A Novel Time–Frequency Parameterization Method for Oscillations in Specific Frequency Bands and Its Application on OPM-MEG
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Simulation and MEG Experiment
2.2.1. Stimulated Neural Data
- Simulation I: Simulated neural data consist of aperiodic and oscillatory activity
- Simulation II: Simulated neural data with empty-room noise in OPM-MEG
2.2.2. Resting-State SQUID-MEG and OPM-MEG
2.2.3. OPM-MEG under Rhythmic Flash Stimulus (FS)
2.2.4. MEG Data Preprocessing
2.2.5. Source Time Series Reconstruction
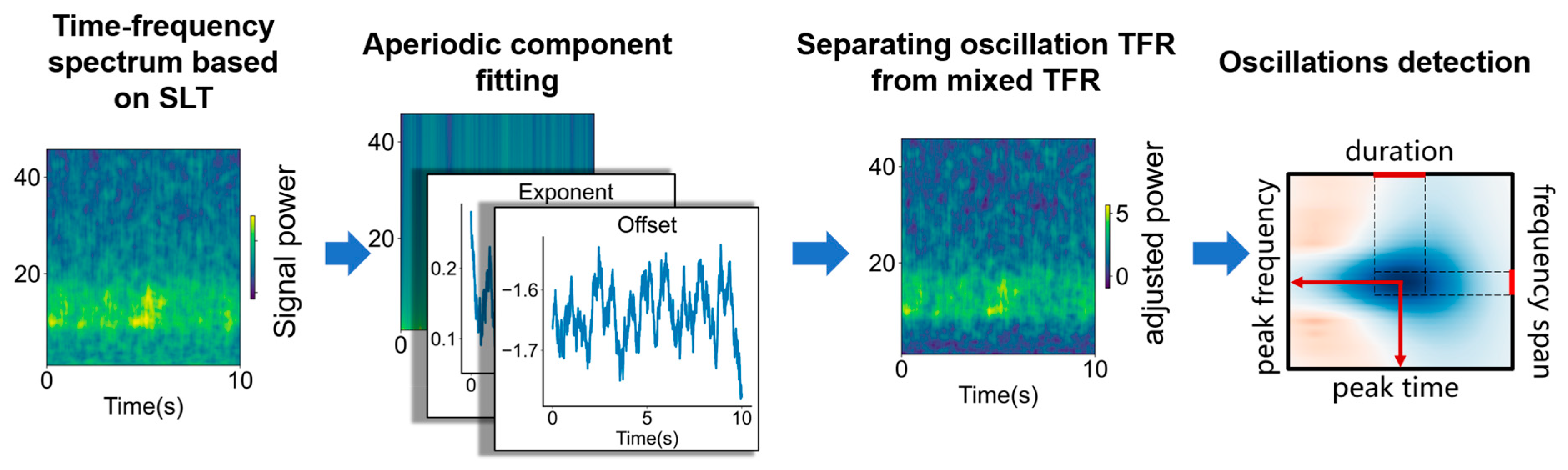
2.3. The Principle of STPPTO
2.3.1. Time–Frequency Spectrum of Signal
2.3.2. Separating Oscillation TFR
2.3.3. Oscillation Detection
2.4. Compared with Existing Methods
The Evaluation of the Performance of the Proposed Algorithm
- The mean absolute error (MAE) of oscillation parameters
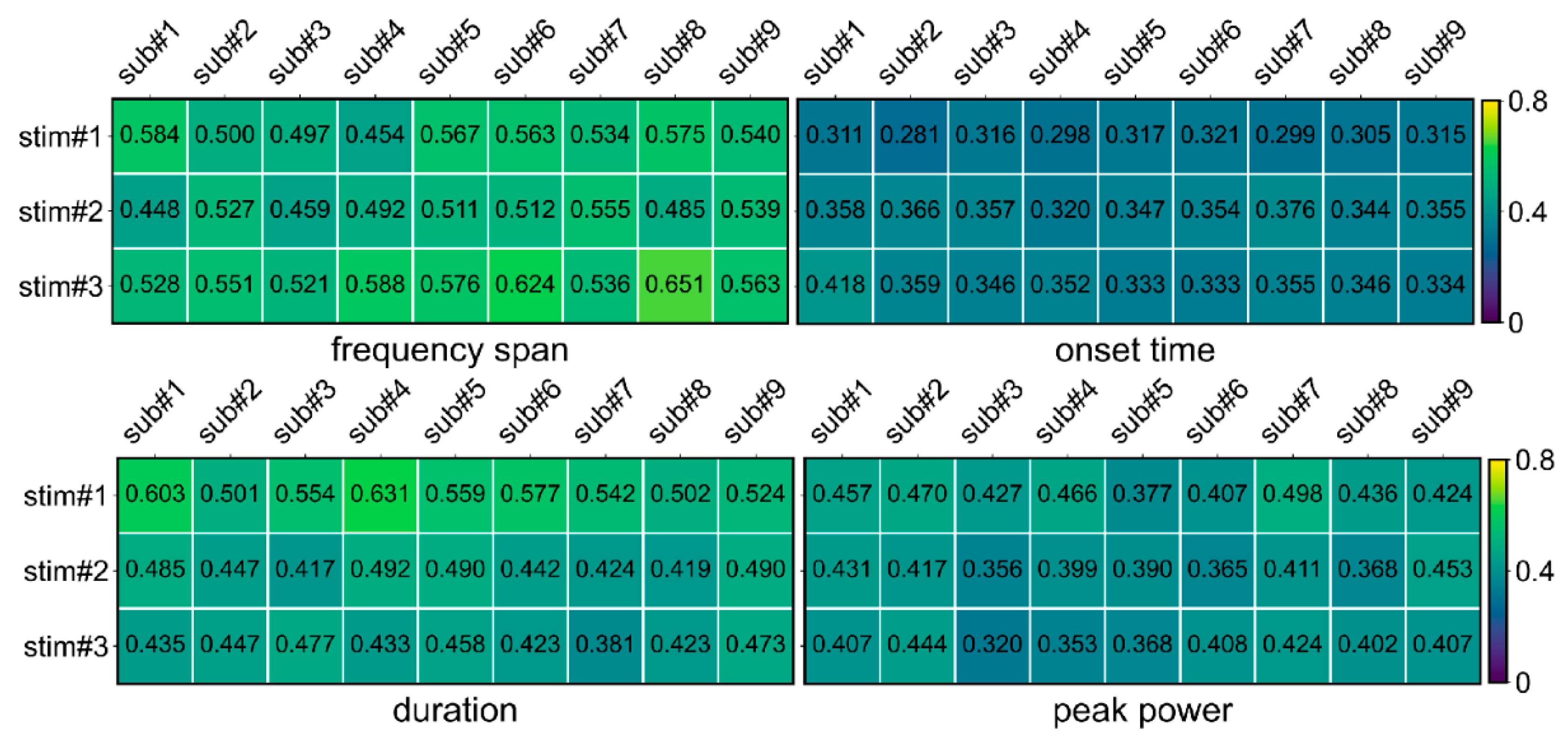
- The coefficient of variation (CV) in multi-trial OPM-MEG oscillation parameters
2.5. Statistical Methods
3. Results
3.1. The Performance of STPPTO against Simulation
3.2. The Application of STPPTO
3.2.1. The Transient Oscillation in the Primary Visual Cortex under a Resting State
3.2.2. The Oscillations in the Visual Cortex under Rhythmic Flash Stimulus OPM-MEG
4. Discussion
- Limitations of the study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engel, A.K.; Fries, P.; Singer, W. Dynamic Predictions: Oscillations and Synchrony in Top–down Processing. Nat. Rev. Neurosci. 2001, 2, 704–716. [Google Scholar] [CrossRef]
- Fries, P. A Mechanism for Cognitive Dynamics: Neuronal Communication through Neuronal Coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef]
- Voytek, B.; Kayser, A.S.; Badre, D.; Fegen, D.; Chang, E.F.; Crone, N.E.; Parvizi, J.; Knight, R.T.; D’Esposito, M. Oscillatory Dynamics Coordinating Human Frontal Networks in Support of Goal Maintenance. Nat. Neurosci. 2015, 18, 1318–1324. [Google Scholar] [CrossRef]
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Rhodes, N.; Rea, M.; Boto, E.; Rier, L.; Shah, V.; Hill, R.M.; Osborne, J.; Doyle, C.; Holmes, N.; Coleman, S.C.; et al. Measurement of Frontal Midline Theta Oscillations Using OPM-MEG. NeuroImage 2023, 271, 120024. [Google Scholar] [CrossRef]
- Tierney, T.M.; Levy, A.; Barry, D.N.; Meyer, S.S.; Shigihara, Y.; Everatt, M.; Mellor, S.; Lopez, J.D.; Bestmann, S.; Holmes, N.; et al. Mouth Magnetoencephalography: A Unique Perspective on the Human Hippocampus. NeuroImage 2021, 225, 117443. [Google Scholar] [CrossRef]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T.; et al. Parameterizing Neural Power Spectra into Periodic and Aperiodic Components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef]
