iPSC Technology Revolutionizes CAR-T Cell Therapy for Cancer Treatment
Abstract
1. Introduction
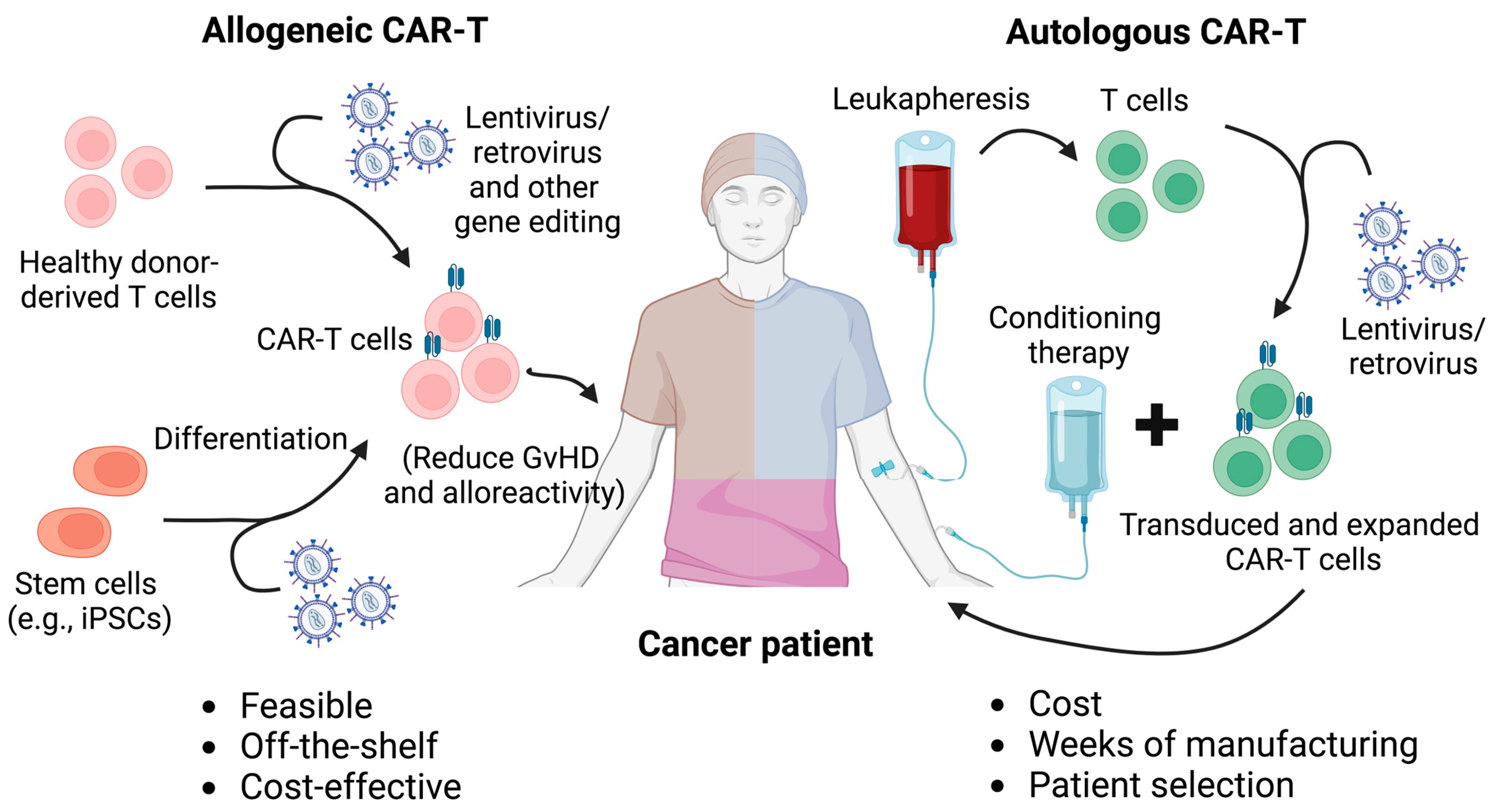
2. Allogeneic CAR-T Cell Therapy
2.1. Conventional Autologous CAR-T Cell Therapy
2.2. Allogeneic TCR-Disrupted CAR-T Cell Therapy
2.3. Limitations of Current CAR-T Cell Therapy
3. iPSC-Derived Allogeneic CAR-T Cell Products
3.1. Generate iPSC-Derived CAR-T Cells Using an OP9-DL1 Feeder-Dependent Culture
3.2. Generate iPSC-Derived CAR-T Cells Using a Stroma-Free Culture
3.3. Generating iPSC-Derived CAR-T Cells Using a 3D-Organoid Culture
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.C.; Maus, M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V. Immunotherapy: Tisagenlecleucel—The first approved CAR-T-cell therapy: Implications for payers and policy makers. Nat. Rev. Clin. Oncol. 2018, 15, 11–12. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Li, Y.-R.; Zhou, K.; Zhu, Y.; Halladay, T.; Yang, L. Breaking the mold: Unconventional T cells in cancer therapy. Cancer Cell 2024. online ahead of print. [Google Scholar] [CrossRef]
- Tchou, J.; Wang, L.-C.; Selven, B.; Zhang, H.; Conejo-Garcia, J.; Borghaei, H.; Kalos, M.; Vondeheide, R.H.; Albelda, S.M.; June, C.H.; et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res. Treat. 2012, 133, 799–804. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. Long-term outcomes following CAR T cell therapy: What we know so far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves second BCMA-targeted CAR-T cell therapy. Nat. Rev. Drug Discov. 2022, 21, 249. [Google Scholar] [CrossRef]
- Wagner, D.L.; Fritsche, E.; Pulsipher, M.A.; Ahmed, N.; Hamieh, M.; Hegde, M.; Ruella, M.; Savoldo, B.; Shah, N.N.; Turtle, C.J.; et al. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 379–393. [Google Scholar] [CrossRef]
- DiNofia, A.M.; Grupp, S.A. Will allogeneic CAR T cells for CD19(+) malignancies take autologous CAR T cells “off the shelf”? Nat. Rev. Clin. Oncol. 2021, 18, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, M.; Zhou, K.; Brown, J.; Tsao, T.; Cen, X.; Husman, T.; Bajpai, A.; Dunn, Z.S.; Yang, L. Engineering Induced Pluripotent Stem Cells for Cancer Immunotherapy. Cancers 2022, 14, 2266. [Google Scholar] [CrossRef] [PubMed]
- Iriguchi, S.; Yasui, Y.; Kawai, Y.; Arima, S.; Kunitomo, M.; Sato, T.; Ueda, T.; Minagawa, A.; Mishima, Y.; Yanagawa, N.; et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun. 2021, 12, 430. [Google Scholar] [CrossRef]
- Themeli, M.; Kloss, C.C.; Ciriello, G.; Fedorov, V.D.; Perna, F.; Gonen, M.; Sadelain, M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 2013, 31, 928–933. [Google Scholar] [CrossRef]
- Jing, R.; Scarfo, I.; Najia, M.A.; Lummertz da Rocha, E.; Han, A.; Sanborn, M.; Bingham, T.; Kubaczka, C.; Jha, D.K.; Falchetti, M.; et al. EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity. Cell Stem Cell 2022, 29, 1181–1196.e6. [Google Scholar] [CrossRef]
- Wang, Z.; McWilliams-Koeppen, H.P.; Reza, H.; Ostberg, J.R.; Chen, W.; Wang, X.; Huynh, C.; Vyas, V.; Chang, W.-C.; Starr, R.; et al. 3D-organoid culture supports differentiation of human CAR+ iPSCs into highly functional CAR T cells. Cell Stem Cell 2022, 29, 515–527.e8. [Google Scholar] [CrossRef]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13, 125. [Google Scholar] [CrossRef]
