Modulating the Release Kinetics of Natural Product Actinomycin from Bacterial Nanocellulose Films and Their Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
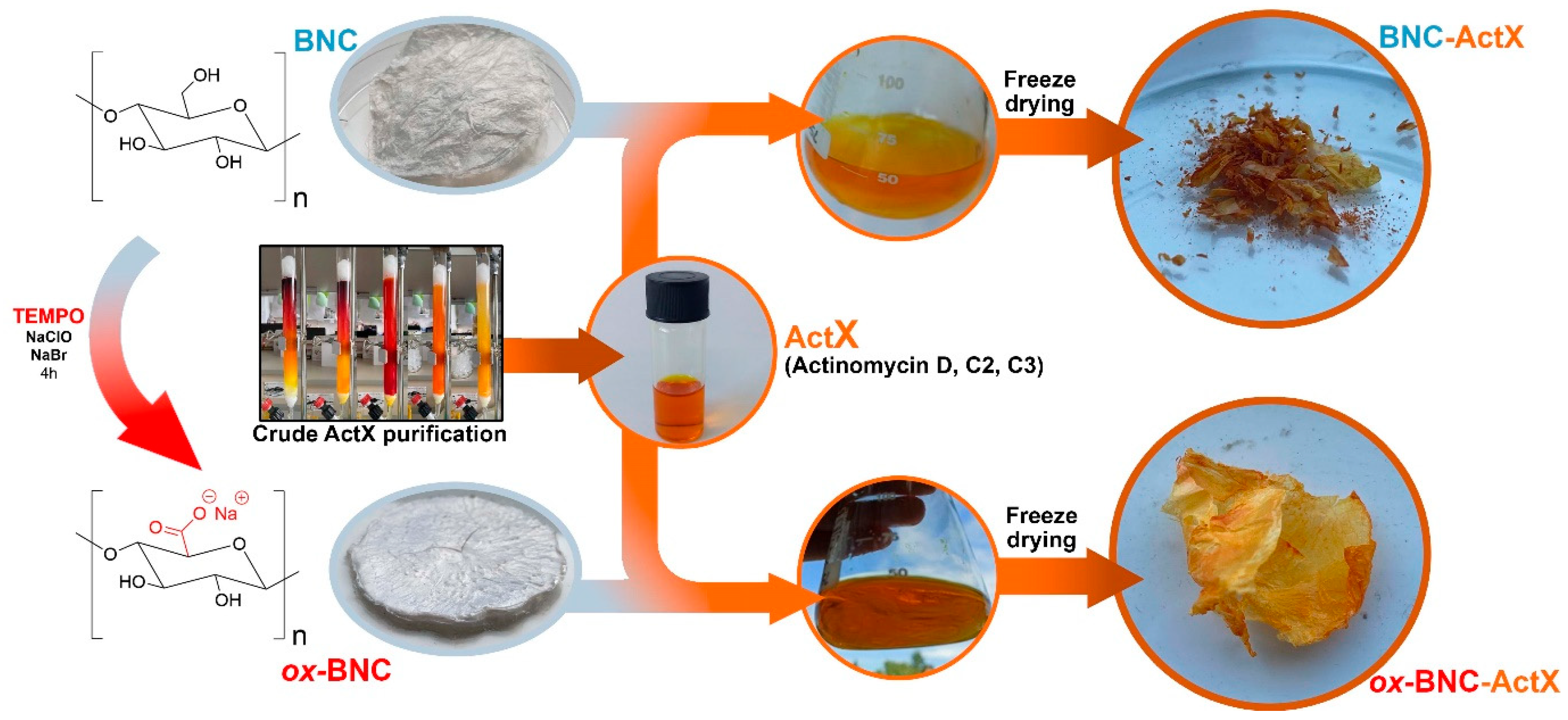
2.2. BNC Production
2.3. TEMPO Oxidation of BNC
2.4. Production and Purification of Actinomycin D Mixture (ActX)
2.5. Immobilization of ActX on BNC (BNC-ActX) and Oxidized BNC (ox-BNC- ActX)
2.6. Efficiency of ActX Immobilization
2.7. Characterization of BNC Samples
2.7.1. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.7.2. Scanning Electron Microscopy (SEM) Analysis
2.7.3. X-ray Diffraction (XRD) Analysis
2.7.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.8. Actinomycin Release
2.9. Antibacterial Activity of ActX—BNC Films
3. Results and Discussion
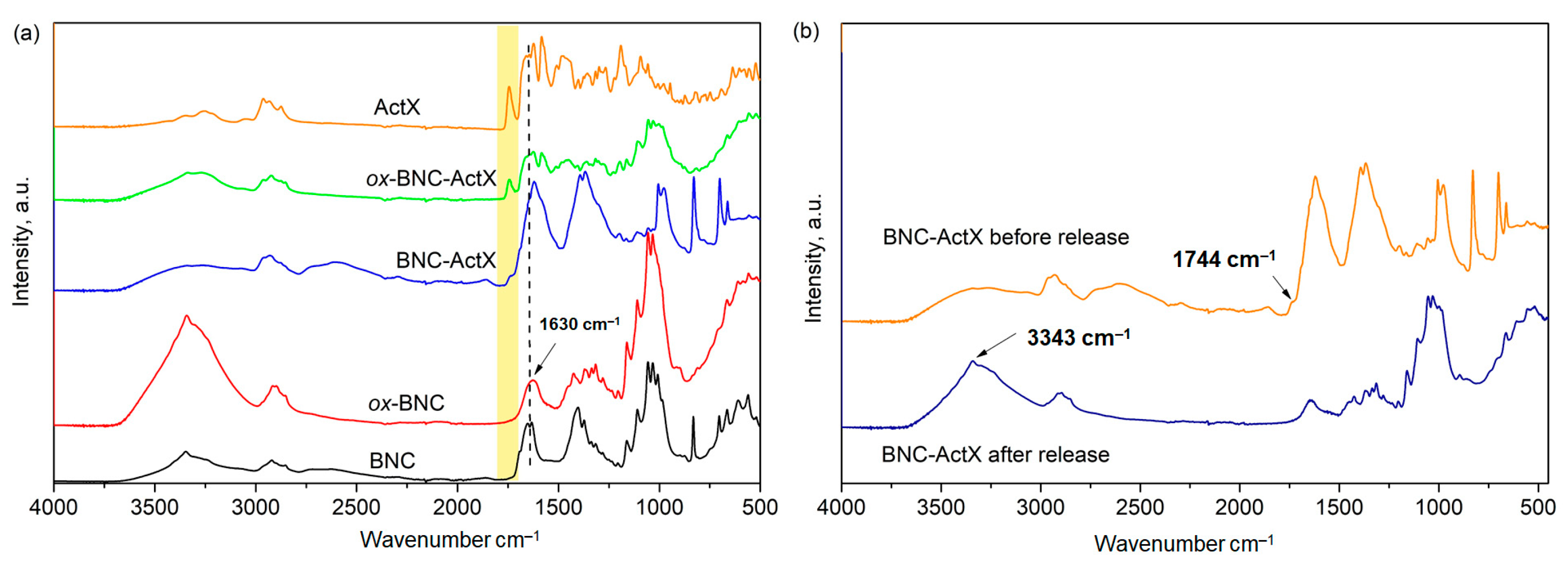
3.1. FTIR Analysis
3.2. SEM Analysis
3.3. XRD and XPS Analysis
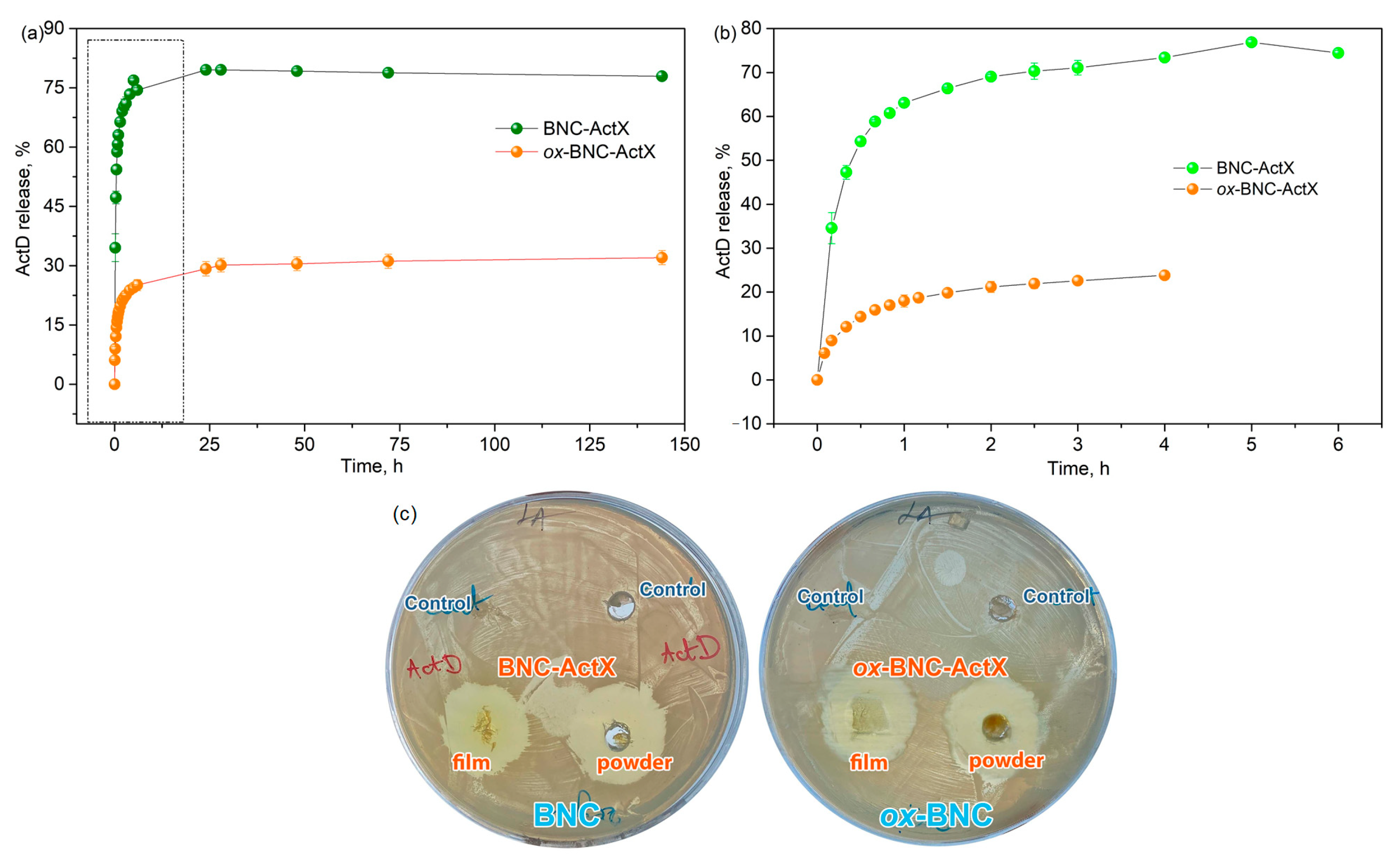
3.4. ActX ActX Release in PBS and Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samyn, P.; Meftahi, A.; Geravand, S.A.; Heravi, M.E.M.; Najarzadeh, H.; Sabery, M.S.K.; Barhoum, A. Opportunities for bacterial nanocellulose in biomedical applications: Review on biosynthesis, modification and challenges. Int. J. Biol. Macromol. 2023, 231, 123316. [Google Scholar] [CrossRef]
- Chandana, A.; Mallick, S.; Dikshit, P.K.; Singh, B.; Sahi, A. Recent Developments in Bacterial Nanocellulose Production and its Biomedical Applications. J. Polym. Environ. 2022, 30, 4040–4067. [Google Scholar] [CrossRef]
- Ullah, M.W.; Alabbosh, K.F.; Fatima, A.; Islam, S.U.; Manan, S.; Ul-Islam, M.; Yang, G. Advanced biotechnological applications of bacterial nanocellulose-based biopolymer nanohybrids: A review. Adv. Ind. Eng. Polym. Res. 2024, 7, 100–121. [Google Scholar] [CrossRef]