- Clements, G.M.; Bowie, D.C.; Gyurkovics, M.; Low, K.A.; Fabiani, M.; Gratton, G. Spontaneous Alpha and Theta Oscillations Are Related to Complementary Aspects of Cognitive Control in Younger and Older Adults. Front. Hum. Neurosci. 2021, 15, 621620. [Google Scholar] [CrossRef]
- Ronconi, L.; Oosterhof, N.N.; Bonmassar, C.; Melcher, D. Multiple Oscillatory Rhythms Determine the Temporal Organization of Perception. Proc. Natl. Acad. Sci. USA 2017, 114, 13435–13440. [Google Scholar] [CrossRef]
- Rier, L.; Rhodes, N.; Pakenham, D.; Boto, E.; Holmes, N.; Hill, R.M.; Rivero, G.R.; Shah, V.; Doyle, C.; Osborne, J.; et al. The Neurodevelopmental Trajectory of Beta Band Oscillations: An OPM-MEG Study. eLife 2024, 13. [Google Scholar] [CrossRef]
- Chota, S.; VanRullen, R.; Gulbinaite, R. Random Tactile Noise Stimulation Reveals Beta-Rhythmic Impulse Response Function of the Somatosensory System. J. Neurosci. 2023, 43, 3107–3119. [Google Scholar] [CrossRef]
- Van Ede, F.; Quinn, A.J.; Woolrich, M.W.; Nobre, A.C. Neural Oscillations: Sustained Rhythms or Transient Burst-Events? Trends Neurosci. 2018, 41, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Kaefer, K.; Stella, F.; Csicsvari, J. Theta Oscillations as a Substrate for Medial Prefrontal-Hippocampal Assembly Interactions. Cell Rep. 2023, 42, 113015. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, D.; Wei, Q.; Wang, G.; Huang, F.; Chen, X.; Muhammad, K.G.; Sun, Y.; Liu, J. Intrinsic Phase–Amplitude Coupling on Multiple Spatial Scales during the Loss and Recovery of Consciousness. Comput. Biol. Med. 2022, 147, 105687. [Google Scholar] [CrossRef]
- Shin, H.; Law, R.; Tsutsui, S.; Moore, C.I.; Jones, S.R. The Rate of Transient Beta Frequency Events Predicts Behavior across Tasks and Species. eLife 2017, 6, e29086. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, C.; Quinn, A.; Vivekananda, U.; Zhan, S.; Liu, W.; Sun, B.; Woolrich, M.; Lu, Q.; Litvak, V. Dynamic Analysis on Simultaneous iEEG-MEG Data via Hidden Markov Model. NeuroImage 2021, 233, 117923. [Google Scholar] [CrossRef]
- Ouyang, G.; Hildebrandt, A.; Schmitz, F.; Herrmann, C.S. Decomposing Alpha and 1/f Brain Activities Reveals Their Differential Associations with Cognitive Processing Speed. NeuroImage 2020, 205, 116304. [Google Scholar] [CrossRef]
- He, B.J.; Zempel, J.M.; Snyder, A.Z.; Raichle, M.E. The Temporal Structures and Functional Significance of Scale-free Brain Activity. Neuron 2010, 66, 353–369. [Google Scholar] [CrossRef]
