Optogenetic Brain–Computer Interfaces
Abstract
1. Introduction
- Targeted regulation of specific kinds of cells or specific locations with high spatial resolution [29].
- The bidirectional regulation of neurons, i.e., activation or inhibition of neurons, and diversity of regulation [30].
- Single-neuron activity manipulation and, in combination with diverse modulation modes of excitation light, multi-scale modulation from cells, loops, and brain regions to the whole brain [31].
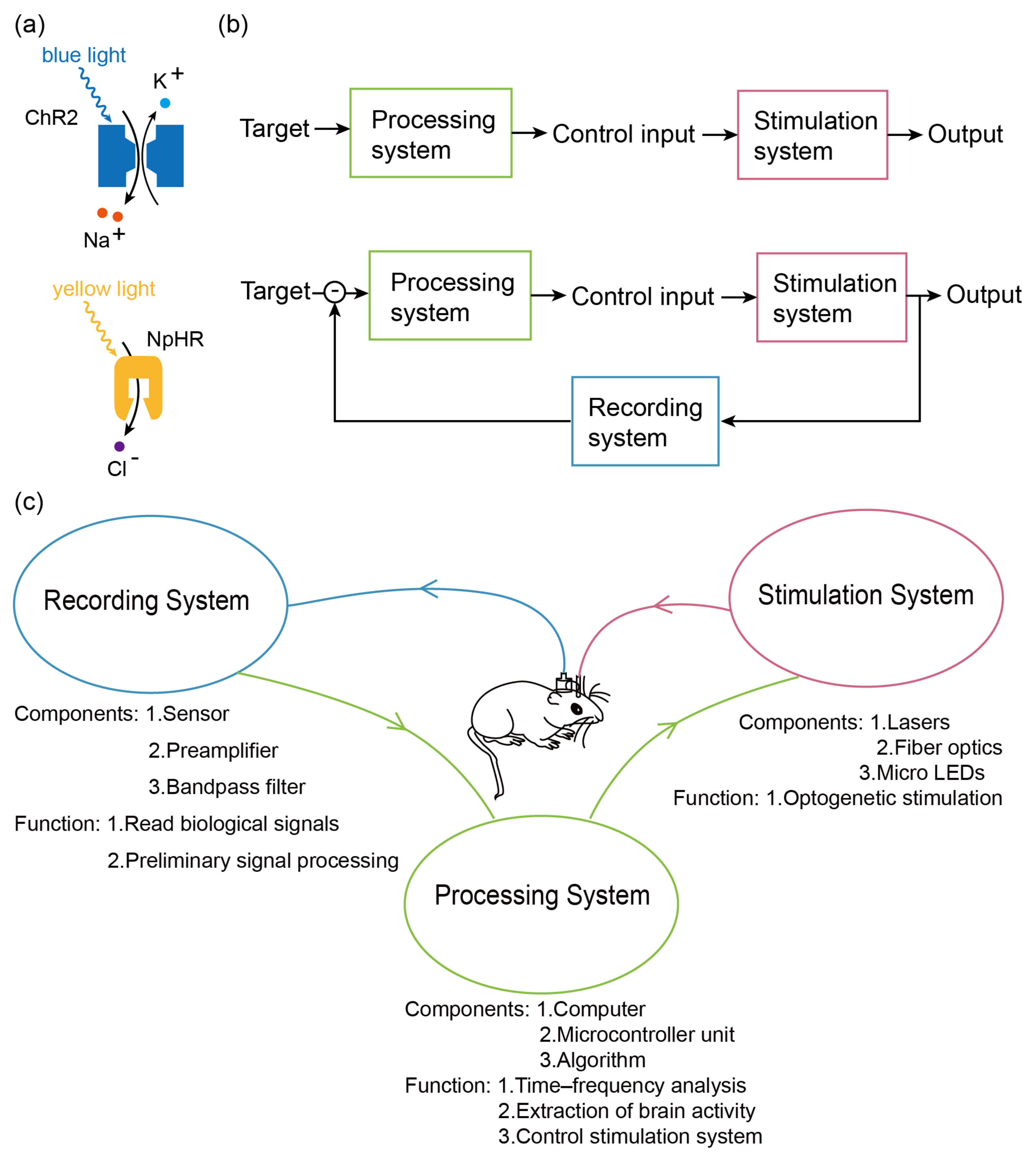
2. Development
2.1. Recording System
2.2. A Processor System
2.3. Stimulus System
3. Applications

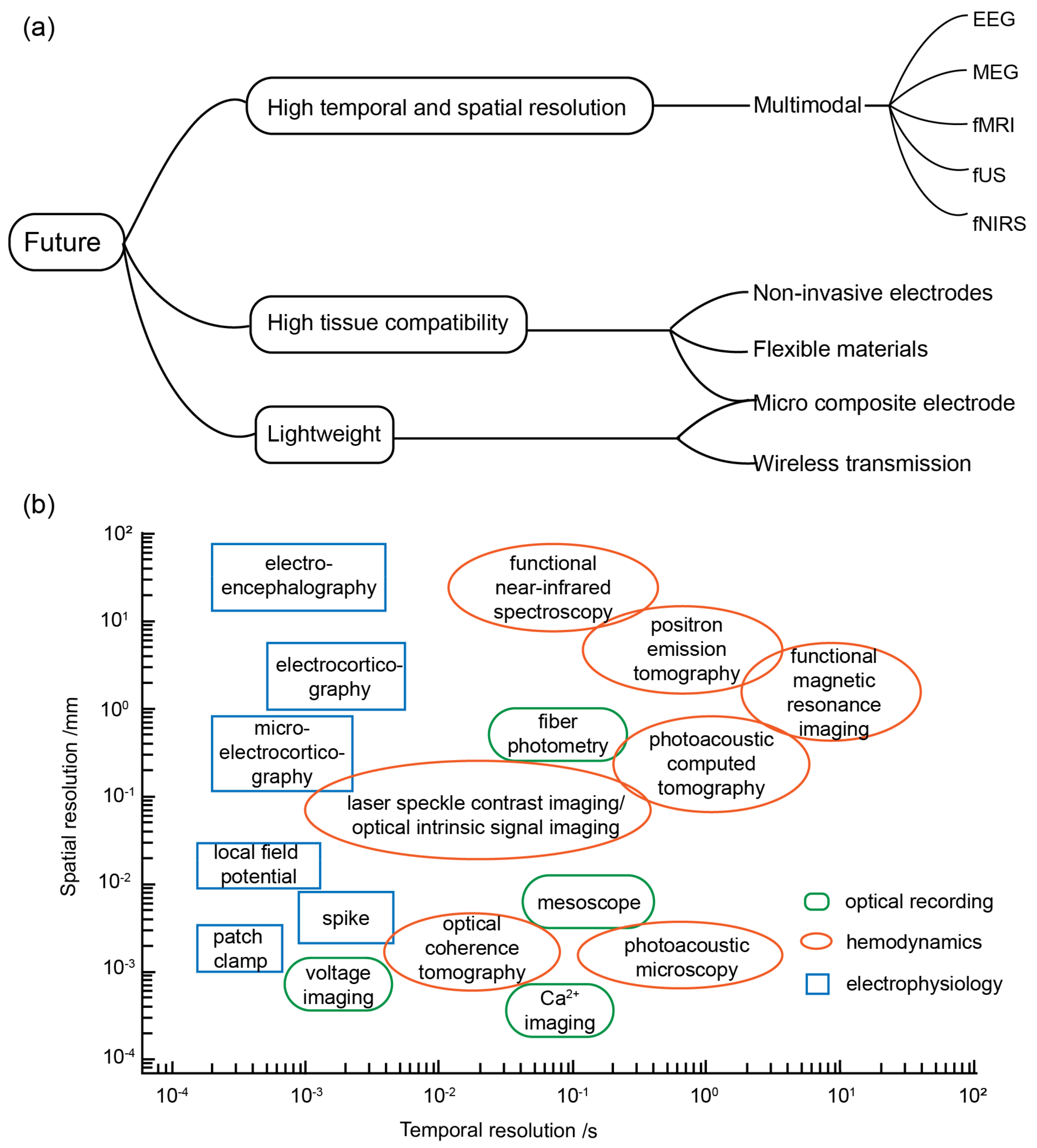
4. Discussion and Prospects
4.1. Software and Hardware
4.2. Application Scenarios
4.3. Multimodal BCI
- (1)
- These techniques have a wide range of adaptations, from simple visual stimulation experiments to detection in intensive care patients, with an adaptation age from premature infants to elderly patients, covering all ages [128].
- (2)
- The two methods complement each other in terms of temporal and spatial resolution [129].
- (3)
- Both the EEG and fNIRS techniques have the advantages of being relatively small and inexpensive devices and can be integrated into portable devices [130].
- (4)
- The fNIRS and EEG techniques are robust to motion artifacts without excessive physical constraints.
- (5)
- Compared to other techniques, non-invasive EEG and fNIRS methods can be performed under conditions close to daily life, providing considerable freedom in experimental design.
- (6)
- The methods are silent, making them more conducive to language research and auditory cognitive experiments.
- (7)
- Integration is relatively simple due to the absence of electro-optic interference [109].
Author Contributions
Funding
Conflicts of Interest
References
- Rao, R.P.; Scherer, R. Brain-computer interfacing. IEEE Signal Process. Mag. 2010, 27, 150–152. [Google Scholar] [CrossRef]
- Vidal, J.J. Toward direct brain-computer communication. Annu. Rev. Biophys. 1973, 2, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Mridha, M.F.; Das, S.C.; Kabir, M.M.; Lima, A.A.; Islam, M.R.; Watanobe, Y. Brain-Computer Interface: Advancement and Challenges. Sensors 2021, 21, 5746. [Google Scholar] [CrossRef] [PubMed]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.S.; Edelman, B.J.; Nesbitt, N.; He, B. Sensorimotor Rhythm BCI with Simultaneous High Definition-Transcranial Direct Current Stimulation Alters Task Performance. Brain Stimul. 2016, 9, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Kosnoff, J.; Yu, K.; Liu, C.; He, B. Transcranial focused ultrasound to V5 enhances human visual motion brain-computer interface by modulating feature-based attention. Nat. Commun. 2024, 15, 4382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.P.; Nie, C.; Jiang, C.T.; Cao, S.H.; Tian, K.X.; Yu, S.; Gu, J.W. Modulating Brain Activity with Invasive Brain-Computer Interface: A Narrative Review. Brain Sci. 2023, 13, 134. [Google Scholar] [CrossRef]
- Lin, B.H.; Ju, M.S.; Lin, C.C.K. Suppression of acute and chronic mesial temporal epilepsy by contralateral sensing and closed-loop optogenetic stimulation with proportional-plus-off control. Biomed. Signal Process. Control 2019, 51, 309–317. [Google Scholar] [CrossRef]
- Bamdad, M.; Zarshenas, H.; Auais, M.A. Application of BCI systems in neurorehabilitation: A scoping review. Disabil. Rehabil. Assist. Technol. 2015, 10, 355–364. [Google Scholar] [CrossRef]
- Herron, J.A.; Thompson, M.C.; Brown, T.; Chizeck, H.J.; Ojemann, J.G.; Ko, A.L. Cortical Brain-Computer Interface for Closed-Loop Deep Brain Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2180–2187. [Google Scholar] [CrossRef]