- Sourkouni, G.; Jeremić, S.; Kalogirou, C.; Höfft, O.; Nenadovic, M.; Jankovic, V.; Rajasekaran, D.; Pandis, P.; Padamati, R.; Nikodinovic-Runic, J.; et al. Study of PLA pre-treatment, enzymatic and model-compost degradation, and valorization of degradation products to bacterial nanocellulose. World J. Microbiol. Biotechnol. 2023, 39, 161. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Taxeidis, G.; Pereira, E.H.D.S.; Azeem, M.; Pantelic, B.; Jeremic, S.; Ponjavic, M.; Chen, Y.; Mojicevic, M.; Nikodinovic-Runic, J.; et al. Biotechnological model for ubiquitous mixed petroleum- and bio-based plastics degradation and upcycling into bacterial nanocellulose. J. Clean. Prod. 2024, 443, 141025. [Google Scholar] [CrossRef]
- Ponjavic, M.; Filipovic, V.; Topakas, E.; Karnaouri, A.; Zivkovic, J.; Krgovic, N.; Mudric, J.; Savikin, K.; Nikodinovic-Runic, J. Two-Step Upcycling Process of Lignocellulose into Edible Bacterial Nanocellulose with Black Raspberry Extract as an Active Ingredient. Foods 2023, 12, 2995. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; da Silva, M.F.; Almeida, F.L.C.; de Mélo, A.H.F.; Forte, M.B.S.; Martín, C.; Barud, H.d.S.; Baudel, H.M.; Goldbeck, R. A green approach to biomass residue valorization: Bacterial nanocellulose production from agro-industrial waste. Biocatal. Agric. Biotechnol. 2024, 56, 103036. [Google Scholar] [CrossRef]
- Stanisławska, A. Bacterial Nanocellulose as a Microbiological Derived Nanomaterial. Adv. Mater. Sci. 2016, 16, 45–57. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Mota, J.P.; Santos de Almeida, T.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B. Nanosize drug delivery system. Curr. Pharm. Biotechnol. 2013, 14, 1221. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Queirós, E.C.; Pinheiro, S.P.; Pereira, J.E.; Prada, J.; Pires, I.; Dourado, F.; Parpot, P.; Gama, M. Hemostatic Dressings Made of Oxidized Bacterial Nanocellulose Membranes. Polysaccharides 2021, 2, 80–99. [Google Scholar] [CrossRef]