- Brady, B.; Power, L.; Bardouille, T. Age-Related Trends in Neuromagnetic Transient Beta Burst Characteristics during a Sensorimotor Task and Rest in the Cam-CAN Open-access Dataset. NeuroImage 2020, 222, 117245. [Google Scholar] [CrossRef]
- Schaworonkow, N.; Voytek, B. Longitudinal Changes in Aperiodic and Periodic Activity in Electrophysiological Recordings in the First Seven Months of Life. Dev. Cogn. Neurosci. 2021, 47, 100895. [Google Scholar] [CrossRef]
- Azami, H.; Zrenner, C.; Brooks, H.; Zomorrodi, R.; Blumberger, D.M.; Fischer, C.E.; Flint, A.; Herrmann, N.; Kumar, S.; Lanctôt, K.; et al. Beta to Theta Power Ratio in EEG Periodic Components as a Potential Biomarker in Mild Cognitive Impairment and Alzheimer’s Dementia. Alzheimer's Res. Ther. 2023, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Wiesman, A.I.; Donhauser, P.W.; Degroot, C.; Diab, S.; Kousaie, S.; Fon, E.A.; Klein, D.; Baillet, S. PREVENT-AD Research Group; Villeneuve, S.; et al. Aberrant Neurophysiological Signaling Associated with Speech Impairments in Parkinson’s Disease. NPJ Park. Dis. 2023, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Brady, B.; Bardouille, T. Periodic/Aperiodic Parameterization of Transient Oscillations (PAPTO)–Implications for Healthy Ageing. Neuro. Image 2022, 251, 118974. [Google Scholar] [CrossRef] [PubMed]
- Waschke, L.; Donoghue, T.; Fiedler, L.; Smith, S.; Garrett, D.D.; Voytek, B.; Obleser, J. Modality-specific Tracking of Attention and Sensory Statistics in the Human Electrophysiological Spectral Exponent. eLife 2021, 10, e70068. [Google Scholar] [CrossRef] [PubMed]
- Kluger, D.S.; Forster, C.; Abbasi, O.; Chalas, N.; Villringer, A.; Gross, J. Modulatory Dynamics of Periodic and Aperiodic Activity in Respiration-Brain Coupling. Nat. Commun. 2023, 14, 4699. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.E.; da Silva Castanheira, J.; Baillet, S. Time-Resolved Parameterization of Aperiodic and Periodic Brain Activity. eLife 2022, 11, e77348. [Google Scholar] [CrossRef]
- Moca, V.V.; Bârzan, H.; Nagy-Dăbâcan, A.; Mureșan, R.C. Time-Frequency Super-Resolution with Superlets. Nat. Commun. 2021, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, J.-M.; Oostenveld, R.; Lam, N.H.L.; Uddén, J.; Hultén, A.; Hagoort, P. A 204-Subject Multimodal Neuroimaging Dataset to Study Language Processing. Sci. Data 2019, 6, 17. [Google Scholar] [CrossRef]
- Cole, S.; Donoghue, T.; Gao, R.; Voytek, B. NeuroDSP: A Package for Neural Digital Signal Processing. JOSS 2019, 4, 1272. [Google Scholar] [CrossRef]
