Singlet Oxygen Energy for Enhancing Physiological Function and Athletic Performance
Abstract
1. Introduction
- We deliberately produced a large amount of SO with no targets to attack, preventing energy transfer. Eventually, the excited singlet oxygen returned to its ground state by releasing 94 kJ/mol of energy, which supported the potential for novel applications and generated unexpected results [22].
- According to the principle of antibodies, when there are few harmful substances invading the human body, the defense capabilities of the immune system can be stimulated. Therefore, a small amount of ROS is indeed beneficial to the body [23].
- A total of 75% of the oxygen humans inhale is exhaled without being utilized. The key point is therefore not the supply or concentration of oxygen in the air but how efficiently the body uses the available oxygen for energy metabolism. That is, instead of the conventional method of increasing oxygen supply, we propose a novel approach to enhancing the energy of the existing oxygen.
2. Materials and Methods
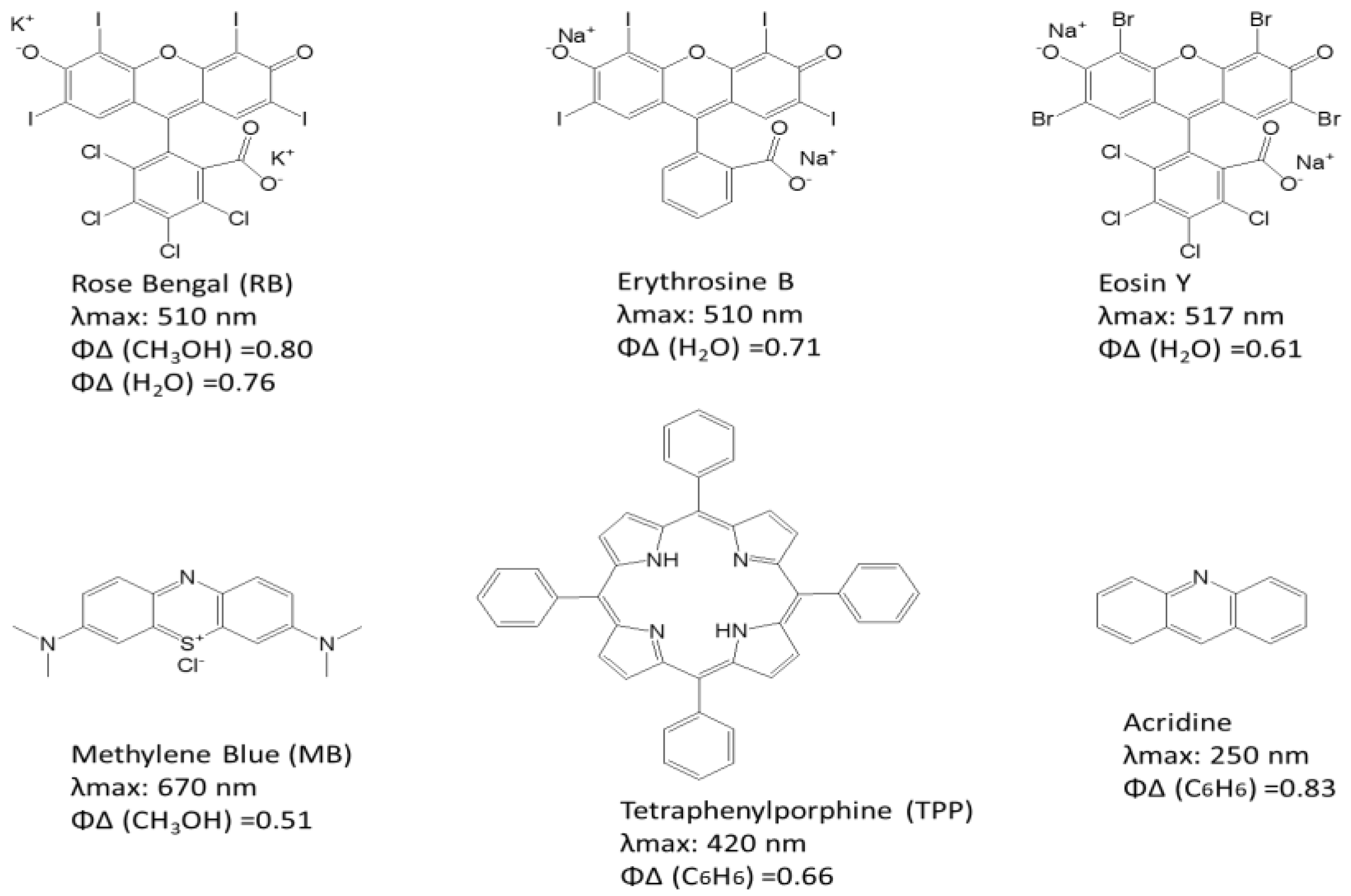
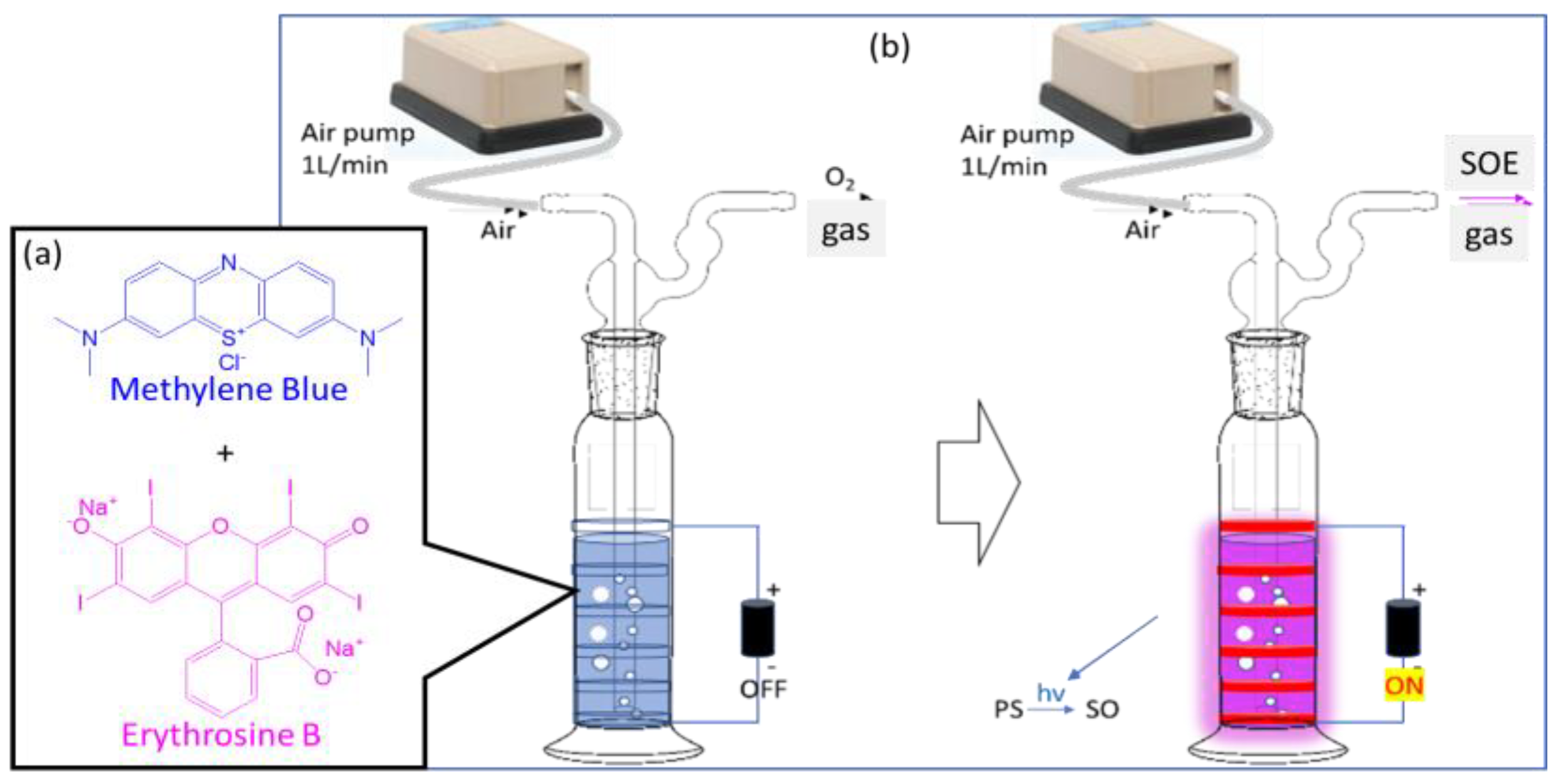
2.1. Photosensitive Solution Kit
2.2. Design of SOEG Device
2.3. Instrumentation for Analysis
2.3.1. Gas Analysis System
2.3.2. Treadmill
2.3.3. Heart Rate Sensor
2.3.4. Blood Lactate Testing
2.4. Methods
2.4.1. Experimental Tests
2.4.2. Submaximal Running Exercise Test
- Initial speed estimation: The starting running speed was determined based on each participant’s usual running pace to ensure comfort and familiarity.
