Fabrication and Dielectric Validation of an Arm Phantom for Electromyostimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Phantom Realization Procedure
2.2. Dielectric Characterization of Phantom Layers
2.3. EMS–EMG Measurement Setup
3. Results
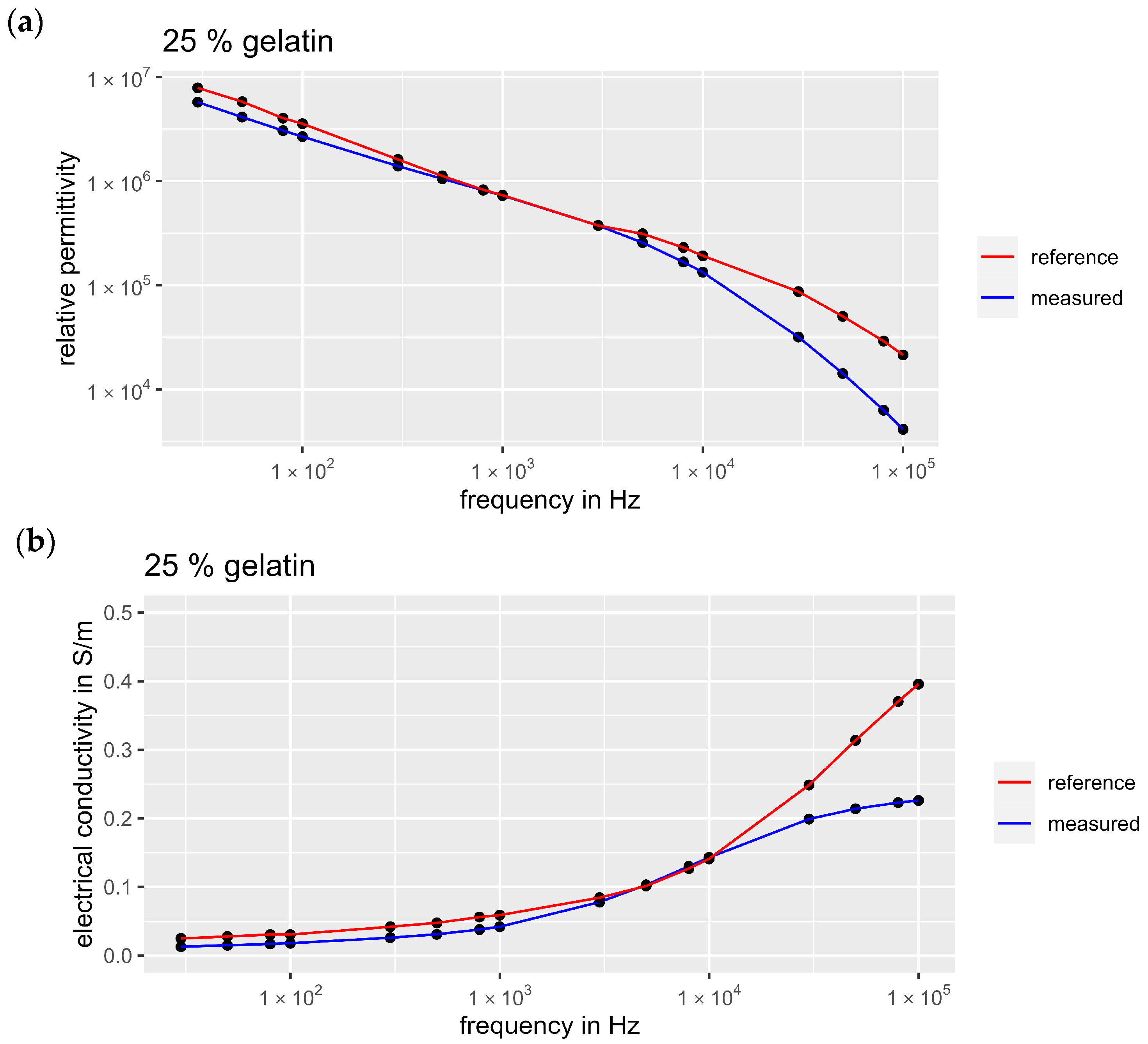
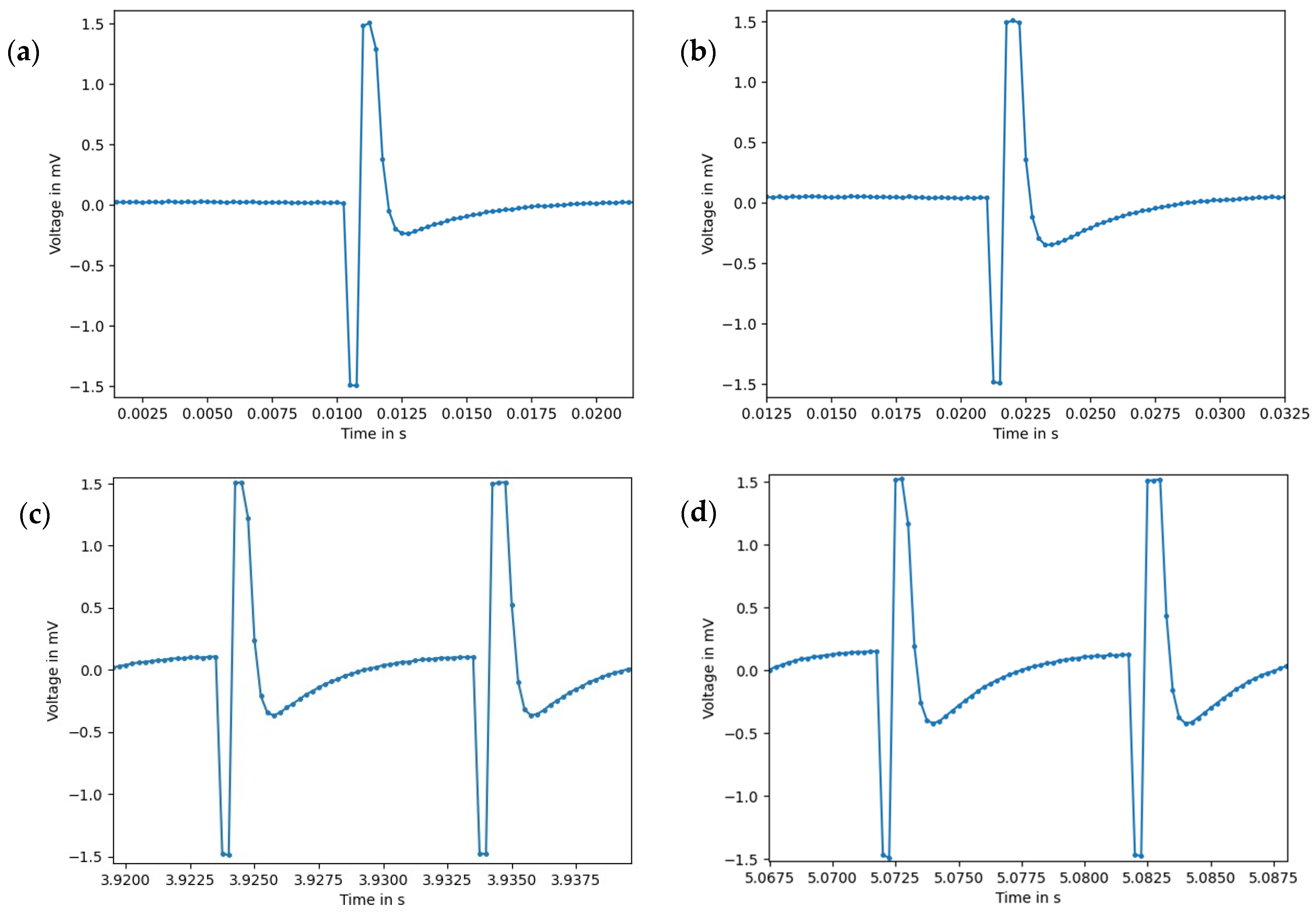
3.1. Validation of the Measurement Setup
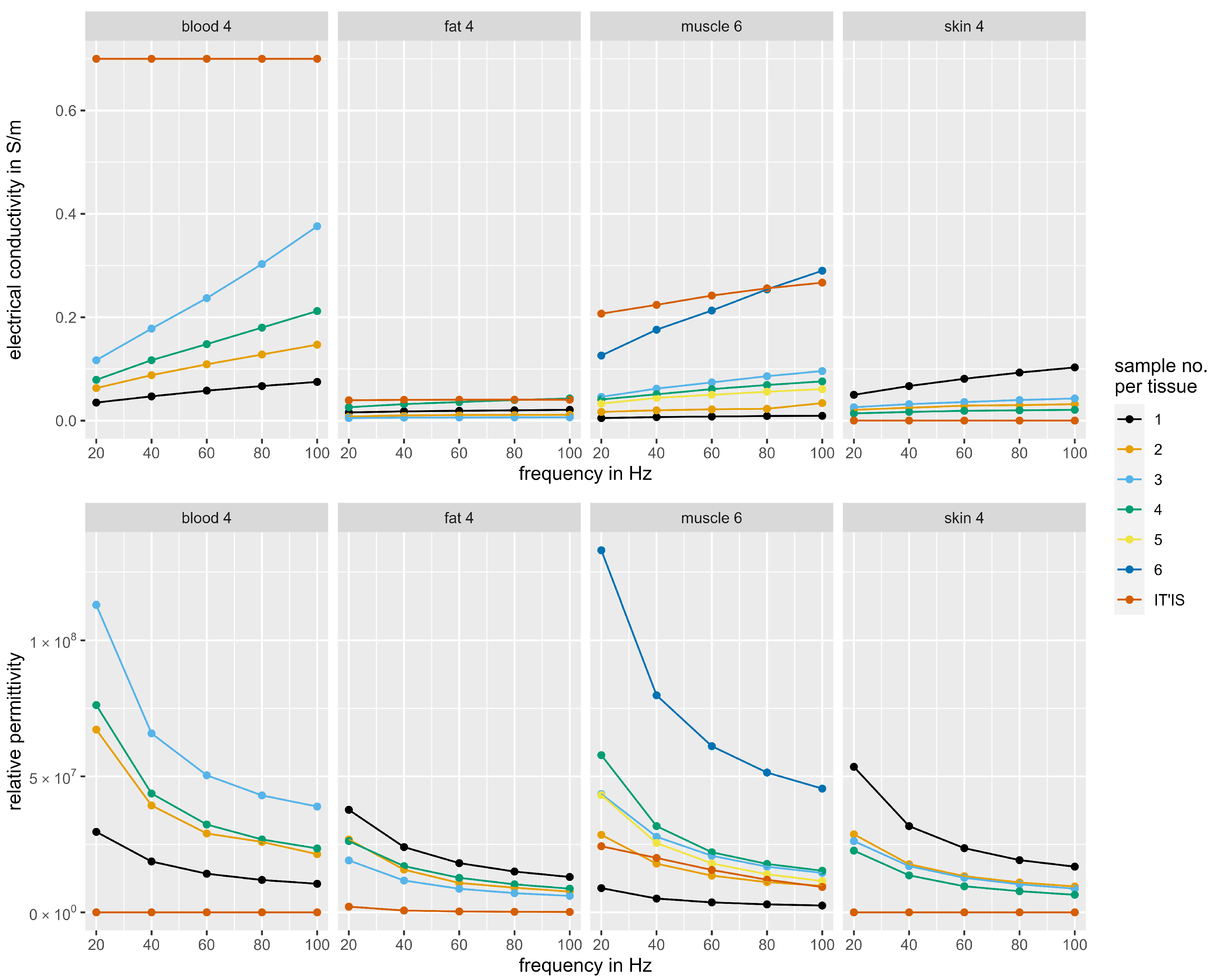
3.2. Dielectric Properties of the Tissue Phantoms
3.3. EMS–EMG Measurement Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemmler, W.; Fröhlich, M.; Eifler, C. Ganzkörper-Elektromyostimulation; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 978-3-662-65205-3. [Google Scholar]
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Aldayel, A.; Jubeau, M.; Chen, T.C. Muscle damage induced by electrical stimulation. Eur. J. Appl. Physiol. 2011, 111, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Teschler, M.; Bebenek, M.; Stengel, S. Hohe Kreatinkinase-Werte nach exzessiver Ganzkörper-Elektromyostimulation: Gesundheitliche Relevanz und Entwicklung im Trainingsverlauf. Wien Med. Wochenschr. 2015, 165, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, Y.; Gomez-Tames, J.; Yu, W. An experimental study on the effect of fat conductivity on voltage distribution and muscle recruitment using tissue-equivalent phantoms. In Proceedings of the 2013 E-Health and Bioengineering Conference (EHB 2013), Iasi, Romania, 21–23 November 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1–4, ISBN 978-1-4799-2373-1. [Google Scholar]
