Orbital Adipose Tissue: The Optimal Control for Back-Table Fluorescence Imaging of Orbital Tumors
Abstract
1. Introduction
2. Methods and Materials
2.1. Ethics
2.2. Back-Table Fluorescence Image Analysis
2.3. Histopathology
3. Results
3.1. Back-Table Fluorescence Imaging Characteristics of Control Tissues
3.2. Effect of Basic Clinical Characteristics of Patients on Fluorescence Imaging Results of Orbital Adipose
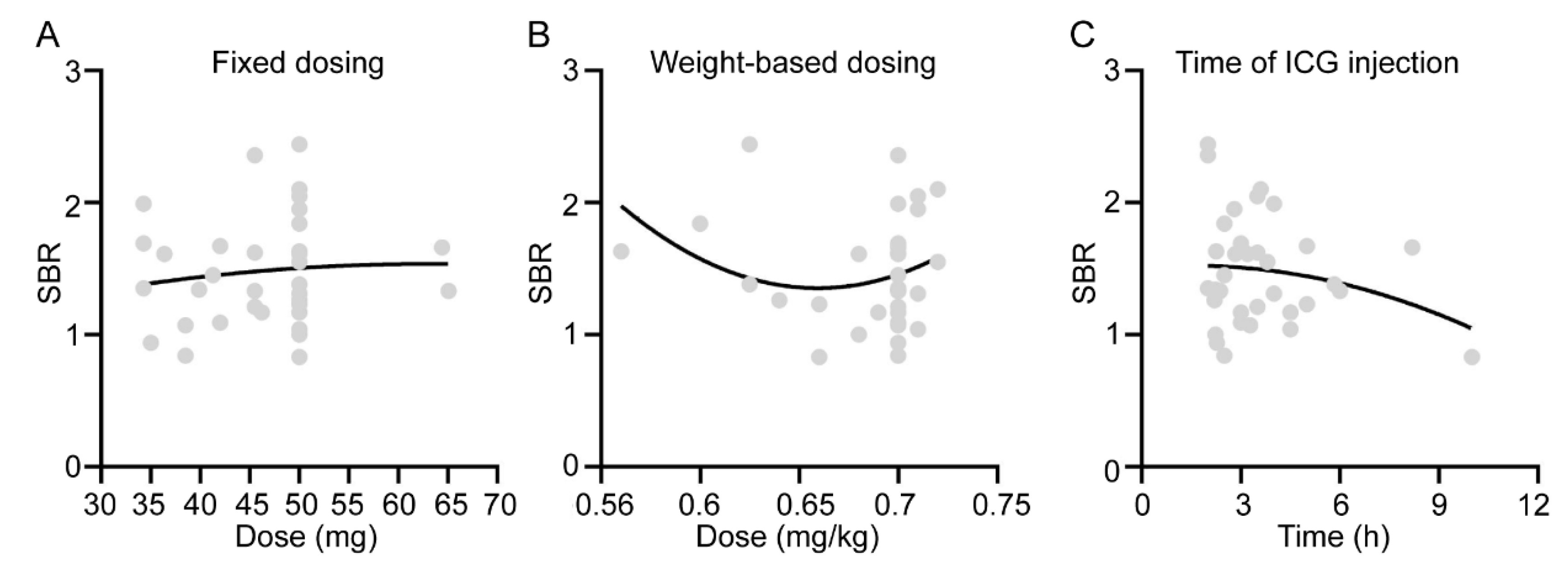
3.3. Effect of ICG Administration Method on Fluorescence Imaging Results of Orbital Adipose
3.4. Effect of Adipose Tissue Size on Its SBR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinkman, D.; Callanan, D.; O’Shea, R.; Jawad, H.; Feeley, L.; Sheahan, P. Impact of 3 mm margin on risk of recurrence and survival in oral cancer. Oral Oncol. 2020, 110, 104883. [Google Scholar] [CrossRef] [PubMed]
- John, A.; John, H.; Catterwell, R.; Selth, L.A.; Callaghan, M.O. Primary gleason grade and gleason grade group at positive surgical margins: A systematic review and meta-analysis. BJU Int. 2021, 127 (Suppl. S1), 13–22. [Google Scholar] [CrossRef] [PubMed]
- Orosco, R.K.; Tapia, V.J.; Califano, J.A.; Clary, B.; Cohen, E.E.W.; Kane, C.; Lippman, S.M.; Messer, K.; Molinolo, A.; Murphy, J.D.; et al. Positive surgical margins in the 10 most common solid cancers. Sci. Rep. 2018, 8, 5686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, Y.; Shi, X.; Wang, K.; Qu, Q.; Liang, Q.; Ma, X.; He, K.; Chi, C.; Tang, J.; et al. NIR-II light in clinical oncology: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2024, 21, 449–467. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Azari, F.; Zhang, K.; Kennedy, G.T.; Chang, A.; Nadeem, B.; Delikatny, E.J.; Singhal, S. Precision surgery guided by intraoperative molecular imaging. J. Nucl. Med. 2022, 63, 1620–1627. [Google Scholar]
- Lee, J.Y.K.; Cho, S.S.; Stummer, W.; Tanyi, J.L.; Vahrmeijer, A.L.; Rosenthal, E.; Smith, B.; Henderson, E.; Roberts, D.W.; Lee, A.; et al. Review of clinical trials in intraoperative molecular imaging during cancer surgery. J. Biomed. Opt. 2019, 24, 1–8. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; De Ravin, E.; Teng, C.W.; Li, C.; Abdullah, K.G.; Buch, L.; Hussain, J.; Ahmed, F.; Dorsey, J.; et al. Near-Infrared imaging with second-window indocyanine green in newly diagnosed high-grade gliomas predicts gadolinium enhancement on postoperative magnetic resonance imaging. Mol. Imaging Biol. 2020, 22, 1427–1437. [Google Scholar] [CrossRef]
- de Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-table fluorescence-guided imaging for circumferential resection margin evaluation using bevacizumab-800CW in patients with locally advanced rectal cancer. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef]
