Formation and Long-Term Culture of hiPSC-Derived Sensory Nerve Organoids Using Microfluidic Devices
Abstract
1. Introduction
2. Materials and Methods
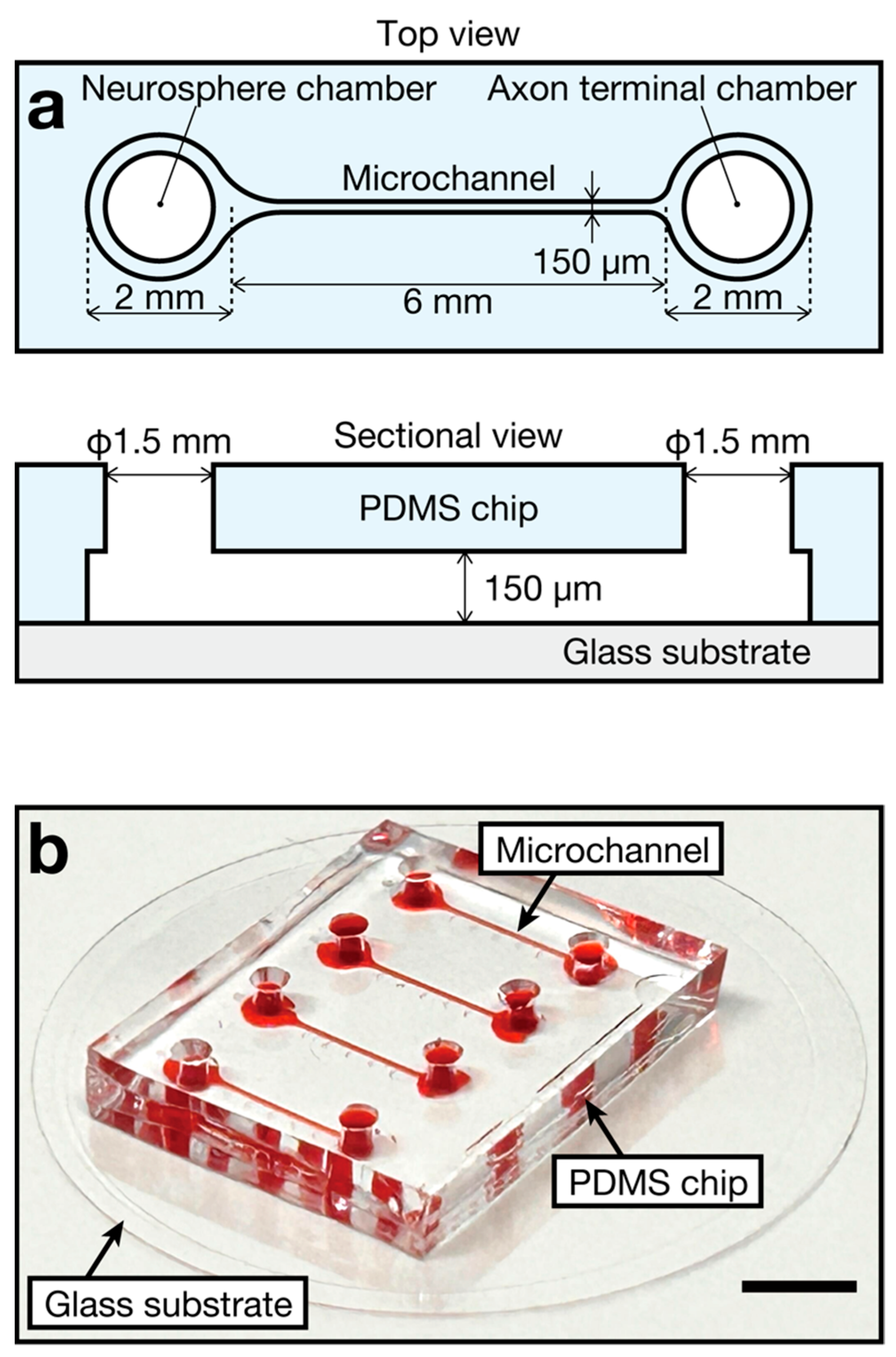
2.1. Device Design and Fabrication
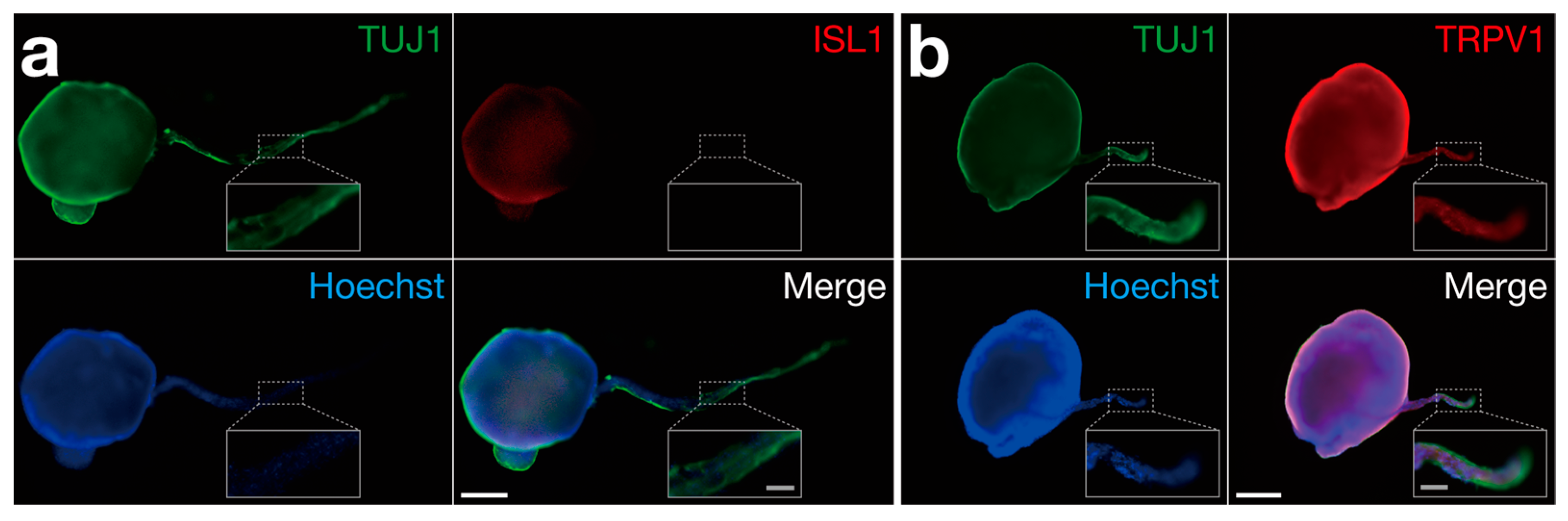
2.2. Cell Culture for Sensory Nerve Organoid Formation and Characterization
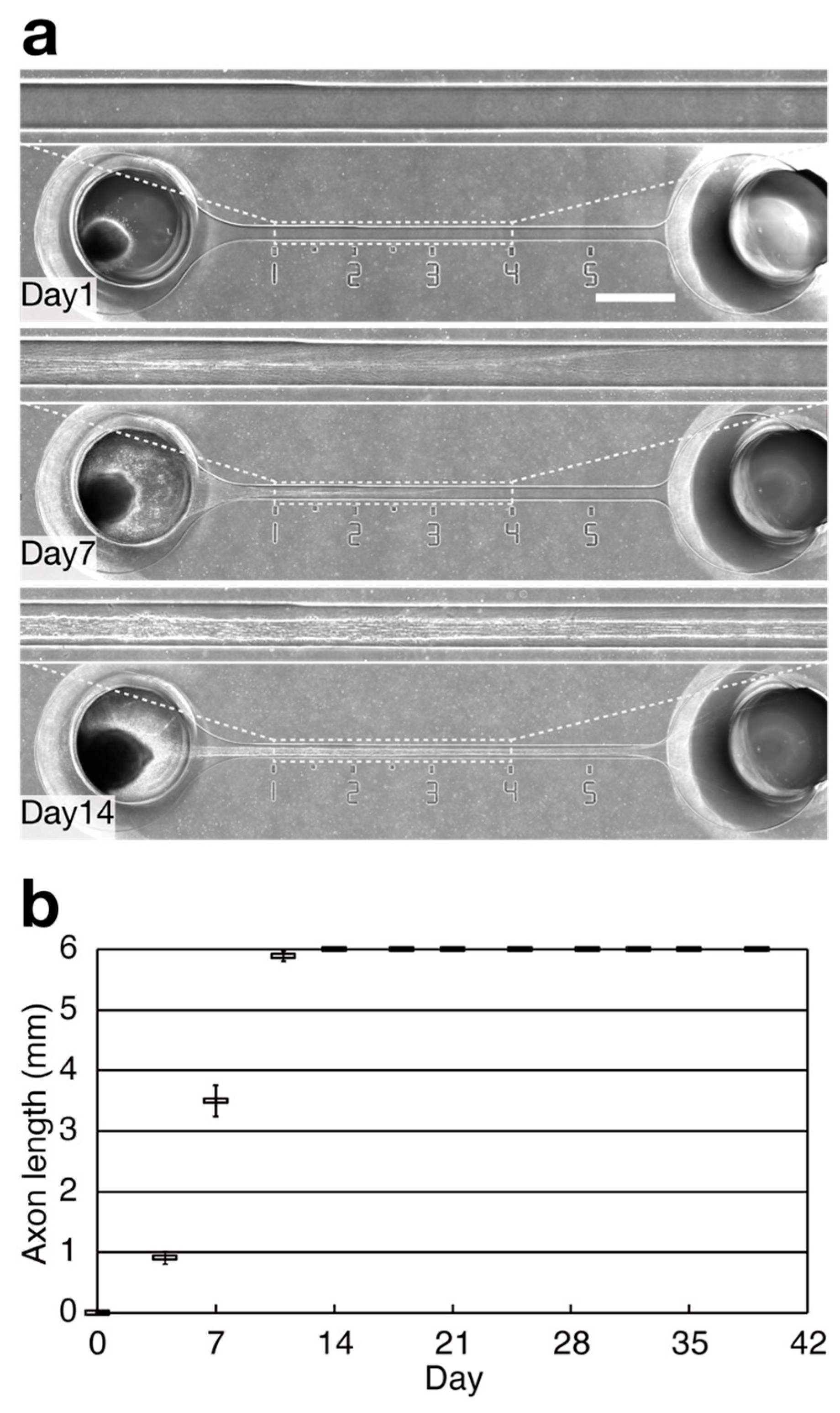
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lampert, A.; Bennett, D.L.; McDermott, L.A.; Neureiter, A.; Eberhardt, E.; Winner, B.; Zenke, M. Human Sensory Neurons Derived from Pluripotent Stem Cells for Disease Modelling and Personalized Medicine. Neurobiol. Pain 2020, 8, 100055. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chao, J.; Shi, Y. Modeling Neurological Diseases Using iPSC-Derived Neural Cells: iPSC Modeling of Neurological Diseases. Cell Tissue Res. 2018, 371, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Karpe, Y.; Chen, Z.; Li, X.-J. Stem Cell Models and Gene Targeting for Human Motor Neuron Diseases. Pharmaceuticals 2021, 14, 565. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; McDonnell, A.; Nitzsche, A.; Alexandrou, A.; Saintot, P.-P.; Loucif, A.J.C.; Brown, A.R.; Young, G.; Mis, M.; Randall, A.; et al. Pharmacological Reversal of a Pain Phenotype in IPSC-Derived Sensory Neurons and Patients with Inherited Erythromelalgia. Sci. Transl. Med. 2016, 8, 335ra56. [Google Scholar] [CrossRef] [PubMed]