- Livshitz, L.M.; Mizrahi, J.; Einziger, P.D. Interaction of array of finite electrodes with layered biological tissue: Effect of electrode size and configuration. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Bliznakova, K. The advent of anthropomorphic three-dimensional breast phantoms for X-ray imaging. Phys. Med. 2020, 79, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, M.E.; Kuipers, H.; Liefers, H.R.; van Es, J.; Simonis, F.F.J.; Greuter, M.J.W.; Slump, C.H.; Slart, R.H.J.A. A Multimodality Myocardial Perfusion Phantom: Initial Quantitative Imaging Results. Bioengineering 2022, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Breslin, T.; Paino, J.; Wegner, M.; Engels, E.; Fiedler, S.; Forrester, H.; Rennau, H.; Bustillo, J.; Cameron, M.; Häusermann, D.; et al. A Novel Anthropomorphic Phantom Composed of Tissue-Equivalent Materials for Use in Experimental Radiotherapy: Design, Dosimetry and Biological Pilot Study. Biomimetics 2023, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Jafary, R.; Forsythe, J.S.; Gregory, S.D. Tissue-Mimicking Materials for Ultrasound-Guided Needle Intervention Phantoms: A Comprehensive Review. Ultrasound Med. Biol. 2023, 49, 18–30. [Google Scholar] [CrossRef]
- Zhong, X.; Cao, Y.; Zhou, P. Thermochromic Tissue-Mimicking Phantoms for Thermal Ablation Based on Polyacrylamide Gel. Ultrasound Med. Biol. 2022, 48, 1361–1372. [Google Scholar] [CrossRef]
- Lai, M.; Skyrman, S.; Kor, F.; Homan, R.; El-Hajj, V.G.; Babic, D.; Edström, E.; Elmi-Terander, A.; Hendriks, B.H.W.; de With, P.H.N. Development of a CT-Compatible, Anthropomorphic Skull and Brain Phantom for Neurosurgical Planning, Training, and Simulation. Bioengineering 2022, 9, 537. [Google Scholar] [CrossRef]
- Guntur, S.R.; Kim, S.-C.; Choi, M.-J. A Cost-Effective Reusable Tissue Mimicking Phantom for High Intensity Focused Ultrasonic Liver Surgery. Bioengineering 2022, 9, 786. [Google Scholar] [CrossRef]
- Boice, E.N.; Berard, D.; Gonzalez, J.M.; Hernandez Torres, S.I.; Knowlton, Z.J.; Avital, G.; Snider, E.J. Development of a Modular Tissue Phantom for Evaluating Vascular Access Devices. Bioengineering 2022, 9, 319. [Google Scholar] [CrossRef]