- Maksimenko, V.A.; van Heukelum, S.; Makarov, V.V.; Kelderhuis, J.; Luttjohann, A.; Koronovskii, A.A.; Hramov, A.E.; van Luijtelaar, G. Absence Seizure Control by a Brain Computer Interface. Sci. Rep. 2017, 7, 2487. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, P.; Hermann, B.; Sitt, J.D.; Naccache, L. Electromagnetic Brain Stimulation in Patients with Disorders of Consciousness. Front. Neurosci. 2019, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, F.; Barsotti, M.; Leonardis, D.; Gabardi, M.; Rosati, G.; Frisoli, A. Haptic Stimulation for Improving Training of a Motor Imagery BCI Developed for a Hand-Exoskeleton in Rehabilitation. IEEE Int. Conf. Rehabil. Robot. 2019, 2019, 1127–1132. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, N.; Wang, Y.; Liu, C.; Hu, S. Transcranial Focused Ultrasound Neuromodulation: A Review of the Excitatory and Inhibitory Effects on Brain Activity in Human and Animals. Front. Hum. Neurosci. 2021, 15, 749162. [Google Scholar] [CrossRef]
- Belkacem, A.N.; Jamil, N.; Khalid, S.; Alnajjar, F. On closed-loop brain stimulation systems for improving the quality of life of patients with neurological disorders. Front. Hum. Neurosci. 2023, 17, 1085173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Bieger, J.; Garcia Molina, G.; Aarts, R.M. A survey of stimulation methods used in SSVEP-based BCIs. Comput. Intell. Neurosci. 2010, 2010, 702357. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.S.; Kouzani, A.Z. Electrophysiology-Based Closed Loop Optogenetic Brain Stimulation Devices: Recent Developments and Future Prospects. IEEE Rev. Biomed. Eng. 2023, 16, 91–108. [Google Scholar] [CrossRef]
- Di Biase, L.; Falato, E.; Di Lazzaro, V. Transcranial Focused Ultrasound (tFUS) and Transcranial Unfocused Ultrasound (tUS) Neuromodulation: From Theoretical Principles to Stimulation Practices. Front. Neurol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Siebner, H.R.; Bergmann, T.O.; Bestmann, S.; Massimini, M.; Johansen-Berg, H.; Mochizuki, H.; Bohning, D.E.; Boorman, E.D.; Groppa, S.; Miniussi, C.; et al. Consensus paper: Combining transcranial stimulation with neuroimaging. Brain Stimul. 2009, 2, 58–80. [Google Scholar] [CrossRef]
- Jo, Y.; Lee, S.M.; Jung, T.; Park, G.; Lee, C.; Im, G.H.; Lee, S.; Park, J.S.; Oh, C.; Kook, G.; et al. General-Purpose Ultrasound Neuromodulation System for Chronic, Closed-Loop Preclinical Studies in Freely Behaving Rodents. Adv. Sci. 2022, 9, e2202345. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Ellis-Davies, G.C.R.; Mourot, A. Optical control of neuronal ion channels and receptors. Nat. Rev. Neurosci. 2019, 20, 514–532. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.P.; Boyden, E.S.; Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 2006, 3, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.L.; Cunha, C.; Zhang, F.; Liu, Q.; Gloss, B.; Deisseroth, K.; Augustine, G.J.; Feng, G.P. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol 2008, 36, 141–154. [Google Scholar] [CrossRef]
- Packer, A.M.; Roska, B.; Hausser, M. Targeting neurons and photons for optogenetics. Nat. Neurosci. 2013, 16, 805–815. [Google Scholar] [CrossRef]
- Welkenhuysen, M.; Hoffman, L.; Luo, Z.; De Proft, A.; Van den Haute, C.; Baekelandt, V.; Debyser, Z.; Gielen, G.; Puers, R.; Braeken, D. An integrated multi-electrode-optrode array for in vitro optogenetics. Sci. Rep. 2016, 6, 20353. [Google Scholar] [CrossRef]
- Ermakova, Y.G.; Lanin, A.A.; Fedotov, I.V.; Roshchin, M.; Kelmanson, I.V.; Kulik, D.; Bogdanova, Y.A.; Shokhina, A.G.; Bilan, D.S.; Staroverov, D.B.; et al. Thermogenetic neurostimulation with single-cell resolution. Nat. Commun. 2017, 8, 15362. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Mackay, M.; Whittaker, R.G. Taking Optogenetics into the Human Brain: Opportunities and Challenges in Clinical Trial Design. Open Access J. Clin. Trials 2020, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, V.; Thompson, K.R.; Zhang, F.; Mogri, M.; Kay, K.; Schneider, M.B.; Deisseroth, K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 2007, 27, 14231–14238. [Google Scholar] [CrossRef]
