Reverse Shoulder Arthroplasty Baseplate Stability Is Affected by Bone Density and the Type and Amount of Augmentation
Abstract
1. Introduction
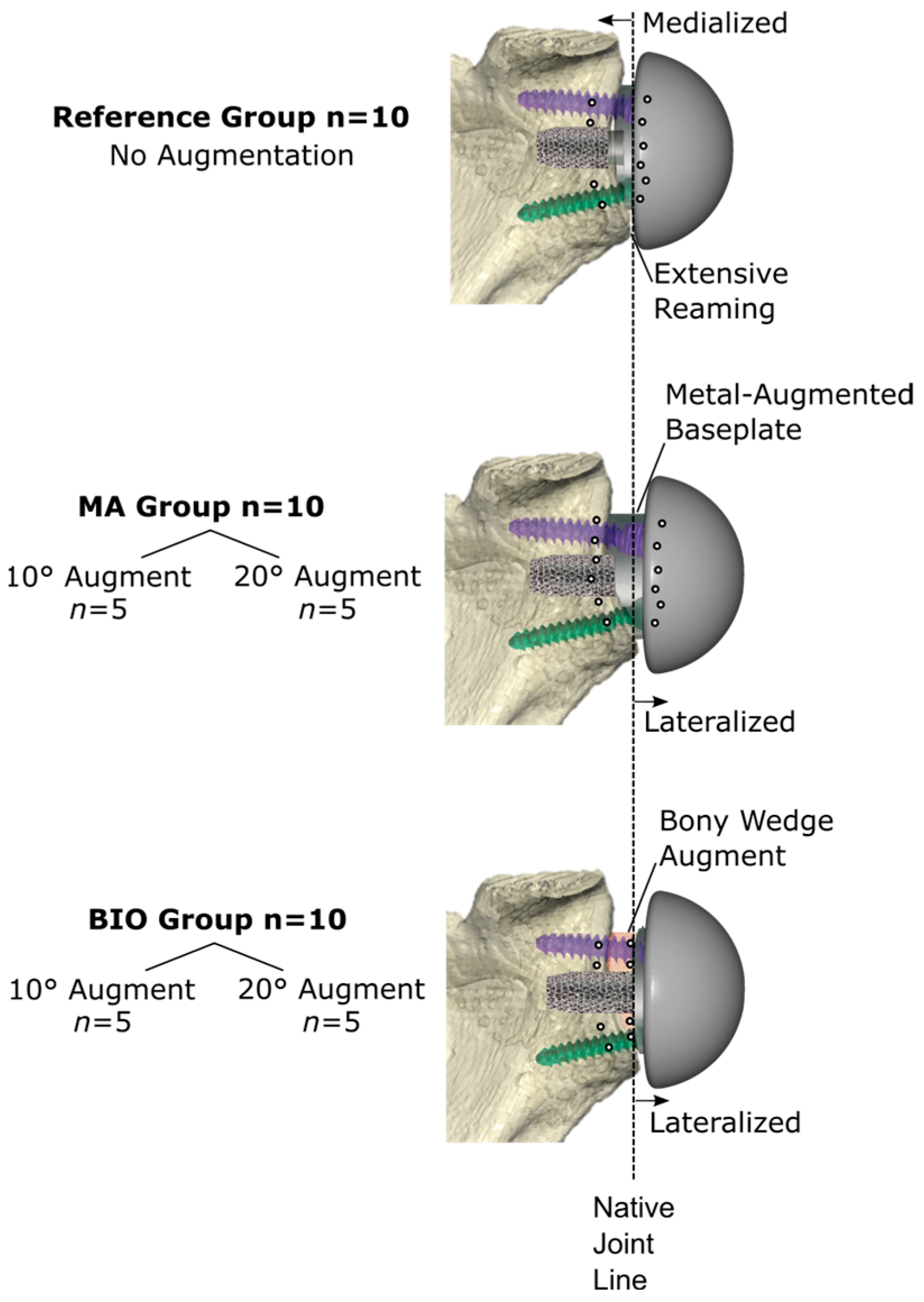
2. Materials and Methods
2.1. Bone Density
Regions of Interest
2.2. Virtual Planning and Surgical Technique
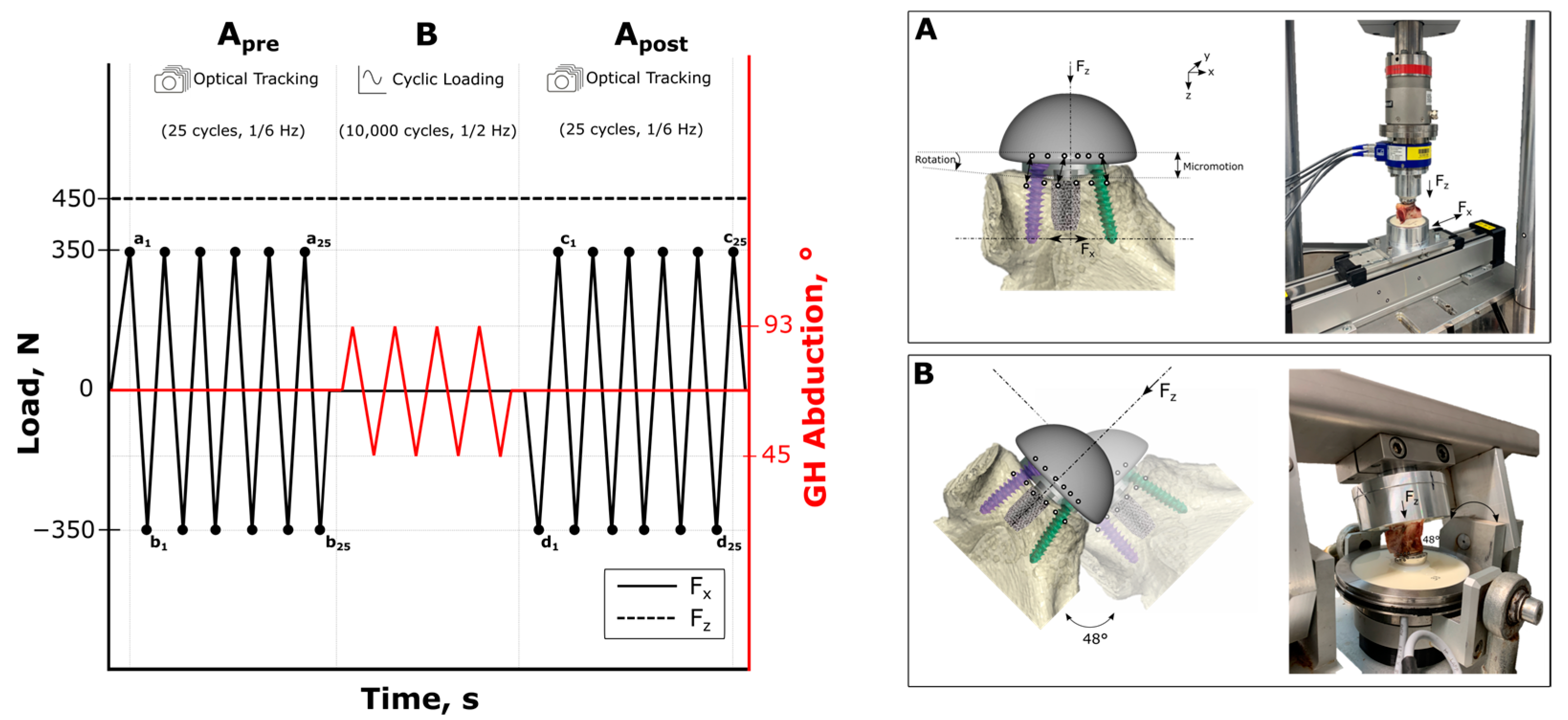
2.3. Biomechanical Testing
2.4. Outcome Variables
2.5. Statistical Analysis
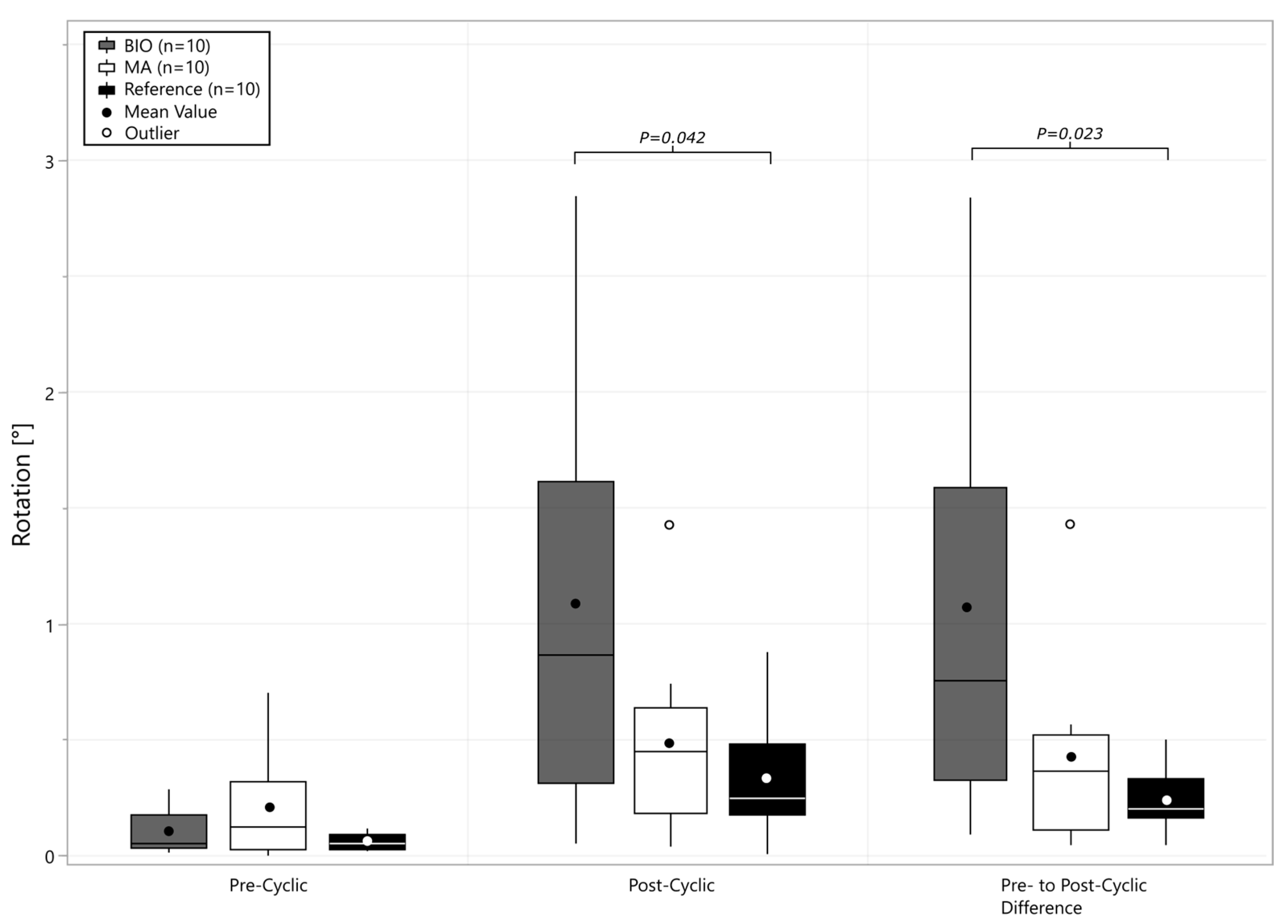
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bacle, G.; Nové-Josserand, L.; Garaud, P.; Walch, G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J. Bone Joint Surg. Am. 2017, 99, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Berhouet, J.; Garaud, P.; Favard, L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J. Shoulder Elbow Surg. 2014, 23, 151–158. [Google Scholar] [CrossRef]
- Levigne, C.; Boileau, P.; Favard, L.; Garaud, P.; Mole, D.; Sirveaux, F.; Walch, G. Scapular notching in reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2008, 17, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Werthel, J.D.; Walch, G.; Vegehan, E.; Deransart, P.; Sanchez-Sotelo, J.; Valenti, P. Lateralization in reverse shoulder arthroplasty: A descriptive analysis of different implants in current practice. Int. Orthop. 2019, 43, 2349–2360. [Google Scholar] [CrossRef]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony Increased-offset Reversed Shoulder Arthroplasty: Minimizing Scapular Impingement While Maximizing Glenoid Fixation. Clin. Orthop. Relat. Res.® 2011, 469, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Morin-Salvo, N.; Bessière, C.; Chelli, M.; Gauci, M.-O.; Lemmex, D.B. Bony increased-offset–reverse shoulder arthroplasty: 5 to 10 years’ follow-up. J. Shoulder Elb. Surg. 2020, 29, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Dimock, R.; Fathi Elabd, M.; Imam, M.; Middleton, M.; Godenèche, A.; Ali Narvani, A. Bony increased-offset reverse shoulder arthroplasty: A meta-analysis of the available evidence. Shoulder Elb. 2021, 13, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Imiolczyk, J.-P.; Audigé, L.; Harzbecker, V.; Moroder, P.; Scheibel, M. Metallic humeral and glenoid lateralized implants in reverse shoulder arthroplasty for cuff tear arthropathy and primary osteoarthritis. JSES Int. 2022, 6, 221–228. [Google Scholar] [CrossRef]