- Seymour, R.A.; Alexander, N.; Mellor, S.; O’Neill, G.C.; Tierney, T.M.; Barnes, G.R.; Maguire, E.A. Interference Suppression Techniques for OPM-Based MEG: Opportunities and Challenges. NeuroImage 2022, 247, 118834. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Dai, C.; Hu, X. Independent Component Analysis Based Algorithms for High-Density Electromyogram Decomposition: Systematic Evaluation through Simulation. Comput. Biol. Med. 2019, 109, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; An, N.; Xu, W.; Wang, W.; Li, W.; Wang, C.; Yang, Y.; Xiang, M.; Gao, Y.; Ning, X. OMMR: Co-registration toolbox of OPM-MEG and MRI. Front. Neurosci. 2022, 16, 984036. [Google Scholar] [CrossRef]
- Cao, F.; An, N.; Xu, W.; Wang, W.; Li, W.; Wang, C.; Xiang, M.; Gao, Y.; Ning, X. Optical Co-Registration Method of Triaxial OPM-MEG and MRI. IEEE Trans. Med. Imaging 2023, 42, 2706–2713. [Google Scholar] [CrossRef]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic Statistical Parametric Mapping: Combining fMRI and MEG for High-Resolution Imaging of Cortical Activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE Software for Processing MEG and EEG Data. NeuroImage 2014, 86, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; William, C.H.; LaMantia, A.-S.; Mooney, R.D.; Platt, M.L.; White, L.E. Neuroscience, 6th ed.; Sinauer Associates of Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic Parcellation of Human Cortical Gyri and Sulci Using Standard Anatomical Nomenclature. NeuroImage 2010, 53, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shechtman, O. The Coefficient of Variation as an Index of Measurement Reliability. In Methods of Clinical Epidemiology; Doi, S.A.R., Williams, G.M., Eds.; Springer Series on Epidemiology and Public Health; Springer: Berlin/Heidelberg, Germany, 2013; pp. 39–49. ISBN 978-3-642-37131-8. [Google Scholar]
- Rouder, J.N.; Speckman, P.L.; Sun, D.; Morey, R.D.; Iverson, G. Bayesian t Tests for Accepting and Rejecting the Null Hypothesis. Psychon. Bull. Rev. 2009, 16, 225–237. [Google Scholar] [CrossRef]
- Gutteling, T.P.; Bonnefond, M.; Clausner, T.; Daligault, S.; Romain, R.; Mitryukovskiy, S.; Fourcault, W.; Josselin, V.; Le Prado, M.; Palacios-Laloy, A.; et al. A New Generation of OPM for High Dynamic and Large Bandwidth MEG: The 4He OPMs—First Applications in Healthy Volunteers. Sensors 2023, 23, 2801. [Google Scholar] [CrossRef]
- Iivanainen, J.; Carter, T.R.; Trumbo, M.; McKay, J.; Taulu, S.; Wang, J.; Stephen, J.; Schwindt, P.D.D.; Borna, A. Single-trial Classification of Evoked Responses to Auditory Tones Using OPM- and SQUID-MEG. J. Neural Eng. 2023, 20, 056032. [Google Scholar] [CrossRef]