- Li, C.; Mi, J.; Wang, Y.; Zhang, Z.; Guo, X.; Zhou, J.; Hu, Z.; Tian, J. New and effective EGFR-targeted fluorescence imaging technology for intraoperative rapid determination of lung cancer in freshly isolated tissue. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 494–507. [Google Scholar] [CrossRef]
- van Keulen, S.; Nishio, N.; Birkeland, A.; Fakurnejad, S.; Martin, B.; Forouzanfar, T.; Cunanan, K.; Colevas, A.D.; S van den Berg, N.; Rosenthal, E. The sentinel margin: Intraoperative ex vivo specimen mapping using relative fluorescence intensity. Clin. Cancer Res. 2019, 25, 4656–4662. [Google Scholar] [CrossRef] [PubMed]
- van Keulen, S.; Nishio, N.; Fakurnejad, S.; Birkeland, A.; Martin, B.A.; Lu, G.; Zhou, Q.; Chirita, S.U.; Forouzanfar, T.; Colevas, A.D.; et al. The clinical application of fluorescence-guided surgery in head and neck cancer. J. Nucl. Med. 2019, 60, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yung, B.C.; Chandra, S.; Niu, G.; Antaris, A.L.; Chen, X. Near-Infrared-II (NIR-II) bioimaging via off-peak NIR-I fluorescence emission. Theranostics 2018, 8, 4141–4151. [Google Scholar] [CrossRef]
- Shi, X.; Xu, P.; Cao, C.; Cheng, Z.; Tian, J.; Hu, Z. PET/NIR-II fluorescence imaging and image-guided surgery of glioblastoma using a folate receptor α-targeted dual-modal nanoprobe. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4325–4337. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; van Driel, P.B.A.A.; Baatenburg de Jong, R.J.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Löwik, C.W.G.M.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021, 22, e186–e195. [Google Scholar] [CrossRef]
- van Keulen, S.; van den Berg, N.S.; Nishio, N.; Birkeland, A.; Zhou, Q.; Lu, G.; Wang, H.W.; Middendorf, L.; Forouzanfar, T.; Martin, B.A.; et al. Rapid, non-invasive fluorescence margin assessment: Optical specimen mapping in oral squamous cell carcinoma. Oral Oncol. 2019, 88, 58–65. [Google Scholar] [CrossRef]
- Nishio, N.; van den Berg, N.S.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Teraphongphom, N.; Chirita, S.U.; Oberhelman, N.J.; Lu, G.; Horton, C.E.; et al. Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat. Commun. 2019, 10, 5044. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, D.; Wang, Z.; Zhang, X.; Zhang, Q.; Wang, Y.; Newton, A.D.; Singhal, S.; Cai, H.; Wang, Y.; et al. Kinetics of indocyanine green: Optimizing tumor to normal tissue fluorescence in image-guided oral cancer surgery applications. Head Neck 2019, 41, 1032–1038. [Google Scholar] [CrossRef]
- Cho, S.S.; Sheikh, S.; Teng, C.W.; Georges, J.; Yang, A.I.; De Ravin, E.; Buch, L.; Li, C.; Singh, Y.; Appelt, D.; et al. Evaluation of diagnostic accuracy following the coadministration of delta-aminolevulinic acid and second window indocyanine green in rodent and human glioblastomas. Mol. Imaging Biol. 2020, 22, 1266–1279. [Google Scholar] [CrossRef]