- Aminzadeh, R.; Saviz, M.; Shishegar, A.A. Theoretical and experimental broadband tissue-equivalent phantoms at microwave and millimetre-wave frequencies. Electron. Lett. 2014, 50, 618–620. [Google Scholar] [CrossRef]
- Costanzo, S.; Cioffi, V.; Qureshi, A.M.; Borgia, A. Gel-Like Human Mimicking Phantoms: Realization Procedure, Dielectric Characterization and Experimental Validations on Microwave Wearable Body Sensors. Biosensors 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, A.E.; Alquaydheb, I.N.; Eltawil, A.M.; Kurdahi, F.J. IBCFAP: Intra-Body Communications Five-Layers Arm Phantom Model. IEEE Access 2019, 7, 93701–93710. [Google Scholar] [CrossRef]
- Toyoda, S.; Yamamoto, T.; Koshiji, K. Prototype and Evaluation of High-Hydrous Gel Phantom for 100 kHz to 1 MHz Using ATO/TiO2. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021. [Google Scholar]
- Anand, G.; Lowe, A.; Al-Jumaily, A. Tissue phantoms to mimic the dielectric properties of human forearm section for multi-frequency bioimpedance analysis at low frequencies. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Borkholder, D.A.; Day, S.W. A biomimetic skin phantom for characterizing wearable electrodes in the low-frequency regime. Sens. Actuators A Phys. 2022, 340, 113513. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Lowe, A.; Anand, G. Bio Phantoms Mimicking the Dielectric and Mechanical Properties of Human Skin Tissue at Low-Frequency Ranges. Mod. Appl. Sci. 2020, 14, 1. [Google Scholar] [CrossRef]
- Yu, Y.; Lowe, A.; Anand, G.; Kalra, A. Tissue phantom to mimic the dielectric properties of human muscle within 20 Hz and 100 kHz for biopotential sensing applications. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 6490–6493. [Google Scholar] [CrossRef]