- Feys, O.; Corvilain, P.; Aeby, A.; Sculier, C.; Holmes, N.; Brookes, M.; Goldman, S.; Wens, V.; De Tiège, X. On-Scalp Optically Pumped Magnetometers versus Cryogenic Magnetoencephalography for Diagnostic Evaluation of Epilepsy in School-aged Children. Radiology 2022, 304, 429–434. [Google Scholar] [CrossRef]
- An, N.; Cao, F.; Li, W.; Wang, W.; Xu, W.; Wang, C.; Xiang, M.; Gao, Y.; Sui, B.; Liang, A.; et al. Imaging somatosensory Cortex Responses Measured by OPM-MEG: Variational Free Energy-Based Spatial Smoothing Estimation Approach. iScience 2022, 25, 103752. [Google Scholar] [CrossRef] [PubMed]
- Mahjoory, K.; Schoffelen, J.-M.; Keitel, A.; Gross, J. The Frequency Gradient of Human Resting-State Brain Oscillations Follows Cortical Hierarchies. eLife 2020, 9, e53715. [Google Scholar] [CrossRef] [PubMed]
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving Magnetoencephalography towards Real-World Applications with a Wearable System. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.; Zhou, H.; Urbach, E.K.; Turner, M.J.; Walsworth, R.L.; Lukin, M.D.; Park, H. Quantum Sensors for Biomedical Applications. Nat. Rev. Phys. 2023, 5, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Houlgreave, M.S.; Morera Maiquez, B.; Brookes, M.J.; Jackson, S.R. The Oscillatory Effects of Rhythmic Median Nerve Stimulation. NeuroImage 2022, 251, 118990. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Clark, G.M.; Bigelow, F.J.; Lum, J.A.G.; Enticott, P.G. Periodic and Aperiodic Neural Activity Displays Age-Dependent Changes across Early-to-Middle Childhood. Dev. Cogn. Neurosci. 2022, 54, 101076. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.L.; Khalil, T.; Kim, L.H.; Noor, M.S.; Luo, F.; Kiss, Z.H. Aperiodic Subthalamic Activity Predicts Motor Severity and Stimulation Response in Parkinson Disease. Park. Relat. Disord. 2023, 110, 105397. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhang, X.; Wu, X.; Valdes-Sosa, P.A. ξ-π: A Nonparametric Model for Neural Power Spectra Decomposition. IEEE J. Biomed. Health Inform. 2024, 28, 2624–2635. [Google Scholar] [CrossRef]
- Neymotin, S.A.; Tal, I.; Barczak, A.; O’Connell, M.N.; McGinnis, T.; Markowitz, N.; Espinal, E.; Griffith, E.; Anwar, H.; Dura-Bernal, S.; et al. Detecting Spontaneous Neural Oscillation Events in Primate Auditory Cortex. eNeuro 2022, 9. [Google Scholar] [CrossRef]
- Neumann, W.-J.; Steiner, L.A.; Milosevic, L. Neurophysiological Mechanisms of Deep Brain Stimulation across Spatiotemporal Resolutions. Brain 2023, 146, 4456–4468. [Google Scholar] [CrossRef] [PubMed]