- Mattis, J.; Tye, K.M.; Ferenczi, E.A.; Ramakrishnan, C.; O’Shea, D.J.; Prakash, R.; Gunaydin, L.A.; Hyun, M.; Fenno, L.E.; Gradinaru, V.; et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 2012, 9, 159–172. [Google Scholar] [CrossRef]
- Grosenick, L.; Marshel, J.H.; Deisseroth, K. Closed-Loop and Activity-Guided Optogenetic Control. Neuron 2015, 86, 106–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Roy, D.S.; Zhu, Y.; Chen, Y.F.; Aida, T.; Hou, Y.Y.; Shen, C.J.; Lea, N.E.; Schroeder, M.E.; Skaggs, K.M.; et al. Targeting thalamic circuits rescues motor and mood deficits in PD mice. Nature 2022, 607, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Chalif, J.I.; Martinez-Silva, M.L.; Pagiazitis, J.G.; Murray, A.J.; Mentis, G.Z. Control of mammalian locomotion by ventral spinocerebellar tract neurons. Cell 2022, 185, 328–344.e26. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Kitamura, T.; Roy, D.S.; Itohara, S.; Tonegawa, S. Ventral CA1 neurons store social memory. Science 2016, 353, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Chen, X.; Gao, S. Interface, interaction, and intelligence in generalized brain-computer interfaces. Trends Cogn. Sci. 2021, 25, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mamun, K.A.; Ahmed, K.; Mostafa, R.; Naik, G.R.; Darvishi, S.; Khandoker, A.H.; Baumert, M. Progress in Brain Computer Interface: Challenges and Opportunities. Front. Syst. Neurosci. 2021, 15, 578875. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; He, J.; Yang, Y.; Zhao, J. Brain-Computer Interfaces in Disorders of Consciousness. Neurosci. Bull. 2023, 39, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Matthews, F.; Pearlmutter, B.A.; Wards, T.E.; Soraghan, C.; Markham, C. Hemodynamics for Brain-Computer Interfaces. IEEE Signal Process. Mag. 2008, 25, 87–94. [Google Scholar] [CrossRef]
- Fukuma, R.; Yanagisawa, T.; Saitoh, Y.; Hosomi, K.; Kishima, H.; Shimizu, T.; Sugata, H.; Yokoi, H.; Hirata, M.; Kamitani, Y.; et al. Corrigendum: Real-Time Control of a Neuroprosthetic Hand by Magnetoencephalographic Signals from Paralysed Patients. Sci. Rep. 2016, 6, 34970. [Google Scholar] [CrossRef]
- Kaas, A.; Goebel, R.; Valente, G.; Sorger, B. Topographic Somatosensory Imagery for Real-Time fMRI Brain-Computer Interfacing. Front. Hum. Neurosci. 2019, 13, 427. [Google Scholar] [CrossRef]
- Khalaf, A.; Sejdic, E.; Akcakaya, M. A novel motor imagery hybrid brain computer interface using EEG and functional transcranial Doppler ultrasound. J. Neurosci. Methods 2019, 313, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liang, Y.; Wang, X.; Lan, H.; Bai, X.; Jin, L.; Guan, B.O. Free-moving-state microscopic imaging of cerebral oxygenation and hemodynamics with a photoacoustic fiberscope. Light Sci. Appl. 2024, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Taheri, B.; Smith, R.L.; Knight, R.T. An active, microfabricated, scalp electrode array for EEG recording. Sens. Actuators A Phys. 1996, 54, 606–611. [Google Scholar] [CrossRef]
- Bertram, E.H.; Williamson, J.M.; Cornett, J.F.; Spradlin, S.; Chen, Z.F. Design and construction of a long-term continuous video-EEG monitoring unit for simultaneous recording of multiple small animals. Brain Res. Protoc. 1997, 2, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Weiergraber, M.; Henry, M.; Hescheler, J.; Smyth, N.; Schneider, T. Electrocorticographic and deep intracerebral EEG recording in mice using a telemetry system. Brain Res. Protoc. 2005, 14, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wais, M.; Sheppy, E.; del Campo, M.; Zhang, L. A glue-based, screw-free method for implantation of intra-cranial electrodes in young mice. J. Neurosci. Methods 2008, 171, 126–131. [Google Scholar] [CrossRef]