- Timmers, M.; Roex, G.; Wang, Y.; Campillo-Davo, D.; Van Tendeloo, V.F.I.; Chu, Y.; Berneman, Z.N.; Luo, F.; Van Acker, H.H.; Anguille, S. Chimeric antigen receptor-modified T cell therapy in multiple myeloma: Beyond B cell maturation antigen. Front. Immunol. 2019, 10, 1613. [Google Scholar] [CrossRef]
- Schoutrop, E.; El-Serafi, I.; Poiret, T.; Zhao, Y.; Gultekin, O.; He, R.; Moyano-Galceran, L.; Carlson, J.W.; Lehti, K.; Hassan, M.; et al. Mesothelin-Specific CAR T Cells Target Ovarian Cancer. Cancer Res. 2021, 81, 3022–3035. [Google Scholar] [CrossRef]
- Mirazee, J.; Shah, N.N. CD70 CAR T cells in AML: Form follows function. Cell Rep. Med. 2022, 3, 100639. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves fourth CAR-T cell therapy. Nat. Rev. Drug Discov. 2021, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, S.; Ritchie, D.S.; Ramsey, S.D.; Turtle, C.J.; Roth, J.A. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant. 2020, 55, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Kenderian, S.S. Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf. BioDrugs 2017, 31, 473–481. [Google Scholar] [CrossRef]
- Han, D.; Xu, Z.; Zhuang, Y.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Hematological Malignancies. J. Cancer 2021, 12, 326–334. [Google Scholar] [CrossRef]
- Li, Y.-R.; Dunn, Z.S.; Yu, Y.; Li, M.; Wang, P.; Yang, L. Advancing cell-based cancer immunotherapy through stem cell engineering. Cell Stem Cell 2023, 30, 592–610. [Google Scholar] [CrossRef]
- Martino, M.; Alati, C.; Canale, F.A.; Musuraca, G.; Martinelli, G.; Cerchione, C. A Review of Clinical Outcomes of CAR T-Cell Therapies for B-Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 2150. [Google Scholar] [CrossRef]
- Li, Y.-R.; Zhou, Y.; Kramer, A.; Yang, L. Engineering stem cells for cancer immunotherapy. Trends Cancer 2021, 7, 1059–1073. [Google Scholar] [CrossRef]
- Lanza, R.; Russell, D.W.; Nagy, A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019, 19, 723–733. [Google Scholar] [CrossRef]
- Li, Y.-R.; Dunn, Z.S.; Zhou, Y.; Lee, D.; Yang, L. Development of Stem Cell-Derived Immune Cells for Off-the-Shelf Cancer Immunotherapies. Cells 2021, 10, 3497. [Google Scholar] [CrossRef]
- Kagoya, Y.; Guo, T.; Yeung, B.; Saso, K.; Anczurowski, M.; Wang, C.-H.; Murata, K.; Sugata, K.; Saijo, H.; Matsunaga, Y.; et al. Genetic Ablation of HLA Class I, Class II, and the T-cell Receptor Enables Allogeneic T Cells to Be Used for Adoptive T-cell Therapy. Cancer Immunol. Res. 2020, 8, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Ciocarlie, O.; Jain, N.; Jabbour, E.J.; Maus, M.V.; Frigault, M.; Boissel, N.; et al. Preliminary Data on Safety, Cellular Kinetics and Anti-Leukemic Activity of UCART19, an Allogeneic Anti-CD19 CAR T-Cell Product, in a Pool of Adult and Pediatric Patients with High-Risk CD19+ Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Blood 2018, 132, 896. [Google Scholar] [CrossRef]