- Gradual speed increase: At the beginning of each subsequent stage, the treadmill speed was increased by 1 to 1.5 km/h to progressively increase exercise intensity.
- Rating of perceived exertion (RPE): At the end of the fourth minute of each stage, participants were asked to report their RPE using the Borg scale, as shown in Table 1. The RPE indicates perceived exertion and overall fatigue throughout the test.
- Physiological measurements: Throughout the test, key respiratory and metabolic parameters were continuously monitored using the MetaMax 3B gas analysis system.
2.4.3. Maximal Oxygen Uptake (VO2 Max) Test
2.4.4. Singlet Oxygen Detection
3. Results
3.1. Detection and Analysis of Singlet Oxygen Generation by Photosensitizer Solution Kit
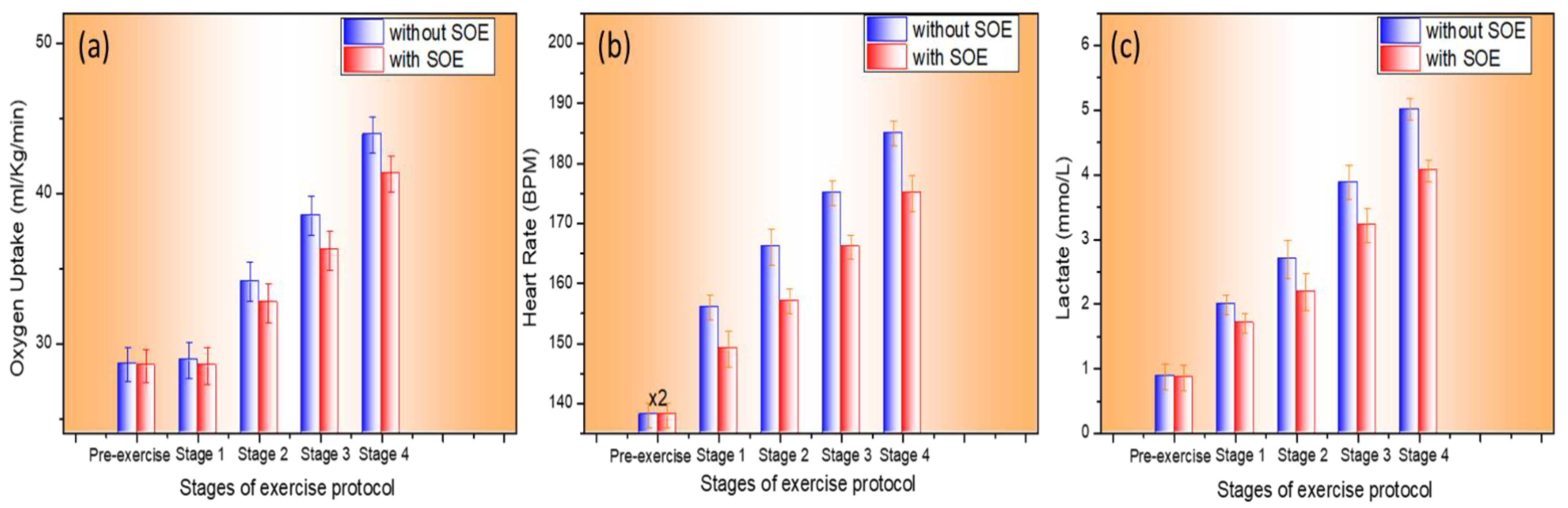
3.2. Comparative Analysis of Exercise Data with and Without the SOEG System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briviba, K.; Klotz, L.O.; Sies, H. Toxic and signaling effects of photochemically or chemically generated singlet oxygen in biological systems. Biol. Chem. 1997, 378, 1259–1265. [Google Scholar] [PubMed]
- Pitts, J.N., Jr.; Khan, A.U.; Smith, E.B.; Wayne, R.P. Singlet oxygen in the environmental sciences. Singlet molecular oxygen and photochemical air pollution. Environ. Sci. Technol. 1969, 3, 241–247. [Google Scholar] [CrossRef]
- Murray, R.W.; Kaplan, M.L. Singlet oxygen sources in ozone chemistry. Chemical oxygenations using the adducts between phosphite esters and ozone. J. Am. Chem. Soc. 1969, 91, 5358–5364. [Google Scholar] [CrossRef]
- Kanofsky, J.R. Singlet oxygen production by biological systems. Chem. Biol. Interact. 1989, 70, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, J.R.; Sima, P. Singlet oxygen production from the reactions of ozone with biological molecules. J. Biol. Chem. 1991, 266, 9039–9042. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Valentine, J.S.; Greenberg, A.; Liebman, J.F. Active Oxygen in Chemistry; Springer Science & Business Media: Berlin, Germany; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Girotti, A.W.; Korytowski, W. Reactions of Singlet Oxygen with Membrane Lipids: Lipid Hydroperoxide Generation, Translocation, Reductive Turnover, and Signaling Activity. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; Volume 1, pp. 409–430. [Google Scholar]
- Leem, J.W.; Kim, S.-R.; Choi, K.-H.; Kim, Y.L. Plasmonic photocatalyst-like fluorescent proteins for generating reactive oxygen species. Nano Converg. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. An Expanded and Revised Compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–677. [Google Scholar] [CrossRef]
- Mostafa, S.; Rosario-Ortiz, F.L. Singlet Oxygen Formation from Wastewater Organic Matter. Environ. Sci. Technol. 2013, 47, 8179–8186. [Google Scholar] [CrossRef]