- Etholm, L.; Arabadzisz, D.; Lipp, H.P.; Heggelund, P. Seizure logging: A new approach to synchronized cable-free EEG and video recordings of seizure activity in mice. J. Neurosci. Methods 2010, 192, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Mickle, A.D.; Won, S.M.; Noh, K.N.; Yoon, J.; Meacham, K.W.; Xue, Y.G.; McIlvried, L.A.; Copits, B.A.; Samineni, V.K.; Crawford, K.E.; et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 2019, 565, 361–365. [Google Scholar] [CrossRef]
- Luo, J.; Firflionis, D.; Turnbull, M.; Xu, W.; Walsh, D.; Escobedo-Cousin, E.; Soltan, A.; Ramezani, R.; Liu, Y.; Bailey, R. The neural engine: A reprogrammable low power platform for closed-loop optogenetics. IEEE Trans. Biomed. Eng. 2020, 67, 3004–3015. [Google Scholar] [CrossRef]
- Yang, G.Y.; Tang, Y.; Lin, T.; Zhong, T.Y.; Fan, Y.W.; Zhang, Y.; Xing, L.L.; Xue, X.Y.; Zhan, Y. A self-powered closed-loop brain-machine-interface system for real-time detecting and rapidly adjusting blood glucose concentration. Nano Energy 2022, 93, 106817. [Google Scholar] [CrossRef]
- Huber, R.; Deboer, T.; Tobler, I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: Empirical data and simulations. Brain Res. 2000, 857, 8–19. [Google Scholar] [CrossRef]
- Gao, D.R.; Li, M.Z.; Li, J.F.; Liu, Z.X.; Yao, D.Z.; Li, G.L.; Liu, T.J. Effects of various typical electrodes and electrode gels combinations on MRI signal-to-noise ratio and safety issues in EEG-fMRI recording. Biocybern. Biomed. Eng. 2016, 36, 9–18. [Google Scholar] [CrossRef]
- Ollikainen, J.O.; Vauhkonen, M.; Karjalainen, P.A.; Kaipio, J.P. Effects of electrode properties on EEG measurements and a related inverse problem. Med. Eng. Phys. 2000, 22, 535–545. [Google Scholar] [CrossRef]
- Seeck, M.; Koessler, L.; Bast, T.; Leijten, F.; Michel, C.; Baumgartner, C.; He, B.; Beniczky, S. The standardized EEG electrode array of the IFCN. Clin. Neurophysiol. 2017, 128, 2070–2077. [Google Scholar] [CrossRef]
- Charvet, G.; Rousseau, L.; Billoint, O.; Gharbi, S.; Rostaing, J.P.; Joucla, S.; Trevisiol, M.; Bourgerette, A.; Chauvet, P.; Moulin, C.; et al. BioMEA (TM): A versatile high-density 3D microelectrode array system using integrated electronics. Biosens. Bioelectron. 2010, 25, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Harimoto, T.; Ishihara, A.; Takei, K.; Kawashima, T.; Usui, S.; Ishida, M. Electrical interfacing between neurons and electronics via vertically integrated sub-4 μm-diameter silicon probe arrays fabricated by vapor–liquid–solid growth. Biosens. Bioelectron. 2010, 25, 1809–1815. [Google Scholar] [CrossRef]
- He, F.; Lycke, R.; Ganji, M.; Xie, C.; Luan, L. Review Ultraflexible Neural Electrodes for Long-Lasting Intracortical Recording. iScience 2020, 23, 101387. [Google Scholar] [CrossRef]
- Stieglitz, T. Electrode materials for recording and stimulation. In Neuroprosthetics: Theory and Practice; World Scientific: Singapore, 2004; pp. 475–516. [Google Scholar]
- Li, P.H.; Huang, J.J.; Li, M.J.; Li, H.J. Evaluation of flexible multi-claw and multi-channel semi-dry electrodes for evoked electroencephalography recording. Sens. Actuators A Phys 2022, 340, 113547. [Google Scholar] [CrossRef]
- Xia, L.; Fattah, N.; Soltan, A.; Jackson, A.; Chester, G.; Degenaar, P. A low power flash-FPGA based brain implant micro-system of PID control. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 173–176. [Google Scholar]
- Wahnoun, R.; Tillery, S.H.; He, J. Neuron selection and visual training for population vector based cortical control. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 4607–4610. [Google Scholar]
- Simon, D. Kalman filtering with state constraints: A survey of linear and nonlinear algorithms. IET Control Theory Appl. 2010, 4, 1303–1318. [Google Scholar] [CrossRef]
- Souza, U.B.D.; Escola, J.P.L.; Brito, L.D. A survey on Hilbert-Huang transform: Evolution, challenges and solutions. Digit. Signal Process. 2022, 120, 103292. [Google Scholar] [CrossRef]
- Yamabe, M.; Horie, K.; Shiokawa, H.; Funato, H.; Yanagisawa, M.; Kitagawa, H. MC-SleepNet: Large-scale Sleep Stage Scoring in Mice by Deep Neural Networks. Sci. Rep. 2019, 9, 15793. [Google Scholar] [CrossRef]