- Orellana, V.D.; Donoghue, J.P.; Vargas-Irwin, C.E. Low Frequency Independent Components: Internal Neuromarkers Linking Cortical LFPs to Behavior. iScience 2024, 27, 108310. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, R.F.; Lendner, J.D.; Knight, R.T. Aperiodic Sleep Networks Promote Memory Consolidation. Trends Cogn. Sci. 2021, 25, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Brookshire, G. Putative Rhythms in Attentional Switching Can Be Explained by Aperiodic Temporal Structure. Nat. Hum. Behav. 2022, 6, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG Differences between Eyes-Closed and Eyes-Open Resting Conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Petro, N.M.; Ott, L.; Penhale, S.; Rempe, M.; Embury, C.; Picci, G.; Wang, Y.-P.; Stephen, J.M.; Calhoun, V.D.; Wilson, T.W. Eyes-Closed versus Eyes-Open Differences in Spontaneous Neural Dynamics during Development. NeuroImage 2022, 258, 119337. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Sorensen, L.B.; Ojemann, J.G.; Den Nijs, M. Power-Law Scaling in the Brain Surface Electric Potential. PLoS Comput. Biol. 2009, 5, e1000609. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Liu, S.; Burkitt, A.N.; Grayden, D.B. Multi-Frequency Steady-State Visual Evoked Potential Dataset. Sci. Data 2024, 11, 26. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Axmacher, N.; Inman, C.S. Modulating Human Memory via Entrainment of Brain Oscillations. Trends Neurosci. 2019, 42, 485–499. [Google Scholar] [CrossRef]





| Peak Frequency Error (Hz) | Frequency Span Error (Hz) | Onset Time Error (s) | Duration Error (s) | |
|---|---|---|---|---|
| SPRiNT [26] | 1.48 ± 0.03 | - | 0.315 ± 0.008 | - |
| s-PAPTO [23] | 0.88 ± 0.02 | 1.89 ± 0.06 | 0.206 ± 0.007 | 0.105 ± 0.003 |
| STPPTO | 0.75 ± 0.02 | 1.43 ± 0.05 | 0.100 ± 0.003 | 0.101 ± 0.002 |
| SPRiNT [26] vs. STPPTO | p < 0.0001 | - | p < 0.0001 | - |
| BF10: >100 | - | BF10: >100 | - | |
| s-PAPTO [23] vs. STPPTO | p < 0.0001 | p < 0.0001 | p < 0.01 | ns. |
| BF10: >100 | BF10: >100 | BF10: >100 | BF10: 0.17 |
| Peak Frequency Error (Hz) | Frequency Span Error (Hz) | Onset Time Error (s) | Duration Error (s) | |
|---|---|---|---|---|
| SPRiNT [26] | 1.40 ± 0.03 | - | 0.314 ± 0.008 | - |
| s-PAPTO [23] | 1.39 ± 0.03 | 3.20 ± 0.07 | 0.399 ± 0.008 | 0.165 ± 0.004 |
| STPPTO | 1.19 ± 0.02 | 2.61 ± 0.06 | 0.231 ± 0.005 | 0.151 ± 0.002 |
| SPRiNT [26] vs. STPPTO | p < 0.0001 | - | p < 0.0001 | - |
| BF10: >100 | - | BF10: >100 | - | |
| s-PAPTO [23] vs. STPPTO | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.01 |
| BF10: 23.98 | BF10: >100 | BF10: >100 | BF10: 0.13 |