- Mis, M.A.; Yang, Y.; Tanaka, B.S.; Gomis-Perez, C.; Liu, S.; Dib-Hajj, F.; Adi, T.; Garcia-Milian, R.; Schulman, B.R.; Dib-Hajj, S.D.; et al. Resilience to Pain: A Peripheral Component Identified Using Induced Pluripotent Stem Cells and Dynamic Clamp. J. Neurosci. 2019, 39, 382–392. [Google Scholar] [CrossRef] [PubMed]
- McDermott, L.A.; Weir, G.; Themistocleous, A.; Segerdahl, A.R.; Blesneac, I.; Baskozos, G.; Clark, A.J.; Millar, V.; Peck, L.; Ebner, D.; et al. Defining the Functional Role of NaV1.7 in Human Nociception. Neuron 2019, 101, 905–919.e8. [Google Scholar] [CrossRef] [PubMed]
- Namer, B.; Schmidt, D.; Eberhardt, E.; Maroni, M.; Dorfmeister, E.; Kleggetveit, I.P.; Kaluza, L.; Meents, J.; Gerlach, A.; Lin, Z.; et al. Pain Relief in a Neuropathy Patient by Lacosamide: Proof of Principle of Clinical Translation from Patient-Specific iPS Cell-Derived Nociceptors. EBioMedicine 2018, 39, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Sances, S.; Bruijn, L.I.; Chandran, S.; Eggan, K.; Ho, R.; Klim, J.R.; Livesey, M.R.; Lowry, E.; Macklis, J.D.; Rushton, D.; et al. Modeling ALS with Motor neurons Derived from Human Induced Pluripotent Stem cells. Nat. Neurosci. 2016, 19, 542–553. [Google Scholar] [CrossRef]
- Imamura, K.; Izumi, Y.; Watanabe, A.; Tsukita, K.; Woltjen, K.; Yamamoto, T.; Hotta, A.; Kondo, T.; Kitaoka, S.; Ohta, A.; et al. The Src/c-Abl Pathway is a Potential Therapeutic Target in Amyotrophic Lateral Sclerosis. Sci. Transl. Med. 2017, 9, eaaf3962. [Google Scholar] [CrossRef]