- IT’IS Foundation. Tissue Properties Database V4.1; IT’IS Foundation: Zürich, Switzerland, 2022. [Google Scholar]
- Khan, M.A.; Gul, H.; Mansor Nizami, S. Determination of Gender from Various Measurements of the Humerus. Cureus 2020, 12, e6598. [Google Scholar] [CrossRef]
- Maughan, R.J.; Watson, J.S.; Weir, J. The relative proportions of fat, muscle and bone in the normal human forearm as determined by computed tomography. Clin. Sci. 1984, 66, 683–689. [Google Scholar] [CrossRef]
- Keysight Technologies. Measuring Dielectric Properties Using Keysight’s Materials Measurements Solutions: Product Brochure; Keysight Technologies: Santa Rosa, CA, USA, 2023. [Google Scholar]
- Franck, A. Dielectric Characterization: APN032. Available online: https://www.tainstruments.com/pdf/literature/APN032%20Dielectric%20characterization_V1_ajf_30SEP12.pdf (accessed on 25 January 2024).
- Cervantes, A.; Paez, G.; Balleza-Ordaz, J.M.; Vargas-Luna, F.M.; Kashina, S. Electrical bioimpedance analysis and comparison in biological tissues through crystalloid solutions implementation. Biosens. Bioelectron. 2024, 246, 115874. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P. EMG-FIBEL: Eine Praxisorientierte Einführung in Die Kinesiologische Elektromyographie. Available online: https://www.velamed.com/wp-content/uploads/EMG-FIBEL-V1.1.pdf (accessed on 16 January 2024).
- Stegman, D.F.; Hermens, H.J. Standards for Surface Electromyography: The European Project Surface EMG for Non-Invasive Assessment of Muscles (SENIAM). Available online: https://www.researchgate.net/publication/228486725_Standards_for_suface_electromyography_The_European_project_Surface_EMG_for_non-invasive_assessment_of_muscles_SENIAM#full-text (accessed on 26 January 2024).