- Niemz, M.H. Laser-Tissue Interactions; Springer: Berlin/Heidelberg, Germany, 2007; Volume 322. [Google Scholar]
- Abaya, T.V.F.; Blair, S.; Tathireddy, P.; Rieth, L.; Solzbacher, F. A 3D glass optrode array for optical neural stimulation. Biomed. Opt. Express 2012, 3, 3087–3104. [Google Scholar] [CrossRef]
- Zhou, X.J.; Tian, P.F.; Sher, C.W.; Wu, J.; Liu, H.Z.; Liu, R.; Kuo, H.C. Growth, transfer printing and colour conversion techniques towards full-colour micro-LED display. Prog. Quantum Electron. 2020, 71, 100263. [Google Scholar] [CrossRef]
- Ronzitti, E.; Ventalon, C.; Canepari, M.; Forget, B.C.; Papagiakoumou, E.; Emiliani, V. Recent advances in patterned photostimulation for optogenetics. J. Opt. 2017, 19, 113001. [Google Scholar] [CrossRef]
- Ricci, P.; Marchetti, M.; Sorelli, M.; Turrini, L.; Resta, F.; Gavryusev, V.; de Vito, G.; Sancataldo, G.; Vanzi, F.; Silvestri, L.; et al. Power-effective scanning with AODs for 3D optogenetic applications. J. Biophotonics 2022, 15, e202100256. [Google Scholar] [CrossRef]
- Junge, S.; Ricci Signorini, M.E.; Al Masri, M.; Gülink, J.; Brüning, H.; Kasperek, L.; Szepes, M.; Bakar, M.; Gruh, I.; Heisterkamp, A.; et al. A micro-LED array based platform for spatio-temporal optogenetic control of various cardiac models. Sci. Rep. 2023, 13, 19490. [Google Scholar] [CrossRef]
- Guo, Z.V.; Hart, A.C.; Ramanathan, S. Optical interrogation of neural circuits in. Nat. Methods 2009, 6, 891–896. [Google Scholar] [CrossRef]
- Shang, C.F.; Wang, Y.F.; Zhao, M.T.; Fan, Q.X.; Zhao, S.; Qian, Y.; Xu, S.J.; Mu, Y.; Hao, J.; Du, J.L. Real-time analysis of large-scale neuronal imaging enables closed-loop investigation of neural dynamics. Nat. Neurosci. 2024, 27, 1014–1018. [Google Scholar] [CrossRef]
- Curtis, J.E.; Koss, B.A.; Grier, D.G. Dynamic holographic optical tweezers. Opt. Commun. 2002, 207, 169–175. [Google Scholar] [CrossRef]
- Adesnik, H.; Abdeladim, L. Probing neural codes with two-photon holographic optogenetics. Nat. Neurosci. 2021, 24, 1356–1366. [Google Scholar] [CrossRef]
- Studer, V.; Bobin, J.; Chahid, M.; Mousavi, H.S.; Candes, E.; Dahan, M. Compressive fluorescence microscopy for biological and hyperspectral imaging. Proc. Natl. Acad. Sci. USA 2012, 109, E1679–E1687. [Google Scholar] [CrossRef]
- Shi, L.; Fu, X.; Gui, S.; Wan, T.; Zhuo, J.; Lu, J.; Li, P. Global spatiotemporal synchronizing structures of spontaneous neural activities in different cell types. Nat. Commun. 2024, 15, 2884. [Google Scholar] [CrossRef]
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammel, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural Neural Projection Dynamics Underlying Social Behavior. Cell 2014, 157, 1535–1551. [Google Scholar] [CrossRef]
- Tsai, H.C.; Zhang, F.; Adamantidis, A.; Stuber, G.D.; Bonci, A.; de Lecea, L.; Deisseroth, K. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science 2009, 324, 1080–1084. [Google Scholar] [CrossRef]
- Lacagnina, A.F.; Brockway, E.T.; Crovetti, C.R.; Shue, F.; McCarty, M.J.; Sattler, K.P.; Lim, S.C.; Santos, S.L.; Denny, C.A.; Drew, M.R. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci. 2019, 22, 753–761. [Google Scholar] [CrossRef]
- Weber, D.; Richter, V.; Rohwedder, A.; Grossjohann, A.; Thum, A.S. Learning and Memory in Drosophila Larvae. Cold Spring Harb. Protoc. 2023, 2023, 107863. [Google Scholar] [CrossRef]
- Li, B.; Ma, C.Y.; Huang, Y.A.; Ding, X.L.; Silverman, D.; Chen, C.W.; Darmohray, D.; Lu, L.H.; Liu, S.Q.; Montaldo, G.; et al. Circuit mechanism for suppression of frontal cortical ignition during NREM sleep. Cell 2023, 186, 5739–5750.e17. [Google Scholar] [CrossRef]
- Li, Y.-D.; Luo, Y.-J.; Su, W.-K.; Ge, J.; Crowther, A.; Chen, Z.-K.; Wang, L.; Lazarus, M.; Liu, Z.-L.; Qu, W.-M.; et al. Anterior cingulate cortex projections to the dorsal medial striatum underlie insomnia associated with chronic pain. Neuron 2024, 112, 1328–1341.e4. [Google Scholar] [CrossRef]