- Svingen, B.A.; O’Neal, F.O.; Aust, S.D. The role of superoxide and singlet oxygen in lipid peroxidation. Photochem. Photobiol. 1978, 28, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yin, J.J.; Cherng, S.H.; Wamer, W.G.; Boudreau, M.; Howard, P.C.; Fu, P.P. UVA photoirradiation of retinyl palmitate--formation of singlet oxygen and superoxide, and their role in induction of lipid peroxidation. Toxicol. Lett. 2006, 163, 30–43. [Google Scholar] [CrossRef]
- Rabek, J.F.; Lucki, J.; Rånby, B. Comparative studies of reactions of commercial polymers with molecular oxygen, singlet oxygen, atomic oxygen and ozone—I. Reactions with cis-1,4-polybutadiene. Eur. Polym. J. 1979, 15, 1089–1100. [Google Scholar] [CrossRef]
- Baier, J.; Maisch, T.; Maier, M.; Engel, E.; Landthaler, M.; Bäumler, W. Singlet Oxygen Generation by UVA Light Exposure of Endogenous Photosensitizers. Biophys. J. 2006, 91, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, R.M. Role for singlet oxygen in biological effects of ultraviolet A radiation. Methods Enzymol. 2000, 319, 290–296. [Google Scholar] [PubMed]
- Kearns, D.R. Physical and chemical properties of singlet molecular oxygen. Chem. Rev. 1971, 71, 395–427. [Google Scholar] [CrossRef]
- Shcherbakov, N.V.; Emel’yanov, A.N.; Khaula, E.V.; Il’ichev, A.N.; Vishnetskaya, M.V.; Rufov, Y.N. Photo-and thermogeneration of singlet oxygen by the metal ions deposited on Al2O3 and SiO2. Russ. J. Phys. Chem. 2006, 80, 799–802. [Google Scholar] [CrossRef]
- Pyryaeva, A.P.; Goldort, V.G.; Kochubei, S.A.; Baklanov, A.V. Singlet oxygen O2(a1Δg) formation via UV-excitation of isoprene-oxygen C5H8–O2 encounter complexes in gas phase. Chem. Phys. Lett. 2014, 610, 8–13. [Google Scholar] [CrossRef]
- Al-Nu’airat, J.; Oluwoye, I.; Zeinali, N.; Altarawneh, M.; Dlugogorski, B.Z. Review of Chemical Reactivity of Singlet Oxygen with Organic Fuels and Contaminants. Chem. Rec. 2021, 21, 315–342. [Google Scholar] [CrossRef]
- Bregnhøj, M.; Thorning, F.; Ogilby, P.R. Singlet oxygen photophysics: From liquid solvents to mammalian cells. Chem. Rev. 2024, 124, 9949–10051. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Buettner, R.; Krutmann, J. Ultraviolet A radiation-induced expression of human genes: Molecular and photobiological mechanisms. Biol. Chem. 1997, 378, 1231–1236. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- 洪敦賓; 張健忠; 林亦香; 巫錦霖. 單重態氧對運動中的生理效益. 大專體育學刊 2024, 26. online. [Google Scholar]
- Wienecke, E. Influence on endurance levels of an Airnergy application prior to exertion. Med. Sports Netw. Available online: https://reurl.cc/oV6dAD (accessed on 3 July 2007).
- Ronald, D. Oxygen Therapies-Activating and Energising Water-Saturated Inhaled Air. The Airnergy Principle-Review and Original Data. Die Naturheilkunde, Offprint from Edition 6/2009. Available online: https://reurl.cc/XZgkvD (accessed on 20 January 2025).