- Cañas-Gutiérrez, A.; Martinez-Correa, E.; Suárez-Avendaño, D.; Arboleda-Toro, D.; Castro-Herazo, C. Influence of bacterial nanocellulose surface modification on calcium phosphates precipitation for bone tissue engineering. Cellulose 2020, 27, 10747–10763. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Alves, A.A.; Silva, W.E.; Belian, M.F.; Lins, L.S.G.; Galembeck, A. Bacterial cellulose membranes for environmental water remediation and industrial wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 3997–4008. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Z.; Li, A.; Chen, N.; Rao, J.; Zeng, Q. Nanocellulose Composite Films in Food Packaging Materials: A Review. Polymers 2024, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Ponjavic, M.; Stevanovic, S.; Nikodinovic-Runic, J.; Jeremic, S.; Cosovic, V.R.; Maksimovic, V. Bacterial nanocellulose as green support of platinum nanoparticles for effective methanol oxidation. Int. J. Biol. Macromol. 2022, 223, 1474–1484. [Google Scholar] [CrossRef]
- Pötzinger, Y.; Kralisch, D.; Fischer, D. Bacterial nanocellulose: The future of controlled drug delivery? Ther. Deliv. 2017, 8, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Shahriari-Khalaji, M.; Zarkesh, M.; Nozhat, Z. Application of Bacterial Nanocellulose in Cancer Drug Delivery: A Review. Curr. Pharm. Des. 2021, 27, 3656–3665. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G. Actinomycin D: A new opening for an old drug. Neuro-Oncol. 2020, 22, 1235–1236. [Google Scholar] [CrossRef]