- Cotofana, S.; Hexsel, D.; Avelar, L.E.; Munia, C.G.; Muniz, M.; Casabona, G.; Schenck, T.L.; Green, J.B.; Lachman, N.; Frank, K. Calculating the Thickness of the Superficial Fatty Layer of the Body Using Age, Gender, and Body Mass Index. J. Drugs Dermatol. 2020, 19, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V.; Ren, Y.; Suchomel, M. Molecular arrangement in water: Random but not quite. J. Phys. Condens. Matter. 2012, 24, 155102. [Google Scholar] [CrossRef]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef]
- Ishai, P.B.; Talary, M.S.; Caduff, A.; Levy, E.; Feldman, Y. Electrode polarization in dielectric measurements: A review. Meas. Sci. Technol. 2013, 24, 102001. [Google Scholar] [CrossRef]
- Sasaki, K.; Wake, K.; Watanabe, S.; Mizuno, M.; Fukunaga, K.; Katayama, K.; Suzuki, H. Dielectric property measurements of biological tissues: Recent activities for development of a novel database. In Proceedings of the 31st URSI General Assembly and Scientific Symposium (URSI GASS), Beijing, China, 16–23 August 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–4, ISBN 978-1-4673-5225-3. [Google Scholar]
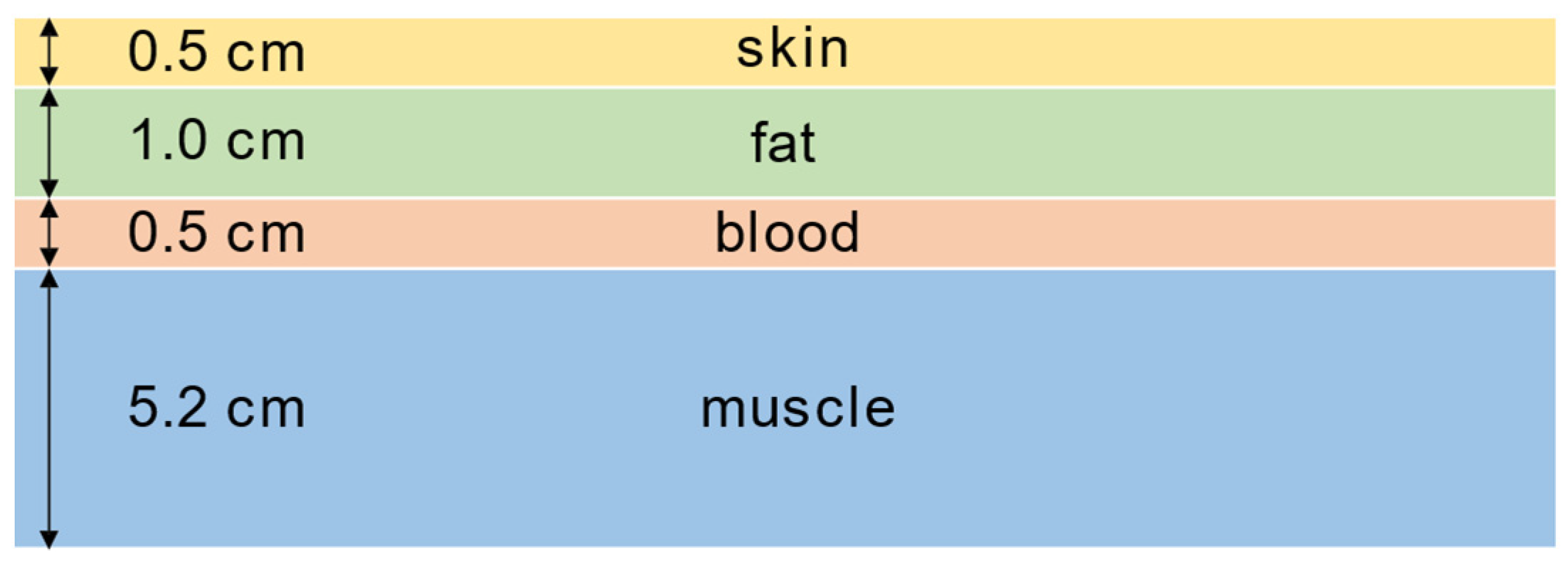
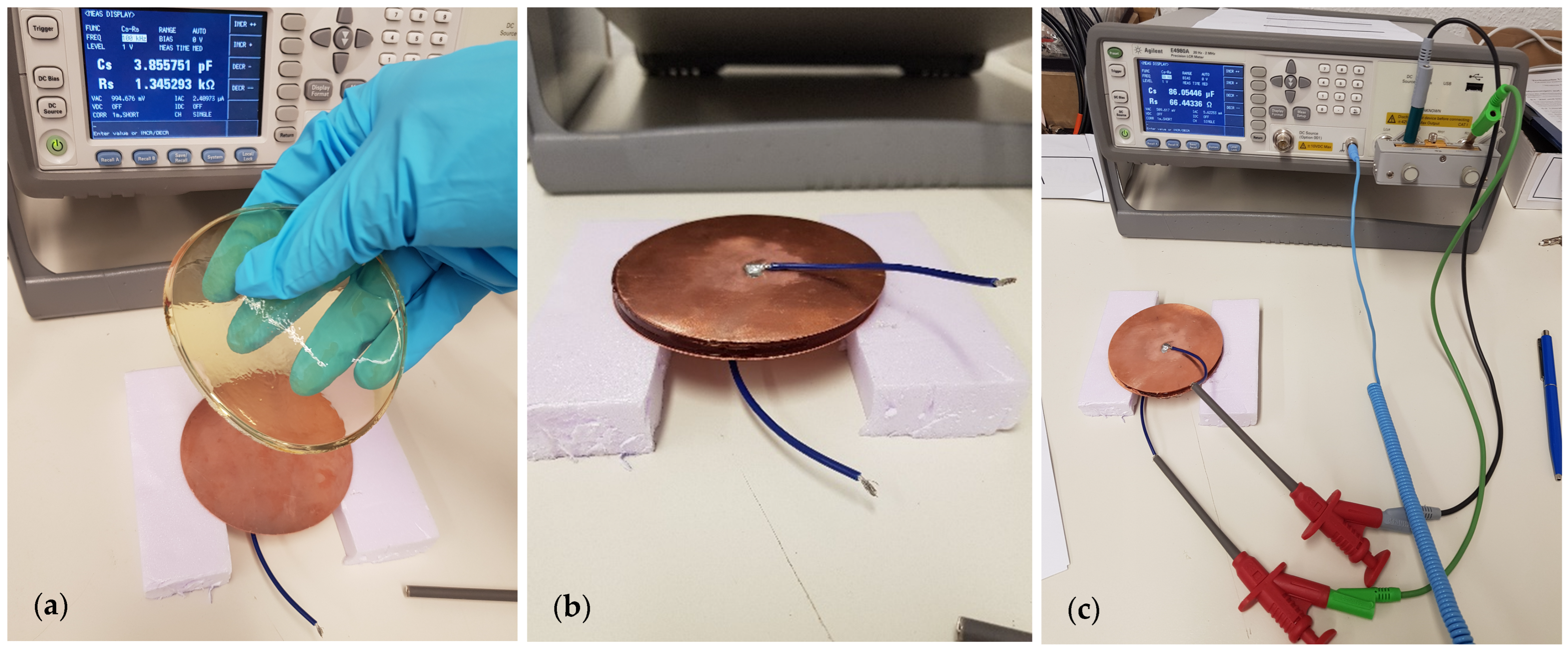
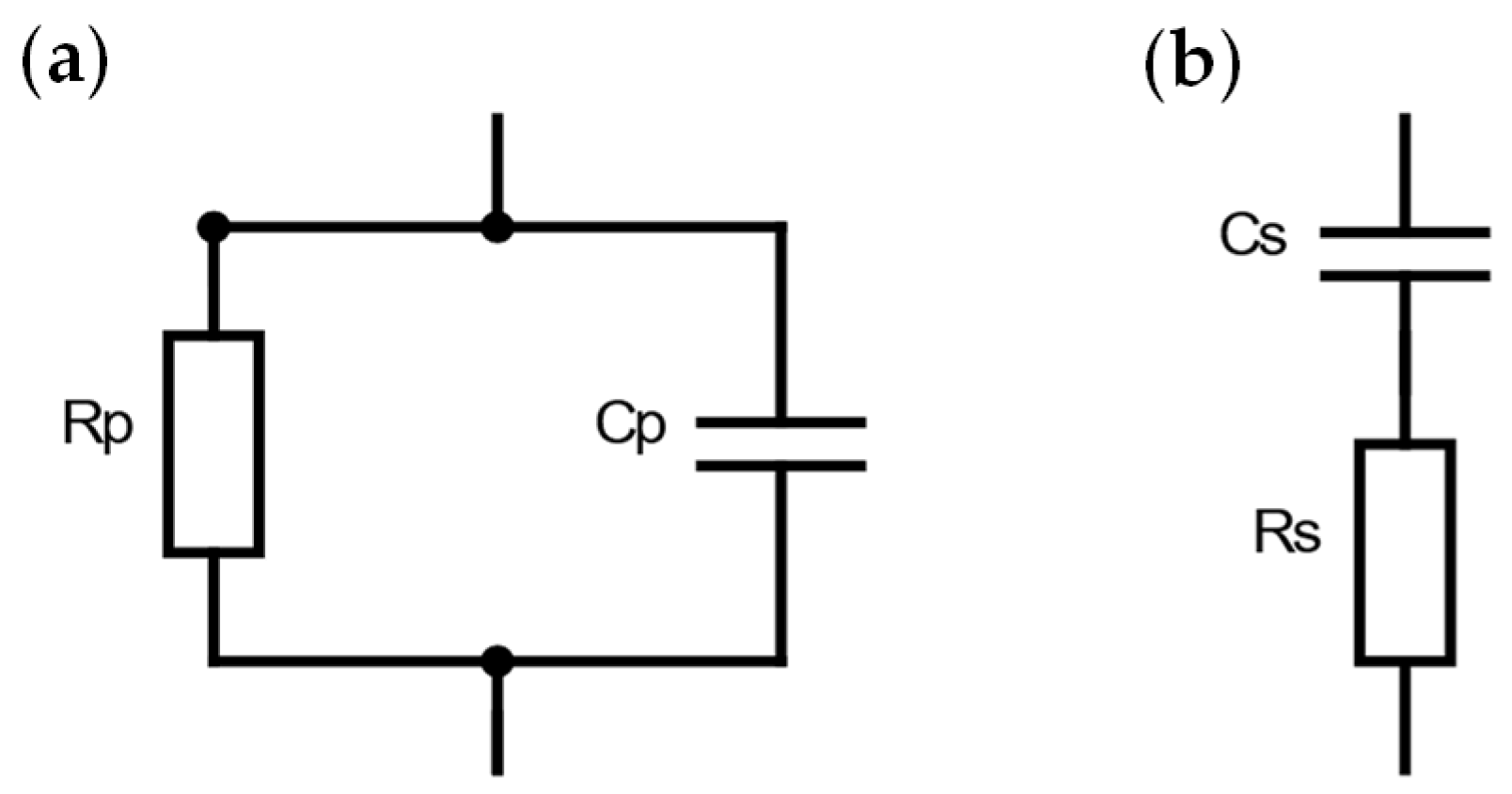
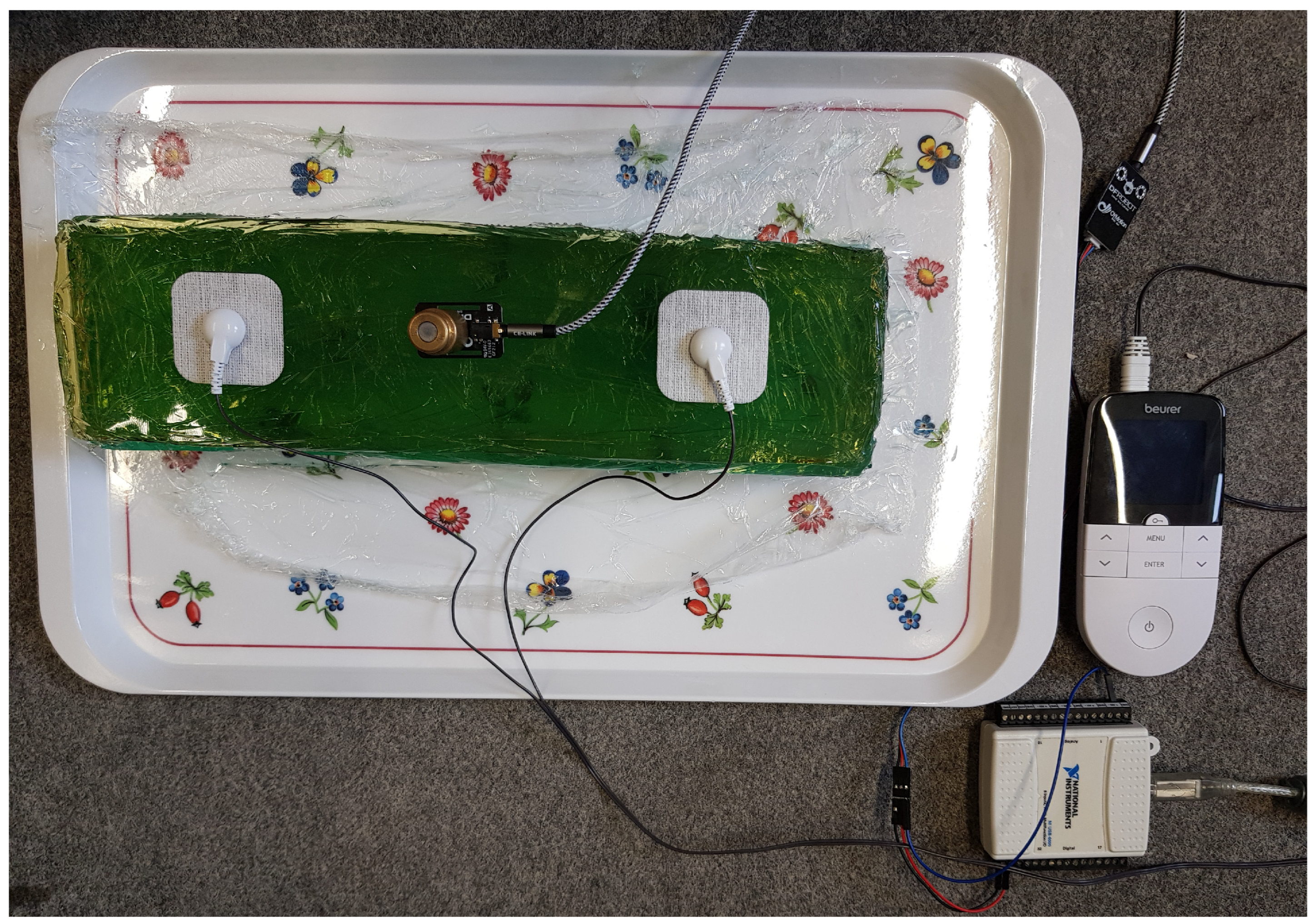