- Merolla, G.; Giorgini, A.; Bonfatti, R.; Micheloni, G.M.; Negri, A.; Catani, F.; Tarallo, L.; Paladini, P.; Porcellini, G. BIO-RSA vs. metal-augmented baseplate in shoulder osteoarthritis with multiplanar glenoid deformity: A comparative study of radiographic findings and patient outcomes. J. Shoulder Elb. Surg. 2023, 32, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Favre, P.; Seebeck, J.; Thistlethwaite, P.A.; Obrist, M.; Steffens, J.G.; Hopkins, A.R.; Hulme, P.A. In vitro initial stability of a stemless humeral implant. Clin. Biomech. 2016, 32, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Peppers, T. Fixation of humeral prostheses and axial micromotion. J. Shoulder Elb. Surg. 1998, 7, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Pilliar, R.M.; Lee, J.M.; Maniatopoulos, C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin. Orthop. Relat. Res. 1986, 208, 108–113. [Google Scholar] [CrossRef]
- Harmer, L.; Throckmorton, T.; Sperling, J.W. Total shoulder arthroplasty: Are the humeral components getting shorter? Curr. Rev. Musculoskelet. Med. 2016, 9, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sperling, J.W.; Haidukewych, G.H.; Cofield, R.H. Periprosthetic Humeral Fractures After Shoulder Arthroplasty. J. Bone Joint Surg. Am. 2004, 86, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Raiss, P.; Edwards, T.B.; Deutsch, A.; Shah, A.; Bruckner, T.; Loew, M.; Boileau, P.; Walch, G. Radiographic changes around humeral components in shoulder arthroplasty. J. Bone Jt. Surg. Am. 2014, 96, e54. [Google Scholar] [CrossRef]
- Casp, A.J.; Montgomery, S.R.J.; Cancienne, J.M.; Brockmeier, S.F.; Werner, B.C. Osteoporosis and Implant-Related Complications After Anatomic and Reverse Total Shoulder Arthroplasty. J. Am. Acad. Orthop. Surg. 2020, 28, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ritter, D.; Denard, P.J.; Raiss, P.; Wijdicks, C.A.; Bachmaier, S. Preoperative 3-dimensional computed tomography bone density measures provide objective bone quality classifications for stemless anatomic total shoulder arthroplasty. J. Shoulder Elb. Surg. 2024, 33, 1503–1511. [Google Scholar] [CrossRef]