- Hadi, L.M.; Yaghini, E.; MacRobert, A.J.; Loizidou, M. Synergy between Photodynamic Therapy and Dactinomycin Chemotherapy in 2D and 3D Ovarian Cancer Cell Cultures. Int. J. Mol. Sci. 2020, 21, 3203. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.F.; Wang, Y.S.; Li, C.; Wei, G.J.; Chen, R.; Dong, D.M.; Yao, M. Actinomycin D inhibits cell proliferations and promotes apoptosis in osteosarcoma cells. Int. J. Clin. Exp. Med. 2015, 8, 1904–1911. [Google Scholar]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.P.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 10666–10671. [Google Scholar] [CrossRef] [PubMed]
- Keller, U.; Lang, M.; Crnovcic, I.; Pfennig, F.; Schauwecker, F. The Actinomycin Biosynthetic Gene Cluster of Streptomyces chrysomallus: A Genetic Hall of Mirrors for Synthesis of a Molecule with Mirror Symmetry. J. Bacteriol. 2010, 192, 2583–2595. [Google Scholar] [CrossRef]
- Koba, M.; Konopa, J. Actinomycin D and its mechanisms of action. Postep. Hig. Med. Dosw. 2005, 59, 290–298. [Google Scholar]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs-The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Tan, T.H.; Lee, H.V.; Yehya Dabdawb, W.A.; Hamid, S.B.B.O.A.A. Chapter 5—A review of nanocellulose in the drug-delivery system. In Materials for Biomedical Engineering; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Berlin/Heidelberg, Germany, 2019; pp. 131–164. [Google Scholar] [CrossRef]
- Solomevich, S.O.; Dmitruk, E.I.; Bychkovsky, P.M.; Nebytov, A.E.; Yurkshtovich, T.L.; Golub, N.V. Fabrication of oxidized bacterial cellulose by nitrogen dioxide in chloroform/cyclohexane as a highly loaded drug carrier for sustained release of cisplatin. Carbohydr. Polym. 2020, 248, 116745. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.-C. Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.A.; dos Santos, G.R.; Soeiro, V.S.; dos Santos, J.R.; Rebelo, M.d.A.; Chaud, M.V.; Gerenutti, M.; Grotto, D.; Pandit, R.; Rai, M.; et al. Bacterial nanocellulose membranes combined with nisin: A strategy to prevent microbial growth. Cellulose 2018, 25, 6681–6689. [Google Scholar] [CrossRef]