| Phantom Type | Sample Number | Reference | Gelatin | Distilled Water | Sunflower Oil | Dishwashing Detergent | Iodized Salt | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | % (w/w) | g | % (w/w) | g | % (w/w) | g | % (w/w) | g | % (w/w) | |||
| Skin | 1 | [16] | 3.4 | 9.6 | 19.4 | 54.6 | 10.9 | 30.7 | 1.0 | 2.8 | 0.8 | 2.3 |
| 2 | [16] | 3.5 | 9.2 | 15.3 | 40.6 | 15.0 | 39.8 | 3.9 | 10.3 | - | - | |
| 3 | [21] | 10 * | 25 | 30 | 75 | - | - | - | - | - | - | |
| 4 ** | [21] | 10 * | 25 | 30 | 75 | - | - | - | - | - | - | |
| Fat | 1 | [16] | 2.2 | 3.8 | 8.2 | 14.2 | 45.5 | 78.9 | 1.8 | 3.1 | - | - |
| 2 ** | [16] | 2.2 | 3.9 | 8.2 | 14.4 | 45.0 | 79.2 | 1.4 | 2.5 | - | - | |
| 3 | [16] | 2.2 | 3.9 | 8.2 | 14.4 | 45.0 | 79.2 | 1.4 | 2.5 | - | - | |
| 4 | [21] | 10 * | 25 | 30 | 75 | - | - | - | - | - | - | |
| Blood | 1 | [16] | 3.3 | 10.3 | 21.8 | 68.3 | 2.4 | 7.5 | 4.0 | 12.5 | 0.4 | 1.3 |
| 2 | [16] | 3.3 | 9.9 | 16.9 | 51.1 | 6.3 | 19.0 | 4.6 | 13.9 | 2.0 | 6.0 | |
| 3 ** | [16] | 5.0 | 13.9 | 17.0 | 47.2 | 6.0 | 16.7 | 4.0 | 11.1 | 4.0 | 11.1 | |
| 4 | - | 5.0 | 13.2 | 30.0 | 78.9 | - | - | 3.0 | 7.9 | - | - | |
| Muscle | 1 | [16] | 2.6 | 5.5 | 14.5 | 30.9 | 28.4 | 60.4 | 1.5 | 3.2 | - | - |
| 2 | [16] | 2.6 | 5.5 | 14.5 | 30.9 | 28.4 | 60.4 | 1.5 | 3.2 | - | - | |
| 3 | [22] | 5.3 | 14.9 | 30.0 | 84.3 | - | - | 0.3 | 0.8 | - | - | |
| 4 | [16] | 2.6 | 5.5 | 14.3 | 30.1 | 28.2 | 59.4 | 1.5 | 3.2 | 0.9 | 1.9 | |