- Ritter, D.; Denard, P.J.; Raiss, P.; Wijdicks, C.A.; Werner, B.C.; Bedi, A.; Müller, P.E.; Bachmaier, S. Machine Learning Models Can Define Clinically Relevant Bone Density Sub-groups based on Patient Specific Calibrated CT Scans in Patients Undergoing Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2024. Epub ahead of printing. [Google Scholar] [CrossRef]
- Hayden, A.; Cotter, E.J.; Hennick, T.; Hetzel, S.; Wollaeger, J.; Anderson, S.; Grogan, B.F. Bone Quality in Total Shoulder Arthroplasty: A Prospective Study Correlating CT Hounsfield Units with Thumb Test and FRAX Score. JSES Int. 2023, 7, 628–635. [Google Scholar] [CrossRef]
- Martin, E.J.; Duquin, T.R.; Ehrensberger, M.T. Reverse Total Shoulder Arthroplasty Baseplate Stability in Superior Bone Loss with Augmented Implant. J. Shoulder Elb. Arthroplast. 2021, 5, 24715492211020689. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.F.; Frankle, M.A.; Cronin, K.J. Maximizing Implant Stability in the Face of Glenoid Bone Stock Deficiency. Orthop. Clin. North. Am. 2024, 55, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.; Stroud, N.; Glattke, K.; Flurin, P.H.; Wright, T.; Zuckerman, J.; Roche, C. The impact of posterior wear on reverse shoulder glenoid fixation. Bull. Hosp. Jt. Dis. 2015, 73, S15–S20. [Google Scholar]
- Ritter, D.; Denard, P.J.; Raiss, P.; Wijdicks, C.A.; Bachmaier, S. A Stemless Anatomic Shoulder Arthroplasty Design Provides Increased Cortical Medial Calcar Bone Loading in Variable Bone Densities Compared to a Short Stem Implant. JSES Int. 2024, 8, 851–858. [Google Scholar] [CrossRef]
- Shah, A.; Werner, B.; Gobezie, R.; Denard, P.; Harmsen, S.; Brolin, T.; Bercik, M.; Thankur, S.; Doody, S.; Knopf, D.; et al. Quantifying bone loss and lateralization with standardized baseplate versus augmented baseplates. JSES Int. 2024, 8, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Lin, A.; Lenters, T.R.; Lutton, D.; Creighton, R.A.; Port, J.; Doody, S.; Metcalfe, N.; Knopf, D. Influence