- Jančič, U.; Trcek, J.; Verestiuc, L.; Vukomanovic, M.; Gorgieva, S. Bacterial nanocellulose loaded with bromelain and nisin as a promising bioactive material for wound debridement. Int. J. Biol. Macromol. 2024, 266, 131329. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Jun, S.H.; Park, S.G.; Kang, N.G. One-Pot Method of Synthesizing TEMPO-Oxidized Bacterial Cellulose Nanofibers Using Immobilized TEMPO for Skincare Applications. Polymers 2019, 11, 1044. [Google Scholar] [CrossRef]
- Usala, E.; Espinosa, E.; El Arfaoui, W.; Morcillo-Martín, R.; Ferrari, B.; González, Z. Antibacterial Aerogels-Based Membranes by Customized Colloidal Functionalization of TEMPO-Oxidized Cellulose Nanofibers Incorporating CuO. Bioengineering 2023, 10, 1312. [Google Scholar] [CrossRef]
- Mujtaba, M.; Negi, A.; King, A.W.T.; Zare, M.; Kuncova-Kallio, J. Surface modifications of nanocellulose for drug delivery applications; a critical review. Curr. Opin. Biomed. Eng. 2023, 28, 100475. [Google Scholar] [CrossRef]
- Jeremic, S.; Djokic, L.; Ajdačić, V.; Božinović, N.; Pavlovic, V.; Manojlović, D.D.; Babu, R.; Senthamaraikannan, R.; Rojas, O.; Opsenica, I.; et al. Production of bacterial nanocellulose (BNC) and its application as a solid support in transition metal catalysed cross-coupling reactions. Int. J. Biol. Macromol. 2019, 129, 351–360. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Characterization of carboxymethyl cellulose-based nanocomposite films reinforced with oxidized nanocellulose isolated using ammonium persulfate method. Carbohydr. Polym. 2017, 174, 484–492. [Google Scholar] [CrossRef]
- ISO 15472:2001; Surface Chemical Analysis—X-ray Photoelectron Spectrometers—Calibration of Energy Scales. International Organization for Standardization: Geneva, Switzerland, 2001.