| 5 ** | [22] | 5.3 | 14.5 | 30.0 | 81.9 | - | - | 1.3 | 3.6 | - | - | |
| 6 | [22] | 5.3 | 14.8 | 30.0 | 83.8 | - | - | 0.5 | 1.4 | - | - | |
| Frequency/Hz | 20 | 40 | 60 | 80 | 100 | |
|---|---|---|---|---|---|---|
| Electrical Conductivity/S/m | Blood Phantom 4 | 7.90 × 10−2 | 1.17 × 10−1 | 1.48 × 10−1 | 1.80 × 10−1 | 2.12 × 10−1 |
| Blood * | 7.00 × 10−1 | 7.00 × 10−1 | 7.00 × 10−1 | 7.00 × 10−1 | 7.00 × 10−1 | |
| Fat Phantom 4 | 2.60 × 10−2 | 3.20 × 10−2 | 3.60 × 10−2 | 3.99 × 10−2 | 4.30 × 10−2 | |
| Fat * | 3.94 × 10−2 | 4.03 × 10−2 | 4.05 × 10−2 | 4.06 × 10−2 | 4.06 × 10−2 | |
| Muscle Phantom 6 | 1.26 × 10−1 | 1.76 × 10−1 | 2.13 × 10−1 | 2.54 × 10−1 | 2.90 × 10−1 | |
| Muscle * | 2.07 × 10−1 | 2.24 × 10−1 | 2.42 × 10−1 | 2.56 × 10−1 | 2.67 × 10−1 | |
| Skin Phantom 4 | 140 × 10−2 | 1.70 × 10−2 | 1.90 × 10−2 | 2.00 × 10−2 | 2.10 × 10−2 | |
| Skin * | 2.00 × 10−4 | 2.00 × 10−4 | 2.00 × 10−4 | 2.00 × 10−4 | 2.00 × 10−4 | |
| Relative Permittivity | Blood Phantom 4 | 7.62 × 107 | 4.37 × 107 | 3.23 × 107 | 2.68 × 107 | 2.35 × 107 |
| Blood * | 5.26 × 103 | 5.26 × 103 | 5.26 × 103 | 5.26 × 103 | 5.26 × 103 | |
| Fat Phantom 4 | 2.62 × 107 | 1.70 × 107 | 1.27 × 107 | 1.03 × 107 | 8.71 × 106 | |
| Fat * | 2.09 × 106 | 6.69 × 105 | 3.37 × 105 | 2.12 × 105 | 1.52 × 105 | |
| Muscle Phantom 6 | 1.33 × 108 | 7.98 × 107 | 6.11 × 107 | 5.14 × 107 | 4.55 × 107 | |