of backside seating parameters and augmented baseplate components in virtual planning for reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2024, 33, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2028-17; Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. Book of ASTM Standards: West Conshohocken, PA, USA, 2017. [CrossRef]
- Colasanti, C.A.; Lin, C.C.; Ross, K.A.; Luthringer, T.; Elwell, J.A.; Roche, C.P.; Virk, M.S.; Simovitch, R.W.; Routman, H.D.; Zuckerman, J.D. Augmented baseplates yield optimum outcomes when compared with bone graft augmentation for managing glenoid deformity during reverse total shoulder arthroplasty: A retrospective comparative study. J. Shoulder Elb. Surg. 2023, 32, 958–971. [Google Scholar] [CrossRef]
- Boileau, P.; Morin-Salvo, N.; Gauci, M.-O.; Seeto, B.L.; Chalmers, P.N.; Holzer, N.; Walch, G. Angled BIO-RSA (bony-increased offset–reverse shoulder arthroplasty): A solution for the management of glenoid bone loss and erosion. J. Shoulder Elb. Surg. 2017, 26, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Simcox, T.G.; Hao, K.A.; Dada, O.; Beason, A.M.; Khlopas, A.; Farmer, K.W.; King, J.J.; Schoch, B.S.; Wright, T.W.; Struk, A.M.; et al. Survivorship and Clinical Outcomes of Reverse Total Shoulder Arthroplasty in Patients with Large Glenoid Defects Using the Stilting Technique and a Baseplate with Central Ingrowth Cage and Peripheral Locking Screws. J. Shoulder Elb. Surg. 2024. Online ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- VandeKleut, M.L.; Yuan, X.; Teeter, M.G.; Athwal, G.S. Bony increased-offset reverse shoulder arthroplasty vs. metal augments in reverse shoulder arthroplasty: A prospective, randomized clinical trial with 2-year follow-up. J. Shoulder Elb. Surg. 2022, 31, 591–600. [Google Scholar] [CrossRef]