- Holt, D.; Parthasarathy, A.B.; Okusanya, O.; Keating, J.; Venegas, O.; Deshpande, C.; Karakousis, G.; Madajewski, B.; Durham, A.; Nie, S.; et al. Intraoperative near-infrared fluorescence imaging and spectroscopy identifies residual tumor cells in wounds. J. Biomed. Opt. 2015, 20, 76002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Keating, J.J.; Jesus, E.M.; Judy, R.P.; Madajewski, B.; Venegas, O.; Okusanya, O.T.; Singhal, S. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 390–400. [Google Scholar] [PubMed]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Konopka, C.J.; Cross, T.-W.L.; Swanson, K.S.; Dobrucki, L.W.; Smith, A.M. Multimodal Nanocarrier Probes Reveal Superior Biodistribution Quantification by Isotopic Analysis over Fluorescence. ACS Nano. 2020, 14, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Hren, R.; Sersa, G.; Simoncic, U.; Milanic, M. Imaging perfusion changes in oncological clinical applications by hyperspectral imaging: A literature review. Radiol. Oncol. 2022, 56, 420–429. [Google Scholar] [CrossRef]
- Nwaiwu, C.A.; McCulloh, C.J.; Skinner, G.; Shah, S.K.; Kim, P.C.W.; Schwaitzberg, S.D.; Wilson, E.B. Real-time First-In-Human Comparison of Laser Speckle Contrast Imaging and ICG in Minimally Invasive Colorectal & Bariatric Surgery. J. Gastrointest. Surg. 2023, 27, 3083–3085. [Google Scholar]
- Du, B.; Chong, Y.; Jiang, X.; Yu, M.; Lo, U.G.; Dang, A.; Chen, Y.A.; Li, S.; Hernandez, E.; Lin, J.C.; et al. Hyperfluorescence imaging of kidney cancer enabled by renal secretion pathway dependent efflux transport. Angew. Chem. Int. Ed. Engl. 2021, 60, 351–359. [Google Scholar] [CrossRef]
- Ishizawa, T.; Masuda, K.; Urano, Y.; Kawaguchi, Y.; Satou, S.; Kaneko, J.; Hasegawa, K.; Shibahara, J.; Fukayama, M.; Tsuji, S.; et al. Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 440–448. [Google Scholar] [CrossRef]
- Chu, T.S.; Tzeng, Y.S.; Wang, C.Y.; Shih, Y.J.; Chen, C.Y.; Wang, C.H. The utility of indocyanine green dye fluorescence lymphography and angiography during wide excision of cutaneous melanoma. Surg. Innov. 2022, 29, 566–572. [Google Scholar] [CrossRef]
- Zhou, L.; Gan, Y.; Wu, Y.; Xue, D.; Hu, J.; Zhang, Y.; Liu, Y.; Ma, S.; Zhou, J.; Luo, G.; et al. Indocyanine green fluorescence imaging in the surgical management of skin squamous cell carcinoma. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3309–3320. [Google Scholar] [CrossRef]