- Srinivasan, S.; Tan, N.; Cheng, H.-Y.; Zhang, Y.; Tacheva-Grigorova, S.; Van Blarcom, T.; Sommer, C.; Nguyen, D.; Sasu, B.; Panowski, S. Investigation of ALLO-316: A Fratricide-Resistant Allogeneic CAR T Targeting CD70 As a Potential Therapy for the Treatment of AML. Blood 2020, 136, 23. [Google Scholar] [CrossRef]
- Jo, S.; Das, S.; Williams, A.; Chretien, A.-S.; Pagliardini, T.; Le Roy, A.; Fernandez, J.P.; Le Clerre, D.; Jahangiri, B.; Chion-Sotinel, I.; et al. Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing. Nat. Commun. 2022, 13, 3453. [Google Scholar] [CrossRef]
- Vormittag, P.; Gunn, R.; Ghorashian, S.; Veraitch, F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018, 53, 164–181. [Google Scholar] [CrossRef]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; van der Stegen, S.J.C.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef]
- Li, Y.-R.; Fang, Y.; Niu, S.; Chen, Y.; Lyu, Z.; Yang, L. Managing allorejection in off-the-shelf CAR-engineered cell therapies. Mol. Ther. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Aftab, B.T.; Sasu, B.; Krishnamurthy, J.; Gschweng, E.; Alcazer, V.; Depil, S. Toward “off-the-shelf” allogeneic CAR T cells. Adv. Cell Gene Ther. 2020, 3, e86. [Google Scholar] [CrossRef]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Mirci-Danicar, O.C.; Lucchini, G.; Pinner, D.; Jain, N.; Kantarjian, H.; Boissel, N.; et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: Results of two phase 1 studies. Lancet 2020, 396, 1885–1894. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Nath, R.; Munoz, J.; Tees, M.; Miklos, D.B.; Frank, M.J.; Malik, S.A.; Stevens, D.; Shin, C.R.; Balakumaran, A.; et al. ALPHA Study: ALLO-501 Produced Deep and Durable Responses in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma Comparable to Autologous CAR T. Blood 2021, 138, 3878. [Google Scholar] [CrossRef]
- Benjamin, R.; Jain, N.; Maus, M.V.; Boissel, N.; Graham, C.; Jozwik, A.; Yallop, D.; Konopleva, M.; Frigault, M.J.; Teshima, T.; et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): A phase 1, dose-escalation trial. Lancet Haematol. 2022, 9, e833–e843. [Google Scholar] [CrossRef] [PubMed]
- Themeli, M.; Rivière, I.; Sadelain, M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell 2015, 16, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, G.; Zhang, J.; Wu, X.; Chen, X. The Dual Roles of Human γδ T Cells: Anti-Tumor or Tumor-Promoting. Front. Immunol. 2020, 11, 619954. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Vo, L.T.; Kinney, M.A.; Liu, X.; Zhang, Y.; Barragan, J.; Sousa, P.M.; Jha, D.K.; Han, A.; Cesana, M.; Shao, Z.; et al. Regulation of embryonic haematopoietic multipotency by EZH1. Nature 2018, 553, 506–510. [Google Scholar] [CrossRef]
- Soto, R.A.; Najia, M.A.T.; Hachimi, M.; Frame, J.M.; Yette, G.A.; Lummertz da Rocha, E.; Stankunas, K.; Daley, G.Q.; North, T.E. Sequential regulation of hemogenic fate and hematopoietic stem and progenitor cell formation from arterial endothelium by Ezh1/2. Stem Cell Rep. 2021, 16, 1718–1734. [Google Scholar] [CrossRef]