- Frattini, E.; Ruggieri, M.; Salani, S.; Faravelli, I.; Zanetta, C.; Nizzardo, M.; Simone, C.; Magri, F.; Corti, S. Pluripotent Stem Cell-Based Models of Spinal Muscular Atrophy. Mol. Cell. Neurosci. 2015, 64, 44–50. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.-J.; Qian, W.-J.; Zhang, Q.-J.; Wang, Z.-F.; Lu, Y.-Q.; Dong, E.-L.; He, J.; Wang, N.; Ma, L.-X.; et al. Modeling the Differential Phenotypes of Spinal Muscular Atrophy with High-Yield Generation of Motor Neurons from Human Induced Pluripotent Stem Cells. Oncotarget 2017, 8, 42030–42042. [Google Scholar] [CrossRef]
- Kawada, J.; Kaneda, S.; Kirihara, T.; Maroof, A.; Levi, T.; Eggan, K.; Fujii, T.; Ikeuchi, Y. Generation of a Motor Nerve Organoid with Human Stem Cell-Derived Neurons. Stem Cell Rep. 2017, 9, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, S.; Kurihara, K.; Suzuki, K.; Takanobu, H.; Ohka, S. Microfluidic Devices for the Generation of Centimetre-Long Motor Nerve Organoids Derived from iPSCs. Micro Nano Lett. 2020, 15, 746–750. [Google Scholar] [CrossRef]
- Osaki, T.; Chow, S.Y.A.; Nakanishi, Y.; Hernández, J.; Kawada, J.; Fujii, T.; Ikeuchi, Y. Three-Dimensional Motor Nerve Organoid Generation. J. Vis. Exp. 2020, 163, e61544. [Google Scholar] [CrossRef]
- Akiyama, T.; Suzuki, N.; Ishikawa, M.; Fujimori, K.; Sone, T.; Kawada, J.; Funayama, R.; Fujishima, F.; Mitsuzawa, S.; Ikeda, K.; et al. Aberrant Axon Branching Via Fos-B Dysregulation in FUS-ALS Motor Neurons. EBioMedicine 2019, 45, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Mitsuzawa, S.; Suzuki, N.; Akiyama, T.; Ishikawa, M.; Sone, T.; Kawada, J.; Funayama, R.; Shirota, M.; Mitsuhashi, H.; Morimoto, S.; et al. Reduced PHOX2B Stability Causes Axonal Growth Impairment in Motor Neurons with TARDBP Mutations. Stem Cell Rep. 2021, 16, 1527–1541. [Google Scholar] [CrossRef]