- Mitrović, A.; Milovanović, J.; Gurgul, J.; Žekić, A.; Nikodinović-Runić, J.; Maslak, V. Enzymatic functionalization of liquid phase exfoliated graphene using horseradish peroxidase and laccase. Enzym. Microb. Technol. 2023, 170, 110293. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Avendaño, D.; Martínez-Correa, E.; Cañas-Gutierrez, A.; Castro-Riascos, M.; Zuluaga-Gallego, R.; Gañán-Rojo, P.; Peresin, M.; Pereira, M.; Castro-Herazo, C. Comparative Study on the Efficiency of Mercury Removal From Wastewater Using Bacterial Cellulose Membranes and Their Oxidized Analogue. Front. Bioeng. Biotechnol. 2022, 10, 815892. [Google Scholar] [CrossRef] [PubMed]
- Pawcenis, D.; Twardowska, E.; Leśniak, M.; Jędrzejczyk, R.; Sitarz, M.; Profic-Paczkowska, J. TEMPO-oxidized cellulose for in situ synthesis of Pt nanoparticles. Study of catalytic and antimicrobial properties. Int. J. Biol. Macromol. 2022, 213, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.E.; Andaya, C.; McClay, K. Evaluation of ATR-FTIR for analysis of bacterial cellulose impurities. J. Microbiol. Methods 2018, 144, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Leung, A.C.W.; Liu, Y.; Majid, E.; Hrapovic, S.; Male, K.B.; Luong, J.H.T. Green Strategy Guided by Raman Spectroscopy for the Synthesis of Ammonium Carboxylated Nanocrystalline Cellulose and the Recovery of Byproducts. ACS Sustain. Chem. Eng. 2013, 1, 278–283. [Google Scholar] [CrossRef]
- Sampaio, E.; Pereira, A.; Barros, M.; Barroso, M.; Lima, H.; Borges, M.; Feitosa, J.; Azeredo, H.; Rosa, M. TEMPO oxidation and high-speed blending as a combined approach to disassemble bacterial cellulose. Cellulose 2019, 26, 2291–2302. [Google Scholar] [CrossRef]
- Chen, W.; Ye, K.; Zhu, X.; Zhang, H.; Si, R.; Chen, J.; Chen, Z.; Song, K.; Yu, Z.; Han, B. Actinomycin X2, an Antimicrobial Depsipeptide from Marine-Derived Streptomyces cyaneofuscatus Applied as a Good Natural Dye for Silk Fabric. Mar. Drugs 2022, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Zulu, B.; Oyewo, O.A.; Sithole, B.; Leswifi, T.Y.; Onyango, M.S. Functionalized Sawdust-Derived Cellulose Nanocrystalline Adsorbent for Efficient Removal of Vanadium From Aqueous Solution. Front. Environ. Sci. 2020, 8, 56. [Google Scholar] [CrossRef]
- Zhou, S.; Peng, H.; Zhao, A.; Zhang, R.; Li, T.; Yang, X.; Lin, D. Synthesis of bacterial cellulose nanofibers/Ag nanoparticles: Structure, characterization and antibacterial activity. Int. J. Biol. Macromol. 2024, 259, 129392. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sadaf, A.; Dubey, S.; Singh, R.P.; Khare, S.K. Production and characterization of Komagataeibacter xylinus SGP8 nanocellulose and its calcite based composite for removal of Cd ions. Environ. Sci. Pollut. Res. 2021, 28, 46423–46430. [Google Scholar] [CrossRef]
