; Zuo, J.; Qin, Y.; Wang, J. Novel Mesoporous Hydroxyapatite/Chitosan Composite for Bone Repair. J. Bionic Eng. 2012, 9, 243–251. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Seol, Y.-J.; Lee, J.Y.; Park, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Lee, S.J.; Han, S.B.; Chung, C.P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.K. In vivo study of chitosan-natural nano hydroxyapatite scaffolds for bone tissue regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef]




| Chitosan | Code | Molecular Weight (kDa) | Viscosity (mPa.s/cps) | Degree of Deacetylation (%) |
|---|---|---|---|---|
| Fungal | MDC * | 200–300 | 600 | 98.1 |
| Crustacean | ADC ** | 330–375 | 2000 | ≥75 |
| Sample Code | Description | TCP (wt)% |
|---|---|---|
| TCP | Tricalcium Phosphate Mineral | - |
| MDC-1/ADC-1 | Freeze-dried Chitosan Scaffold | 0 |
| MDC-2/ADC-2 | 10(wt)% TCP mineral loaded chitosan scaffold | 10 |
| MDC-3/ADC-3 | 20(wt)% TCP mineral loaded chitosan scaffold | 20 |
| MDC-4/ADC-4 | 30(wt)% TCP mineral loaded chitosan scaffold | 30 |
| Grade Reactivity | Description |
|---|---|
| 0 | None: No detectable zone around or under specimen |
| 1 | Slight: Some malformed or degraded cell under specimen |
| 2 | Mild: Zone limited to area under specimen |
| 3 | Moderate: Zone extending specimen size up to 1 cm |
| 4 | Severe: Zone extending farther than 1 cm |
| Bond | Peak Type | Range (cm−1) | Ref. |
|---|---|---|---|
| O-H and N-H | Stretching vibrations due to intermolecular hydrogen bonding | 3200–3500 | [3,36,37,38,39] |
| C-H | Stretching vibrations in the CH2 and CH3 | 2870–2920 | |
| C=O | Amide I: Stretching vibration in the amide group | 1600–1650 | |
| N-H and C-N | Amide II: Bending and stretching vibrations, respectively | 1550–1590 | |
| CH3 | Symmetric bending vibrations | 1370–1390 | |
| C-O-C | Stretching vibrations in the glycosidic linkage | 1150–1100 | |
| C-O | Stretching vibrations in the pyranose ring | 1030–1060 | |
| υ4 | Phosphate bending vibrations | 560–600 | [3,33,34,35] |
| υ1 | Symmetric stretching vibrations | 960–962 | |
| υ3 | Asymmetric stretching vibrations | 1020–1100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N.; Ganguly, P.; Yildizbakan, L.; Raif, E.M.; Jones, E.; Giannoudis, P.V.; Jha, A. Chitosan Scaffolds from Crustacean and Fungal Sources: A Comparative Study for Bone-Tissue-Engineering Applications. Bioengineering 2024, 11, 720. https://doi.org/10.3390/bioengineering11070720
Iqbal N, Ganguly P, Yildizbakan L, Raif EM, Jones E, Giannoudis PV, Jha A. Chitosan Scaffolds from Crustacean and Fungal Sources: A Comparative Study for Bone-Tissue-Engineering Applications. Bioengineering. 2024; 11(7):720. https://doi.org/10.3390/bioengineering11070720
Chicago/Turabian StyleIqbal, Neelam, Payal Ganguly, Lemiha Yildizbakan, El Mostafa Raif, Elena Jones, Peter V. Giannoudis, and Animesh Jha. 2024. "Chitosan Scaffolds from Crustacean and Fungal Sources: A Comparative Study for Bone-Tissue-Engineering Applications" Bioengineering 11, no. 7: 720. https://doi.org/10.3390/bioengineering11070720
APA StyleIqbal, N., Ganguly, P., Yildizbakan, L., Raif, E. M., Jones, E., Giannoudis, P. V., & Jha, A. (2024). Chitosan Scaffolds from Crustacean and Fungal Sources: A Comparative Study for Bone-Tissue-Engineering Applications. Bioengineering, 11(7), 720. https://doi.org/10.3390/bioengineering11070720