- Rauhala, E. Some Physiological Effects of Breathing Singlet Oxygen Activated Air. An Experimental Pilot Study with Ergospirometry. Available online: https://reurl.cc/r328ME (accessed on 1 July 1995).
- Martusevich, A.A.; Solov’ieva, A.G.; Martusevich, A.K. Influence of singlet oxygen inhalation on the state of blood pro- and antioxidant systems and energy metabolism. Bull. Exp. Biol. Med. 2013, 156, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Martusevich, A.; Perunova, A.; Karuzin, C.; Bocharin, I.; Nikolaeva, A. Therapeutic effect of singlet oxygen administration on crystallization of rats’ blood serum at thermal trauma. Archiv. Uromedic. 2021, 11, 60–61. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242–272. [Google Scholar] [CrossRef]
- World Health Organization Model List of Essential Medicines: 21st List 2019; World Health Organization: Geneva, Switzerland, 2019.
- Levitan, H. Food, drug, and cosmetic dyes: Biological effects related to lipid solubility. Proc. Natl. Acad. Sci. USA 1977, 74, 2914. [Google Scholar] [CrossRef]
- Center for Food Safety and Applied Nutrition 2023. FD&C Red No. 3 FDA. Available online: https://www.fda.gov/industry/color-additives/fdc-red-no-3 (accessed on 15 January 2025).
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 2020, 204, 111787. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Thomason, H.; Roberts, E. Fractional utilization of the aerobic capacity during distance running. Med. Sci. Sports 1973, 5, 248–252. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef] [PubMed]



| Stage | Duration (Minutes) | Speed Increase (km/h) | RPE Assessment Time |
|---|---|---|---|
| 1 | 0–4 | Baseline | 4th min |
| 2 | 4–8 | +1.0 to 1.5 | 8th min |
| 3 | 4–8 | +1.0 to 1.5 | 12th min |
| 4 | 12–16 | +1.0 to 1.5 | 16th min |
| Oxygen Uptake (ml/Kg/min) | Heart Rate (Beats/min) | Lactate (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Without SOE | With SOE | Improve ∆ (%) | Without SOE | With SOE | Improve ∆ (%) | Without SOE | With SOE | Improve ∆ (%) | |
| Pre-exercise | 28.6 ± 1.1 | 28.5 ± 1.4 | - | 69 ± 2 | 69 ± 2 | - | 0.88 ± 0.15 | 0.86 ± 0.1 | - |
| Stage1 | 28.9 ± 1.2 | 28.5 ± 1.1 | 1.38 | 156 ± 2 | 149 ± 3 | 4.49 | 1.99 ± 0.14 | 1.80 ± 0.2 | 9.55 |
| Stage2 | 34.1 ± 1.3 | 32.2 ± 1.3 | 4.10 | 166 ± 3 | 157 ± 2 | 5.42 | 2.69 ± 0.29 | 2.35 ± 0.2 | 12.63 |
| Stage3 | 38.5 ± 1.3 | 36.2 ± 1.5 | 5.97 | 175 ± 2 | 166 ± 2 | 5.14 | 3.88 ± 0.26 | 3.41 ± 0.3 | 12.11 |
| Stage4 | 43.9 ± 1.2 | 41.3 ± 1.6 | 5.92 | 185 ± 2 | 175 ± 3 | 5.41 | 5.01 ± 0.29 | 4.36 ± 0.2 | 12.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-F.; Huang, C.-T.; Chang, C.-C.; Hung, T.-P. Singlet Oxygen Energy for Enhancing Physiological Function and Athletic Performance. Bioengineering 2025, 12, 118. https://doi.org/10.3390/bioengineering12020118
Hsieh C-F, Huang C-T, Chang C-C, Hung T-P. Singlet Oxygen Energy for Enhancing Physiological Function and Athletic Performance. Bioengineering. 2025; 12(2):118. https://doi.org/10.3390/bioengineering12020118
Chicago/Turabian StyleHsieh, Chia-Feng, Chun-Ta Huang, Cheng-Chung Chang, and Tun-Pin Hung. 2025. "Singlet Oxygen Energy for Enhancing Physiological Function and Athletic Performance" Bioengineering 12, no. 2: 118. https://doi.org/10.3390/bioengineering12020118
APA StyleHsieh, C.-F., Huang, C.-T., Chang, C.-C., & Hung, T.-P. (2025). Singlet Oxygen Energy for Enhancing Physiological Function and Athletic Performance. Bioengineering, 12(2), 118. https://doi.org/10.3390/bioengineering12020118