- Seet, C.S.; He, C.; Bethune, M.T.; Li, S.; Chick, B.; Gschweng, E.H.; Zhu, Y.; Kim, K.; Kohn, D.B.; Baltimore, D.; et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Seet, C.S.; Li, S.; Chick, B.; Zhu, Y.; Chang, P.; Tsai, S.; Sun, V.; Lopez, S.; Chen, H.-C.; et al. Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells. Cell Stem Cell 2019, 24, 376–389.e8. [Google Scholar] [CrossRef]
- Li, Y.-R.; Zhou, Y.; Kim, Y.J.; Zhu, Y.; Ma, F.; Yu, J.; Wang, Y.-C.; Chen, X.; Li, Z.; Zeng, S.; et al. Development of allogeneic HSC-engineered iNKT cells for off-the-shelf cancer immunotherapy. Cell Rep. Med. 2021, 2, 100449. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Rezvani, K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front. Immunol. 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Song, L.; Wei, J.; Courtney, A.N.; Gao, X.; Marinova, E.; Guo, L.; Heczey, A.; Asgharzadeh, S.; Kim, E.; et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J. Clin. Investig. 2012, 122, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Dunn, Z.S.; Jr, G.G.; Carmona, C.; Zhou, Y.; Lee, D.; Yu, J.; Huang, J.; Kim, J.T.; Arumugaswami, V.; et al. Development of off-the-shelf hematopoietic stem cell-engineered invariant natural killer T cells for COVID-19 therapeutic intervention. Stem Cell Res. Ther. 2022, 13, 112. [Google Scholar] [CrossRef]
- Momparler, R.L.; Côté, S. Targeting of cancer stem cells by inhibitors of DNA and histone methylation. Expert Opin. Investig. Drugs 2015, 24, 1031–1043. [Google Scholar] [CrossRef]
- Hoyos, V.; Savoldo, B.; Quintarelli, C.; Mahendravada, A.; Zhang, M.; Vera, J.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K.; Dotti, G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010, 24, 1160–1170. [Google Scholar] [CrossRef]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017, 20, 3025–3033. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, L.; Dai, X.; Yu, H.; Wang, J.; Lei, A.; Zhu, M.; Xu, J.; Zhao, W.; Zhu, Y.; et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 2020, 13, 153. [Google Scholar] [CrossRef]
- Abdin, S.M.; Paasch, D.; Kloos, A.; Oliveira, M.C.; Jang, M.-S.; Ackermann, M.; Stamopoulou, A.; Mroch, P.J.; Falk, C.S.; von Kaisenberg, C.S.; et al. Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia. J. Immunother. Cancer 2023, 11, e007705. [Google Scholar] [CrossRef]
- Yano, H.; Koga, K.; Sato, T.; Shinohara, T.; Iriguchi, S.; Matsuda, A.; Nakazono, K.; Shioiri, M.; Miyake, Y.; Kassai, Y.; et al. Human iPSC-derived CD4(+) Treg-like cells engineered with chimeric antigen receptors control GvHD in a xenograft model. Cell Stem Cell 2024, 31, 795–802.e6. [Google Scholar] [CrossRef] [PubMed]
- Uslu, U.; Castelli, S.; June, C.H. CAR T cell combination therapies to treat cancer. Cancer Cell 2024, 42, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Lukacs, J.D.; Johnston, B. The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead. Cancers 2021, 13, 5174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Zhou, Y.; Wilson, M.; Kramer, A.; Hon, R.; Zhu, Y.; Fang, Y.; Yang, L. Tumor-Localized Administration of α-GalCer to Recruit Invariant Natural Killer T Cells and Enhance Their Antitumor Activity against Solid Tumors. Int. J. Mol. Sci. 2022, 23, 7547. [Google Scholar] [CrossRef]