- Tovote, P.; Fadok, J.P.; Lüthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015, 16, 317–331. [Google Scholar] [CrossRef]
- Jarrin, S.; Finn, D.P. Optogenetics and its application in pain and anxiety research. Neurosci. Biobehav. Rev. 2019, 105, 200–211. [Google Scholar] [CrossRef]
- Li, S.Y.; Feng, X.L.; Bian, H. Optogenetics: Emerging strategies for neuropathic pain treatment. Front. Neurol. 2022, 13, 982223. [Google Scholar] [CrossRef]
- Xu, M.; Chung, S.J.; Zhang, S.Y.; Zhong, P.; Ma, C.Y.; Chang, W.C.; Weissbourd, B.; Sakai, N.; Luo, L.Q.; Nishino, S.J.; et al. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 2015, 18, 1641–1647. [Google Scholar] [CrossRef]
- Prestori, F.; Montagna, I.; D’Angelo, E.; Mapelli, L. The Optogenetic Revolution in Cerebellar Investigations. Int. J. Mol. Sci. 2020, 21, 2494. [Google Scholar] [CrossRef]
- Ramirez, S.; Liu, X.; Lin, P.A.; Suh, J.; Pignatelli, M.; Redondo, R.L.; Ryan, T.J.; Tonegawa, S. Creating a False Memory in the Hippocampus. Science 2013, 341, 387–391. [Google Scholar] [CrossRef]
- Sohal, V.S.; Zhang, F.; Yizhar, O.; Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459, 698–702. [Google Scholar] [CrossRef]
- Siegle, J.H.; Wilson, M.A. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. eLife 2014, 3, e03061. [Google Scholar] [CrossRef]
- Mehmet, B. Optogenetic stimulation of serotonin nuclei retrieve the lost memory in Alzheimer’s disease. J. Cell. Physiol. 2020, 235, 836–847. [Google Scholar] [CrossRef]
- Lu, C.B.; Wu, X.L.; Ma, H.Z.; Wang, Q.C.; Wang, Y.K.; Luo, Y.; Li, C.; Xu, H. Optogenetic Stimulation Enhanced Neuronal Plasticities in Motor Recovery after Ischemic Stroke. Neural Plast. 2019, 2019, 5271573. [Google Scholar] [CrossRef]
- Li, Y.D.; Luo, Y.J.; Xie, L.; Tart, D.S.; Sheehy, R.N.; Zhang, L.B.; Coleman, L.G.; Chen, X.; Song, J. Activation of hypothalamic-enhanced adult-born neurons restores cognitive and affective function in Alzheimer?s disease. Cell Stem Cell 2023, 30, 415–432. [Google Scholar] [CrossRef]
- Gao, Z.; Pang, Z.; Chen, Y.; Lei, G.; Zhu, S.; Li, G.; Shen, Y.; Xu, W. Restoring After Central Nervous System Injuries: Neural Mechanisms and Translational Applications of Motor Recovery. Neurosci. Bull. 2022, 38, 1569–1587. [Google Scholar] [CrossRef]
- Ehmann, N.; Pauls, D. Optogenetics: Illuminating neuronal circuits of memory formation. J. Neurogenet. 2020, 34, 47–54. [Google Scholar] [CrossRef]
- Shirai, F.; Hayashi-Takagi, A. Optogenetics: Applications in psychiatric research. Psychiatry Clin. Neurosci. 2017, 71, 363–372. [Google Scholar] [CrossRef]
- Bentley, J.N.; Chestek, C.; Stacey, W.C.; Patil, P.G. Optogenetics in epilepsy. Neurosurg. Focus 2013, 34, E4. [Google Scholar] [CrossRef]
- Beaudry, H.; Daou, I.; Ribeiro-da-Silva, A.; Séguéla, P. Will optogenetics be used to treat chronic pain patients? Pain Manag. 2017, 7, 269–278. [Google Scholar] [CrossRef]
- Pashaie, R.; Baumgartner, R.; Richner, T.J.; Brodnick, S.K.; Azimipour, M.; Eliceiri, K.W.; Williams, J.C. Closed-Loop Optogenetic Brain Interface. IEEE Trans. Biomed. Eng. 2015, 62, 2327–2337. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Hu, S.L.; Talay, R.; Xiao, Z.D.; Rosenberg, D.; Liu, Y.L.; Sun, G.H.; Li, A.N.; Caravan, B.; Singh, A.; et al. A prototype closed-loop brain-machine interface for the study and treatment of pain. Nat. Biomed. Eng. 2021, 7, 533–545. [Google Scholar] [CrossRef]
- Abbasi, A.; Goueytes, D.; Shulz, D.E.; Ego-Stengel, V.; Estebanez, L. A fast intracortical brain-machine interface with patterned optogenetic feedback. J. Neural Eng. 2018, 15, 046011. [Google Scholar] [CrossRef]
- Lu, L.H.; Wang, R.Y.; Luo, M.M. An optical brain-to-brain interface supports rapid information transmission for precise locomotion control. Sci. China Life Sci. 2020, 63, 875–885. [Google Scholar] [CrossRef]