- Moore, L.S.; Rosenthal, E.L.; Chung, T.K.; de Boer, E.; Patel, N.; Prince, A.C.; Korb, M.L.; Walsh, E.M.; Young, E.S.; Stevens, T.M.; et al. Characterizing the utility and limitations of repurposing an open-field optical imaging device for fluorescence-guided surgery in head and neck cancer Patients. J. Nucl. Med. 2017, 58, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.W.; Spruit, M.A.; Reynaert, N.L.; Franssen, F.M.E.; Wouters, E.F.M.; Theunis, J.; De Boever, P. Skin auto-fluorescence as a measure of advanced glycation end-products is associated with microvascular health in patients with COPD. Microvasc. Res. 2020, 132, 104053. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.M.; de Boer, E.; van Dam, G.M.; Moore, L.S.; Bevans, S.L.; Walsh, E.M.; Young, E.S.; Carroll, W.R.; Stevens, T.M.; Rosenthal, E.L. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J. Pathol. Clin. Res. 2016, 2, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Teraphongphom, N.T.; van den Berg, N.S.; Martin, B.A.; Oberhelman, N.J.; Divi, V.; Kaplan, M.J.; Hong, S.S.; Lu, G.; Ertsey, R.; et al. Determination of tumor margins with surgical specimen mapping using near-infrared fluorescence. Cancer Res. 2018, 78, 5144–5154. [Google Scholar] [CrossRef] [PubMed]


| Variable | Mean SBR | p | |
|---|---|---|---|
| Age | ≤40 years (26%) | 1.30 | N.S. |
| >40 years (74%) | 1.52 | ||
| Gender | Female (50%) | 1.41 | N.S. |
| Male (50%) | 1.52 | ||
| Weight | ≤65 kg (44%) | 1.41 | N.S. |
| >65 kg (56%) | 1.50 | ||
| Course | ≤2 years (68%) | 1.33 | N.S. |
| >2 years (32%) | 1.34 | ||
| Type of Disease | Originating from the lacrimal gland (21%) | 1.33 | N.S. |
| Originating from the nerve (23%) | 1.47 | ||
| Originating from the lymphatic system (23%) | 1.62 | ||
| Others (33%) | 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Zhang, W.; Wang, X.; Guo, L.; Liu, W.; Li, Y.; Ma, R.; Hei, Y.; Yang, X.; Zhang, Z.; et al. Orbital Adipose Tissue: The Optimal Control for Back-Table Fluorescence Imaging of Orbital Tumors. Bioengineering 2024, 11, 922. https://doi.org/10.3390/bioengineering11090922
Yao L, Zhang W, Wang X, Guo L, Liu W, Li Y, Ma R, Hei Y, Yang X, Zhang Z, et al. Orbital Adipose Tissue: The Optimal Control for Back-Table Fluorescence Imaging of Orbital Tumors. Bioengineering. 2024; 11(9):922. https://doi.org/10.3390/bioengineering11090922
Chicago/Turabian StyleYao, Lan, Wenhua Zhang, Xuedong Wang, Lishuang Guo, Wenlu Liu, Yueyue Li, Rui Ma, Yan Hei, Xinji Yang, Zeyu Zhang, and et al. 2024. "Orbital Adipose Tissue: The Optimal Control for Back-Table Fluorescence Imaging of Orbital Tumors" Bioengineering 11, no. 9: 922. https://doi.org/10.3390/bioengineering11090922
APA StyleYao, L., Zhang, W., Wang, X., Guo, L., Liu, W., Li, Y., Ma, R., Hei, Y., Yang, X., Zhang, Z., & Wu, W. (2024). Orbital Adipose Tissue: The Optimal Control for Back-Table Fluorescence Imaging of Orbital Tumors. Bioengineering, 11(9), 922. https://doi.org/10.3390/bioengineering11090922