- Li, Y.-R.; Zhou, Y.; Yu, J.; Zhu, Y.; Lee, D.; Zhu, E.; Li, Z.; Kim, Y.J.; Zhou, K.; Fang, Y.; et al. Engineering Allorejection-Resistant CAR-NKT Cells from Hematopoietic Stem Cells for Off-The-Shelf Cancer Immunotherapy. Mol. Ther. 2024, 32, 1849–1874. [Google Scholar] [CrossRef]
- Li, Y.-R.; Zhou, Y.; Yu, J.; Kim, Y.J.; Li, M.; Lee, D.; Zhou, K.; Chen, Y.; Zhu, Y.; Wang, Y.-C.; et al. Generation of allogeneic CAR-NKT cells from hematopoietic stem and progenitor cells using a clinically guided culture method. Nat. Biotechnol. 2024. online ahead of print. [Google Scholar] [CrossRef]
| iPSC-Derived CAR-T Cell Platforms | iPSC Source | iPSC Technology | Differentiation Approach | CAR Engineering | Characterization of iPSC-Derived CAR-T Cells | Limitations | Year and Reference |
|---|---|---|---|---|---|---|---|
| Platform 1 | T cell-derived iPSCs (T-iPSCs) | Peripheral blood T lymphocytes are transduced with two retroviral vectors, each encoding two of the reprogramming factors KLF4, SOX2, OCT-4, and C-MYC | Mesoderm formation, hematopoietic specification and expansion, and T-lymphoid commitment (OP9-DL1 culture) | CD19-targeting CAR engineering on T-iPSCs | Display a phenotype resembling that of innate γδ T cells, and elicit strong anti-tumor responses in vivo | The generated iPSC-derived CAR-T cells have the properties of γδ T cells, and the OP9-DL1 culture involves murine-derived feeder cells | 2013 [14] |
| Platform 2 | CD62L+ naive and memory T cell-derived iPSCs | The T cells are transduced and reprogramed by episomal plasmids encoding KLF4, SOX2, OCT-4, C-MYC, and LIN28, along with P53 shRNA | Mesoderm induction, hematopoietic induction, T cell differentiation (3D-organoid culture), and expansion | CD19-targeting CAR engineering on iPSCs | Show antigen-specific activation, degranulation, cytotoxicity, and cytokine secretion, and mediate potent antitumor activity in vivo | The 3D-organoid culture involves murine-derived feeder cells | 2022 [16] |
| Platform 3 | Human erythroblast-derived iPSCs (cell line 1157) | NA | Embryoid body formation, EZH1 repression, and T cell differentiation (stroma-free culture) | CD19-targeting CAR engineering on iPSC-derived T cells | Give rise to memory-like T cells upon activation, and display enhanced antitumor activity in vitro and in vivo | iPSC-derived EZ-T cells predominantly consist of CD8 cytotoxic T cells, and a large amount of CAR viruses are utilized | 2022 [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, J.; Li, Y.-R. iPSC Technology Revolutionizes CAR-T Cell Therapy for Cancer Treatment. Bioengineering 2025, 12, 60. https://doi.org/10.3390/bioengineering12010060
Zong J, Li Y-R. iPSC Technology Revolutionizes CAR-T Cell Therapy for Cancer Treatment. Bioengineering. 2025; 12(1):60. https://doi.org/10.3390/bioengineering12010060
Chicago/Turabian StyleZong, Jiepu, and Yan-Ruide Li. 2025. "iPSC Technology Revolutionizes CAR-T Cell Therapy for Cancer Treatment" Bioengineering 12, no. 1: 60. https://doi.org/10.3390/bioengineering12010060
APA StyleZong, J., & Li, Y.-R. (2025). iPSC Technology Revolutionizes CAR-T Cell Therapy for Cancer Treatment. Bioengineering, 12(1), 60. https://doi.org/10.3390/bioengineering12010060