- Naci, L.; Monti, M.M.; Cruse, D.; Kübler, A.; Sorger, B.; Goebel, R.; Kotchoubey, B.; Owen, A.M. Brain-computer interfaces for communication with nonresponsive patients. Ann. Neurol. 2012, 72, 312–323. [Google Scholar] [CrossRef]
- Hong, G.S.; Lieber, C.M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 2019, 20, 376. [Google Scholar] [CrossRef]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef]
- Jastrzebska-Perfect, P.; Chowdhury, S.; Spyropoulos, G.D.; Zhao, Z.F.; Cea, C.; Gelinas, J.N.; Khodagholy, D. Translational Neuroelectronics. Adv. Funct. Mater. 2020, 30, 1909165. [Google Scholar] [CrossRef]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, E.K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2018, 2, 52. [Google Scholar] [CrossRef]
- Woods, G.A.; Rommelfanger, N.J.; Hong, G.S. Bioinspired Materials for Bioelectronic Neural Interfaces. Matter 2020, 3, 1087–1113. [Google Scholar] [CrossRef]
- Vázquez-Guardado, A.; Yang, Y.; Bandodkar, A.J.; Rogers, J.A. Recent advances in neurotechnologies with broad potential for neuroscience research. Nat. Neurosci. 2020, 23, 1522–1536. [Google Scholar] [CrossRef]
- Putze, F.; Hesslinger, S.; Tse, C.Y.; Huang, Y.Y.; Herff, C.; Guan, C.T.; Schultz, T. Hybrid fNIRS- EEG based classification of auditory and visual perception processes. Front. Neurosci. 2014, 8, 373. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Sirowatka, B.; Weber, A.; Li, W. Opto-μECoG array: A hybrid neural interface with transparent μECoG electrode array and integrated LEDs for optogenetics. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 593–600. [Google Scholar] [CrossRef]
- Lee, J.; Ozden, I.; Song, Y.K.; Nurmikko, A.V. Transparent intracortical microprobe array for simultaneous spatiotemporal optical stimulation and multichannel electrical recording. Nat. Methods 2015, 12, 1157–1162. [Google Scholar] [CrossRef]
- Qiang, Y.; Artoni, P.; Seo, K.J.; Culaclii, S.; Hogan, V.; Zhao, X.Y.; Zhong, Y.D.; Han, X.; Wang, P.M.; Lo, Y.K.; et al. Transparent arrays of bilayer-nanomesh microelectrodes for simultaneous electrophysiology and two-photon imaging in the brain. Sci. Adv. 2018, 4, eaat0626. [Google Scholar] [CrossRef]
- Park, D.W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, D.; Matsuhisa, N.; Nagase, M.; Sekino, M.; Malliaras, G.G.; Yokota, T.; Someya, T. Transparent, conformable, active multielectrode array using organic electrochemical transistors. Proc. Natl. Acad. Sci. USA 2017, 114, 10554–10559. [Google Scholar] [CrossRef]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydin, C.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Avaliani, N.; Sorensen, A.T.; Ledri, M.; Bengzon, J.; Koch, P.; Brustle, O.; Deisseroth, K.; Andersson, M.; Kokaia, M. Optogenetics Reveal Delayed Afferent Synaptogenesis on Grafted Human-Induced Pluripotent Stem Cell-Derived Neural Progenitors. Stem Cells 2014, 32, 3088–3098. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Johnson, H.J.; Chen, J.; Schaffer, D.V. Optogenetic Application to Investigating Cell Behavior and Neurological Disease. Front. Cell. Neurosci. 2022, 16, 811493. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pang, R.; Chen, S.; Chen, H.; Xie, Y.; Chen, D.; Wu, K.; Liang, J.; Yan, K.; Hao, Z. Near-infrared spectroscopy as a promising tool in stroke: Current applications and future perspectives. J. Innov. Opt. Health Sci. 2021, 14, 2130006. [Google Scholar] [CrossRef]
- Zhang, X.R.; Song, M.; Li, J.; Jiang, T.Z. EM-fMRI: A Promising Method for Mapping the Brain Functional Connectome. Neurosci. Bull. 2023, 39, 707–709. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of Functional Magnetic Resonance Imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Griggs, W.S.; Norman, S.L.; Deffieux, T.; Segura, F.; Osmanski, B.F.; Chau, G.; Christopoulos, V.; Liu, C.R.; Tanter, M.; Shapiro, M.G.; et al. Decoding motor plans using a closed-loop ultrasonic brain-machine interface. Nat. Neurosci. 2024, 27, 196–207. [Google Scholar] [CrossRef]