- Wittmann, T.; Denard, P.J.; Werner, B.C.; Raiss, P. Glenoid lateralization in reverse shoulder arthroplasty: Metal vs. bone offset in different implant designs. JSES Int. 2024, 8, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, O.H.; Aspenberg, P. Inter-trabecular bone formation: A specific mechanism for healing of cancellous bone. Acta Orthop. 2016, 87, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Achors, K.; Diaz, M.A.; Simon, P.; Hill, B.; Christmas, K.N.; Cronin, K.J.; Frankle, M.A. Avoiding Glenoid Baseplate Fixation Failure by Altering Surgical Technique for Varying Bone Densities. JBJS Open Access 2022, 7, e22. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, F.; Verdonschot, N.; van der Linden, Y.; Tanck, E. Calibration with or without phantom for fracture risk prediction in cancer patients with femoral bone metastases using CT-based finite element models. PLoS ONE 2019, 14, e0220564. [Google Scholar] [CrossRef] [PubMed]
- Free, J.; Eggermont, F.; Derikx, L.; van Leeuwen, R.; van der Linden, Y.; Jansen, W.; Raaijmakers, E.; Tanck, E.; Kaatee, R. The effect of different CT scanners, scan parameters and scanning setup on Hounsfield units and calibrated bone density: A phantom study. Biomed. Phys. Eng. Express 2018, 4, 12. [Google Scholar] [CrossRef]
- Tabarestani, T.Q.; Levin, J.M.; Warren, E.; Boadi, P.; Twomey-Kozak, J.; Wixted, C.; Goltz, D.E.; Wickman, J.; Hurley, E.T.; Anakwenze, O.; et al. Preoperative glenoid bone density is associated with systemic osteoporosis in primary shoulder arthroplasty. Semin. Arthroplast. JSES 2023, 33, 727–734. [Google Scholar] [CrossRef]
- Levin, J.M.; Rodriguez, K.; Polascik, B.A.; Zeng, S.; Warren, E., Jr.; Rechenmacher, A.; Helmkamp, J.; Goltz, D.E.; Wickman, J.; Klifto, C.S.; et al. Simple preoperative radiographic and computed tomography measurements predict adequate bone quality for stemless total shoulder arthroplasty. J. Shoulder Elb. Surg. 2022, 31, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.J.; Vaughan, A.; Tzeuton, S.; Abboud, J.A. Prospective assessment of osteoporosis in total shoulder arthroplasty. Semin. Arthroplast. JSES 2023, 33, 15–21. [Google Scholar] [CrossRef]
- Lee, D.C.; Hoffmann, P.F.; Kopperdahl, D.L.; Keaveny, T.M. Phantomless calibration of CT scans for measurement of BMD and bone strength-Inter-operator reanalysis precision. Bone 2017, 103, 325–333. [Google Scholar] [CrossRef] [PubMed]