- Nishijima, T.; Okuyama, K.; Shibata, S.; Kimura, H.; Shinozaki, M.; Ouchi, T.; Mabuchi, Y.; Ohno, T.; Nakayama, J.; Hayatsu, M.; et al. Novel Artificial Nerve Transplantation of Human iPSC-Derived Neurite Bundles Enhanced Nerve Regeneration after Peripheral Nerve Injury. Inflamm. Regen. 2024, 44, 6. [Google Scholar] [CrossRef]
- Kárai, L.J.; Russell, J.T.; Iadarola, M.J.; Oláh, Z. Vanilloid Receptor 1 Regulates Multiple Calcium Compartments and Contributes to Ca2+-induced Ca2+ Release in Sensory Neurons. J. Biol. Chem. 2004, 279, 16377–16387. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.-I.; et al. A More Efficient Method to Generate Integration-Free Human iPS Cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef]
- Chambers, S.M.; Qi, Y.; Mica, Y.; Lee, G.; Zhang, X.-J.; Niu, L.; Bilsland, J.; Cao, L.; Stevens, E.; Whiting, P.; et al. Combined Small-Molecule Inhibition Accelerates Developmental Timing and Converts Human Pluripotent Stem Cells into Nociceptors. Nat. Biotechnol. 2012, 30, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.-I.; Muguruma, K.; et al. A ROCK Inhibitor Permits Survival of Dissociated Human Embryonic Stem Cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K. Biomarker Analysis on a Power-free Microfluidic Chip Driven by Degassed Poly(dimethylsiloxane). Anal. Sci. 2021, 37, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Goparaju, S.K.; Kohda, K.; Ibata, K.; Soma, A.; Nakatake, Y.; Akiyama, T.; Wakabayashi, S.; Matsushita, M.; Sakota, M.; Kimura, H.; et