| Peak Frequency (Hz) | Frequency Span (Hz) | Duration (s) | Peak Power | ||
|---|---|---|---|---|---|
| alpha | SQUID-MEG | 9.44 ± 0.01 | 5.67 ± 0.01 | 0.204 ± 0.001 | 3.63 ± 0.02 |
| OPM-MEG | 9.85 ± 0.04 | 4.82 ± 0.07 | 0.227 ± 0.005 | 4.36 ± 0.04 | |
| BF10 | 0.52 | 41.97 | 0.24 | 16.57 | |
| Difference | 4% | 15% | 9% | 19% | |
| beta | SQUID-MEG | 19.69 ± 0.02 | 6.86 ± 0.02 | 0.181 ± 0 | 3.15 ± 0 |
| OPM-MEG | 19.84 ± 0.08 | 5.54 ± 0.06 | 0.205 ± 0.005 | 3.91 ± 0.03 | |
| BF10 | 0.37 | 66.34 | 0.29 | >100 | |
| Difference | 1% | 19% | 13% | 24% |
| ROI | Harmonic Order | Frequency Span (Hz) | Onset Time(s) | Duration | Peak Power |
|---|---|---|---|---|---|
| Right V1 + O6 | 1st | 3.74 ± 0.04 | 1.338 ± 0.009 | 0.574 ± 0.006 | 3.03 ± 0.03 |
| 2nd | 4.63 ± 0.06 | 1.368 ± 0.010 | 0.665 ± 0.007 | 2.69 ± 0.02 | |
| 3rd | 5.30 ± 0.07 | 1.386 ± 0.105 | 0.644 ± 0.006 | 2.70 ± 0.02 | |
| Left V1 + O6 | 1st | 3.78 ± 0.04 | 1.313 ± 0.009 | 0.614 ± 0.006 | 2.99 ± 0.03 |
| 2nd | 4.80 ± 0.06 | 1.359 ± 0.011 | 0.650 ± 0.006 | 2.75 ± 0.03 | |
| 3rd | 5.30 ± 0.07 | 1.389 ± 0.011 | 0.640 ± 0.006 | 2.70 ± 0.02 |
| Stimulus-ROI | Frequency Span (Hz) | Onset Time (s) | Duration | Peak Power |
|---|---|---|---|---|
| Right eye-Left V1 + O6 | 3.74 ± 0.04 | 1.338 ± 0.009 | 0.574 ± 0.006 | 3.03 ± 0.03 |
| Left eye-RightV1 + O6 | 3.78 ± 0.04 | 1.313 ± 0.009 | 0.614 ± 0.006 | 2.99 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, R.; Wu, H.; Ma, Y.; Liu, C.; Gao, Y.; Yu, D.; Ning, X. A Novel Time–Frequency Parameterization Method for Oscillations in Specific Frequency Bands and Its Application on OPM-MEG. Bioengineering 2024, 11, 773. https://doi.org/10.3390/bioengineering11080773
Liang X, Wang R, Wu H, Ma Y, Liu C, Gao Y, Yu D, Ning X. A Novel Time–Frequency Parameterization Method for Oscillations in Specific Frequency Bands and Its Application on OPM-MEG. Bioengineering. 2024; 11(8):773. https://doi.org/10.3390/bioengineering11080773
Chicago/Turabian StyleLiang, Xiaoyu, Ruonan Wang, Huanqi Wu, Yuyu Ma, Changzeng Liu, Yang Gao, Dexin Yu, and Xiaolin Ning. 2024. "A Novel Time–Frequency Parameterization Method for Oscillations in Specific Frequency Bands and Its Application on OPM-MEG" Bioengineering 11, no. 8: 773. https://doi.org/10.3390/bioengineering11080773
APA StyleLiang, X., Wang, R., Wu, H., Ma, Y., Liu, C., Gao, Y., Yu, D., & Ning, X. (2024). A Novel Time–Frequency Parameterization Method for Oscillations in Specific Frequency Bands and Its Application on OPM-MEG. Bioengineering, 11(8), 773. https://doi.org/10.3390/bioengineering11080773