| Bone Density Variables | MA | BIO | Reference | MA vs. BIO p-Value | MA vs. Ref. p-Value | BIO vs. Ref. p-Value |
|---|---|---|---|---|---|---|
| Subchondral Cylinder BMD [mgHA/cm3] | 217 ± 34 | 211 ± 16 | 238 ± 37 | 0.905 | 0.256 | 0.121 |
| Glenoid Cylinder [mgHA/cm3] | 409 ± 49 | 385 ± 32 | 397 ± 57 | 0.515 | 0.837 | 0.852 |
| Glenoid Global [mgHA/cm3] | 371 ± 58 | 347 ± 30 | 386 ± 35 | 0.447 | 0.697 | 0.121 |
| Subchondral Cylinder BV/TV | 0.62 ± 0.15 | 0.59 ± 0.18 | 0.66 ± 0.14 | 0.906 | 0.820 | 0.568 |
| Glenoid Cylinder BV/TV | 0.72 ± 0.13 | 0.69 ± 0.11 | 0.73 ± 0.12 | 0.811 | 0.987 | 0.722 |
| Glenoid Global BV/TV | 0.78 ± 0.13 | 0.73 ± 0.11 | 0.80 ± 0.10 | 0.548 | 0.976 | 0.426 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, D.; Raiss, P.; Denard, P.J.; Werner, B.C.; Kistler, M.; Lesnicar, C.; van der Merwe, M.; Müller, P.E.; Woiczinski, M.; Wijdicks, C.A.; et al. Reverse Shoulder Arthroplasty Baseplate Stability Is Affected by Bone Density and the Type and Amount of Augmentation. Bioengineering 2025, 12, 42. https://doi.org/10.3390/bioengineering12010042
Ritter D, Raiss P, Denard PJ, Werner BC, Kistler M, Lesnicar C, van der Merwe M, Müller PE, Woiczinski M, Wijdicks CA, et al. Reverse Shoulder Arthroplasty Baseplate Stability Is Affected by Bone Density and the Type and Amount of Augmentation. Bioengineering. 2025; 12(1):42. https://doi.org/10.3390/bioengineering12010042
Chicago/Turabian StyleRitter, Daniel, Patric Raiss, Patrick J. Denard, Brian C. Werner, Manuel Kistler, Celina Lesnicar, Micheal van der Merwe, Peter E. Müller, Matthias Woiczinski, Coen A. Wijdicks, and et al. 2025. "Reverse Shoulder Arthroplasty Baseplate Stability Is Affected by Bone Density and the Type and Amount of Augmentation" Bioengineering 12, no. 1: 42. https://doi.org/10.3390/bioengineering12010042
APA StyleRitter, D., Raiss, P., Denard, P. J., Werner, B. C., Kistler, M., Lesnicar, C., van der Merwe, M., Müller, P. E., Woiczinski, M., Wijdicks, C. A., & Bachmaier, S. (2025). Reverse Shoulder Arthroplasty Baseplate Stability Is Affected by Bone Density and the Type and Amount of Augmentation. Bioengineering, 12(1), 42. https://doi.org/10.3390/bioengineering12010042