al. Rapid Differentiation of Human Pluripotent Stem Cells into Functional Neurons by mRNAs Encoding Transcription Factors. Sci. Rep. 2017, 7, 42367. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS Cell-Derived Dopaminergic Neurons Function in a Primate Parkinson’s Disease Model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, T.; Yamada, S.; Fukushi, S.; Imai, Y.; Kawada, J.; Ikeda, K.; Ohka, S.; Kaneda, S. Formation and Long-Term Culture of hiPSC-Derived Sensory Nerve Organoids Using Microfluidic Devices. Bioengineering 2024, 11, 794. https://doi.org/10.3390/bioengineering11080794
Ogawa T, Yamada S, Fukushi S, Imai Y, Kawada J, Ikeda K, Ohka S, Kaneda S. Formation and Long-Term Culture of hiPSC-Derived Sensory Nerve Organoids Using Microfluidic Devices. Bioengineering. 2024; 11(8):794. https://doi.org/10.3390/bioengineering11080794
Chicago/Turabian StyleOgawa, Takuma, Souichi Yamada, Shuetsu Fukushi, Yuya Imai, Jiro Kawada, Kazutaka Ikeda, Seii Ohka, and Shohei Kaneda. 2024. "Formation and Long-Term Culture of hiPSC-Derived Sensory Nerve Organoids Using Microfluidic Devices" Bioengineering 11, no. 8: 794. https://doi.org/10.3390/bioengineering11080794
APA StyleOgawa, T., Yamada, S., Fukushi, S., Imai, Y., Kawada, J., Ikeda, K., Ohka, S., & Kaneda, S. (2024). Formation and Long-Term Culture of hiPSC-Derived Sensory Nerve Organoids Using Microfluidic Devices. Bioengineering, 11(8), 794. https://doi.org/10.3390/bioengineering11080794