- Gullo, M.; Sola, A.; Zanichelli, G.; Montorsi, M.; Messori, M.; Giudici, P. Increased production of bacterial cellulose as starting point for scaled-up applications. Appl. Microbiol. Biotechnol. 2017, 101, 8115–8127. [Google Scholar] [CrossRef] [PubMed]
- Rusdi, R.A.A.; Halim, N.A.; Norizan, M.N.; Abidin, Z.H.Z.; Abdullah, N.; Che Ros, F.; Ahmad, N.; Azmi, A.F.M. Pre-treatment effect on the structure of bacterial cellulose from Nata de Coco (Acetobacter xylinum). Polimery 2022, 67, 110–118. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Chatelier, R.C.; Griesser, H.J. Characterization of the Ageing of Plasma-deposited Polymer Films: Global Analysis of X-ray Photoelectron Spectroscopy Data. Surf. Interface Anal. 1996, 24, 271–281. [Google Scholar] [CrossRef]
- Maurya, M.R.; Chauhan, A.; Avecilla, F. Synthesis, Characterization and Biomimetic Activity of Heterogenized Dioxidomolybdenum(VI) and Analogous Homogeneous Complexes. ChemistrySelect 2022, 7, e202202327. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Kutty, R.V.; Tay, C.Y.; Yuan, X.; Xie, J.; Leong, D.T. Novel theranostic DNA nanoscaffolds for the simultaneous detection and killing of Escherichia coli and Staphylococcus aureus. ACS Appl. Mater. Interfaces 2014, 6, 21822–21831. [Google Scholar] [CrossRef]
- Cano, A.; Rodríguez-Hernández, J.; Shchukarev, A.; Reguera, E. Intercalation of pyrazine in layered copper nitroprusside: Synthesis, crystal structure and XPS study. J. Solid State Chem. 2019, 273, 1–10. [Google Scholar] [CrossRef]
- Michl, T.D.; Barz, J.; Giles, C.; Haupt, M.; Henze, J.H.; Mayer, J.; Futrega, K.; Doran, M.R.; Oehr, C.; Vasilev, K.; et al. Plasma Polymerization of TEMPO Yields Coatings Containing Stable Nitroxide Radicals for Controlling Interactions with Prokaryotic and Eukaryotic Cells. ACS Appl. Nano Mater. 2018, 1, 6587–6595. [Google Scholar] [CrossRef]
- Gonçalves, I.S.; Lima, L.R.; Berretta, A.A.; Amorim, N.A.; Pratavieira, S.; Corrêa, T.Q.; Nogueira, F.A.R.; Barud, H.S. Antimicrobial Formulation of a Bacterial Nanocellulose/Propolis-Containing Photosensitizer for Biomedical Applications. Polymers 2023, 15, 987. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-Based Composite Materials Used in Drug Delivery Systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Liu, J.; Huang, L.; Zhang, X.; Deng, Y.; Li, F.; Liu, Z.; Huang, R. Molecular Details of Actinomycin D-Treated MRSA Revealed via High-Dimensional Data. Mar. Drugs 2022, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, F.; Javeed, A.; Wang, M.; Si, R.; Li, J.; Han, B. Preparation and Characterization of a Silk Fibroin/Polyurethane Fiber Blend Membrane Containing Actinomycin X2 with Excellent Mechanical Properties and Enhanced Antibacterial Activities. Chem. Biodivers. 2023, 20, e202300445. [Google Scholar] [CrossRef]