| Muscle * | 2.43 × 107 | 2.00 × 107 | 1.56 × 107 | 1.20 × 107 | 9.33 × 106 | |
| Skin Phantom 4 | 2.27 × 107 | 1.36 × 107 | 9.58 × 106 | 7.78 × 106 | 6.46 × 106 | |
| Skin * | 1.14 × 103 | 1.14 × 103 | 1.14 × 103 | 1.14 × 103 | 1.14 × 103 | |
| RMS Amplitude in mV | |||
|---|---|---|---|
| EMS Parameters | Phantom | Human | Relative Deviation in % |
| 50 Hz, I 9 | 0.35 | 0.36 | 2.8 |
| 50 Hz, I 15 | 0.36 | 0.37 | 2.7 |
| 50 Hz, I 23 | 0.38 | 0.42 | 9.5 |
| 100 Hz, I 23 | 0.53 | 0.54 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhrhan, K.; Schwindt, E.; Witte, H. Fabrication and Dielectric Validation of an Arm Phantom for Electromyostimulation. Bioengineering 2024, 11, 724. https://doi.org/10.3390/bioengineering11070724
Uhrhan K, Schwindt E, Witte H. Fabrication and Dielectric Validation of an Arm Phantom for Electromyostimulation. Bioengineering. 2024; 11(7):724. https://doi.org/10.3390/bioengineering11070724
Chicago/Turabian StyleUhrhan, Katja, Esther Schwindt, and Hartmut Witte. 2024. "Fabrication and Dielectric Validation of an Arm Phantom for Electromyostimulation" Bioengineering 11, no. 7: 724. https://doi.org/10.3390/bioengineering11070724
APA StyleUhrhan, K., Schwindt, E., & Witte, H. (2024). Fabrication and Dielectric Validation of an Arm Phantom for Electromyostimulation. Bioengineering, 11(7), 724. https://doi.org/10.3390/bioengineering11070724