- Fu, Y.F.; Xiong, X.; Jiang, C.H.; Xu, B.L.; Li, Y.C.; Li, H.Y. Imagined Hand Clenching Force and Speed Modulate Brain Activity and Are Classified by NIRS Combined With EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1641–1652. [Google Scholar] [CrossRef]
- Villringer, A.; Chance, B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997, 20, 435–442. [Google Scholar] [CrossRef]
- Atkinson, J.; Campos, D. Improving BCI-based emotion recognition by combining EEG feature selection and kernel classifiers. Expert Syst. Appl. 2016, 47, 35–41. [Google Scholar] [CrossRef]
- Fantini, S.; Frederick, B.; Sassaroli, A. Perspective: Prospects of non-invasive sensing of the human brain with diffuse optical imaging. APL Photonics 2018, 3, 110901. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okumura, Y.; Kita, Y.; Oi, Y.; Shinoda, H.; Inagaki, M. The relationship between the superior frontal cortex and alpha oscillation in a flanker task: Simultaneous recording of electroencephalogram (EEG) and near infrared spectroscopy (NIRS). Neurosci. Res. 2018, 131, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wallois, F.; Mahmoudzadeh, M.; Patil, A.; Grebe, R. Usefulness of simultaneous EEG-NIRS recording in language studies. Brain Lang. 2012, 121, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Jöbsis, F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef]
- Shiga, T.; Tanabe, K.; Nakase, Y.; Shida, T.; Chance, B. Development of a portable tissue oximeter using near infra-red spectroscopy. Med. Biol. Eng. Comput. 1995, 33, 622–626. [Google Scholar] [CrossRef]
- Rivnay, J.; Wang, H.L.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649. [Google Scholar] [CrossRef]
- Collins, F.S.; Tabak, L.A. NIH plans to enhance reproducibility. Nature 2014, 505, 612–613. [Google Scholar] [CrossRef]
| Reference | Parts | Target | Advantages |
|---|---|---|---|
| Alizadeh-Taheri et al. (1996) [43] | Electrode only | Human | Non-invasive; Good electrical performance; No conductive paste. |
| Bertram et al. (1997) [44] | Recording system | Mice | Video signals combined with electrical signals; Suitable for long-term experiments. |
| Weiergräber et al. (2005) [45] | Recording system | Mice | Wireless data transmission; Suitable for long-term experiments. |
| Wu et al. (2008) [46] | Recording system | Mice | Suitable for young mice; High durability and low cost; Suitable for long-term experiments. |
| Etholm et al. (2010) [47] | Recording system | Mice | Wireless data transmission; High sampling rate; Light weight; Reusable device. |
| Mickle et al. (2019) [48] | The whole system | Mice | Fully implantable; High integration capacity; Flexible; Wireless data transmission and charging. |
| Luo et al. (2020) [49] | The whole system | Mice, human | Partially implantable; Reprogrammable; Wireless data transmission; Low transmission delay; Low power consumption. |
| Yang et al. (2022) [50] | The whole system | Mice, human | Non-invasive; High integration capacity; Flexible; Wireless data transmission; Self-powered. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, F.; Yan, F.; Zhong, Y.; Li, J.; Gong, H.; Li, X. Optogenetic Brain–Computer Interfaces. Bioengineering 2024, 11, 821. https://doi.org/10.3390/bioengineering11080821
Tang F, Yan F, Zhong Y, Li J, Gong H, Li X. Optogenetic Brain–Computer Interfaces. Bioengineering. 2024; 11(8):821. https://doi.org/10.3390/bioengineering11080821
Chicago/Turabian StyleTang, Feifang, Feiyang Yan, Yushan Zhong, Jinqian Li, Hui Gong, and Xiangning Li. 2024. "Optogenetic Brain–Computer Interfaces" Bioengineering 11, no. 8: 821. https://doi.org/10.3390/bioengineering11080821
APA StyleTang, F., Yan, F., Zhong, Y., Li, J., Gong, H., & Li, X. (2024). Optogenetic Brain–Computer Interfaces. Bioengineering, 11(8), 821. https://doi.org/10.3390/bioengineering11080821