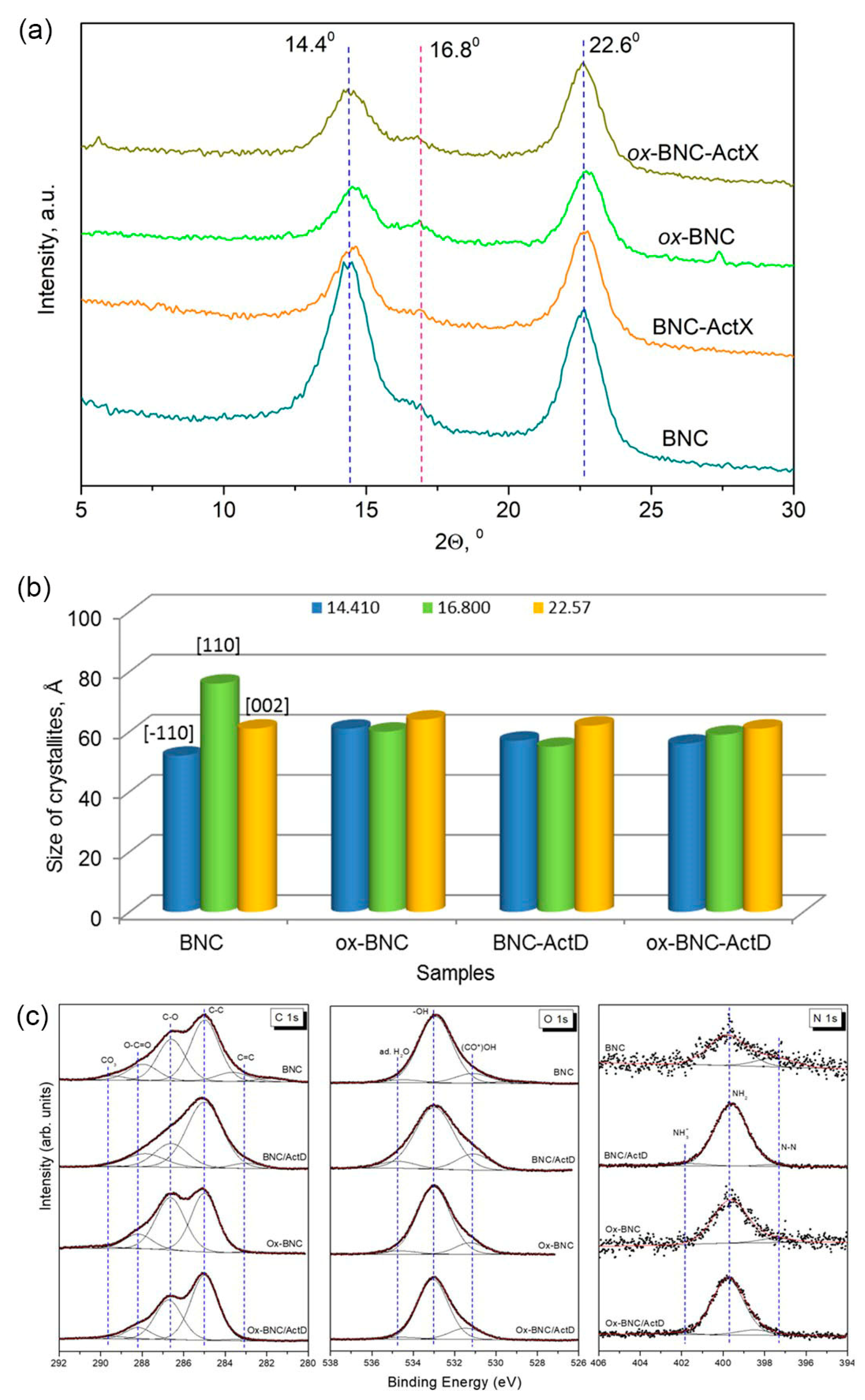
| Sample | 2ϴ° | FWHM | XC002, % | ||||
|---|---|---|---|---|---|---|---|
| (–110) | (110) | (002) | (−110) | (110) | (002) | ||
| BNC | 14.41 | 16.80 | 22.57 | 1.60 | 1.11 | 1.39 | 70.48 |
| ox-BNC | 14.57 | 16.84 | 22.75 | 1.37 | 1.40 | 1.32 | 65.79 |
| BNC-ActX | 14.47 | 16.88 | 22.67 | 1.46 | 1.51 | 1.37 | 69.40 |
| ox-BNC-ActX | 14.41 | 16.76 | 22.62 | 1.50 | 1.42 | 1.38 | 66.17 |
| Sample | τ50%, min | τ60%, min | τ70%, min | c24h, % | ||
|---|---|---|---|---|---|---|
| BNC-ActX | 15 | 20 | 40 | 79.5 | ||
| ox-BNC-ActX | 55 | 80 | 180 | 29.2 | ||
| Sample | Peppas model | Higuchi model | Mechanism | |||
| k | n | R | k | R | ||
| BNC-ActX | 16.897 | 0.2711 | 0.9870 | 14.049 | 0.8466 | Fickian diffusion |
| ox-BNC-ActX | 17.495 | 0.3457 | 0.9944 | 17.395 | 0.9618 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimowska, K.; Filipovic, V.; Nikodinovic-Runic, J.; Simic, J.; Ilic-Tomic, T.; Zimowska, M.; Gurgul, J.; Ponjavic, M. Modulating the Release Kinetics of Natural Product Actinomycin from Bacterial Nanocellulose Films and Their Antimicrobial Activity. Bioengineering 2024, 11, 847. https://doi.org/10.3390/bioengineering11080847
Zimowska K, Filipovic V, Nikodinovic-Runic J, Simic J, Ilic-Tomic T, Zimowska M, Gurgul J, Ponjavic M. Modulating the Release Kinetics of Natural Product Actinomycin from Bacterial Nanocellulose Films and Their Antimicrobial Activity. Bioengineering. 2024; 11(8):847. https://doi.org/10.3390/bioengineering11080847
Chicago/Turabian StyleZimowska, Katarzyna, Vuk Filipovic, Jasmina Nikodinovic-Runic, Jelena Simic, Tatjana Ilic-Tomic, Malgorzata Zimowska, Jacek Gurgul, and Marijana Ponjavic. 2024. "Modulating the Release Kinetics of Natural Product Actinomycin from Bacterial Nanocellulose Films and Their Antimicrobial Activity" Bioengineering 11, no. 8: 847. https://doi.org/10.3390/bioengineering11080847
APA StyleZimowska, K., Filipovic, V., Nikodinovic-Runic, J., Simic, J., Ilic-Tomic, T., Zimowska, M., Gurgul, J., & Ponjavic, M. (2024). Modulating the Release Kinetics of Natural Product Actinomycin from Bacterial Nanocellulose Films and Their Antimicrobial Activity. Bioengineering, 11(8), 847. https://doi.org/10.3390/bioengineering11080847










