Pulsed Field Ablation: A Review of Preclinical and Clinical Studies
Abstract
1. Introduction
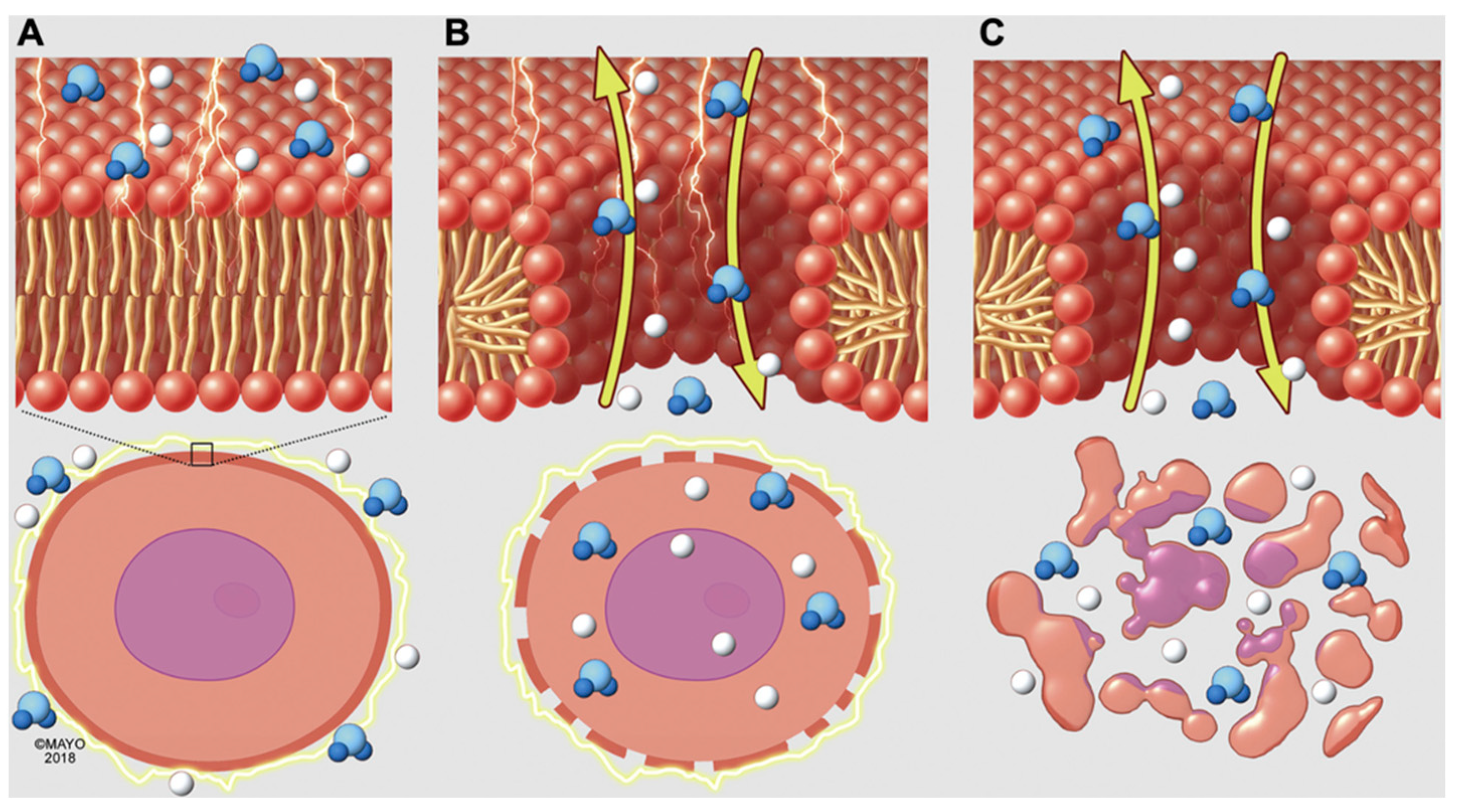
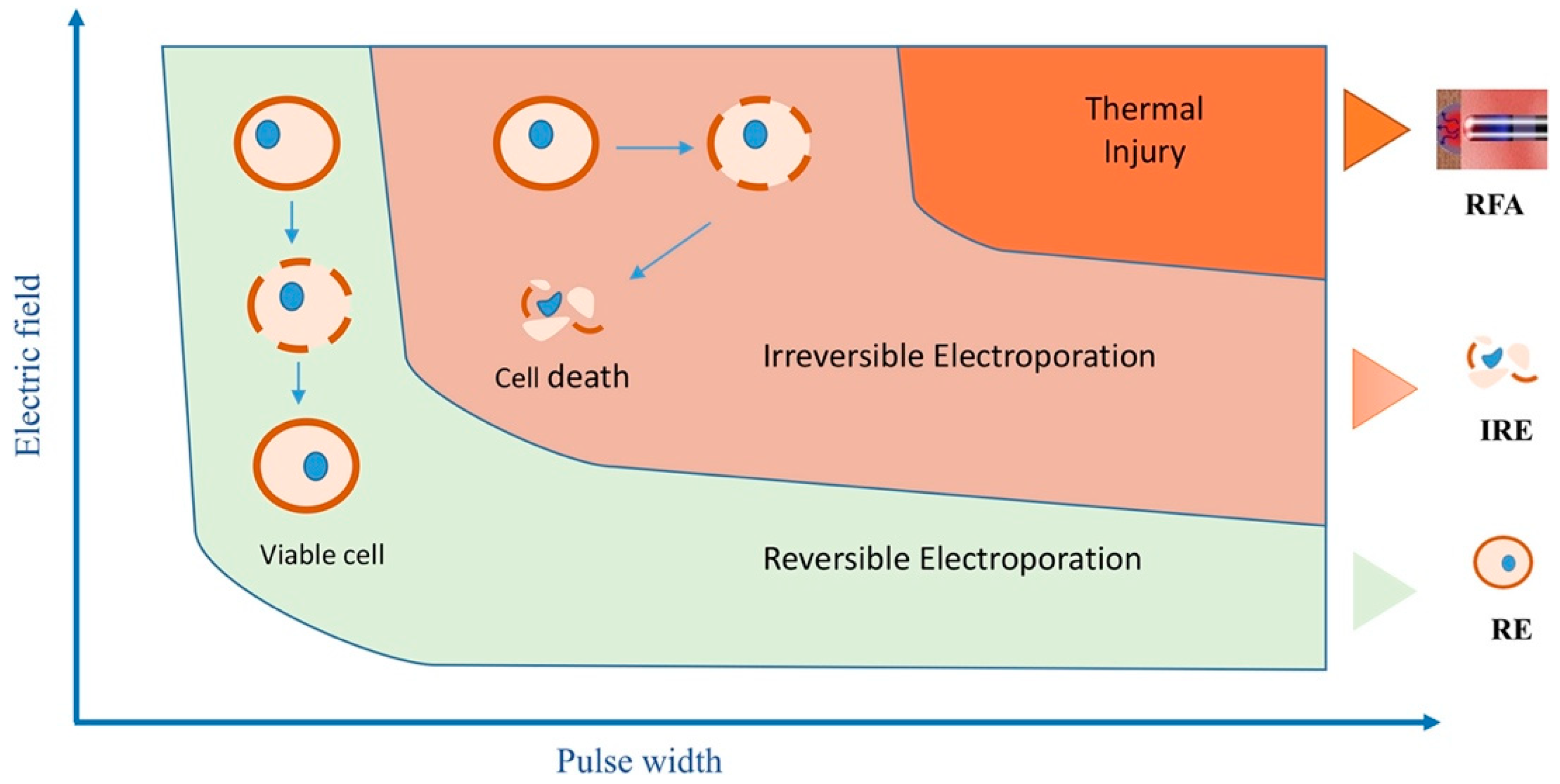
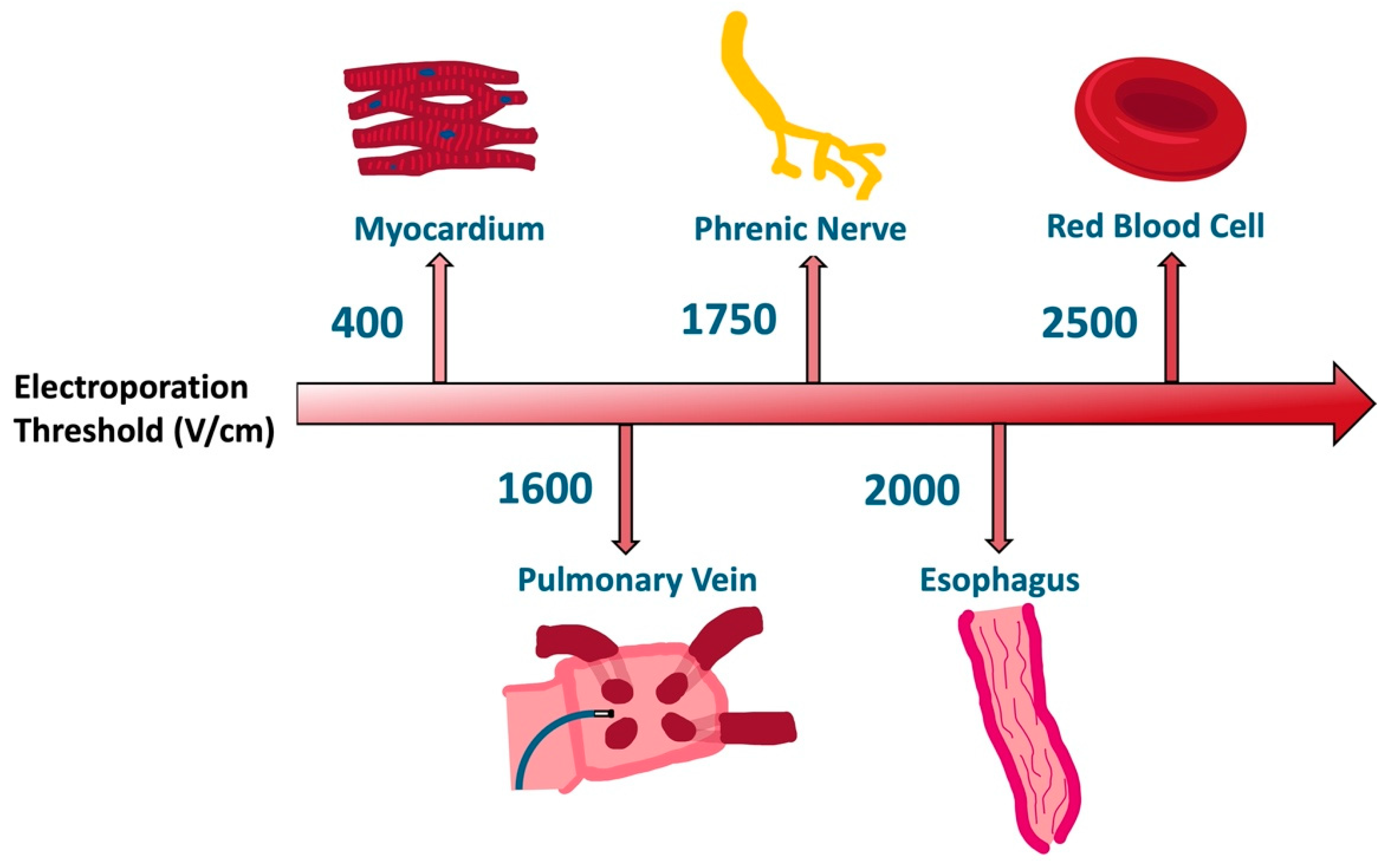
2. Electroporation Biophysics
3. Pulsed Field Ablation Technology
4. Preclinical Studies
5. Clinical Studies
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheinman, M.M.; Morady, F.; Hess, D.S.; Gonzales, R. Catheter-Induced Ablation of the Atrioventricular Junction to Control Refractory Supraventricular Arhythmias. JAMA 1982, 248, 851–855. [Google Scholar] [CrossRef]
- Gallagher, J.J.; Svenson, R.H.; Kasell, J.H.; German, L.D.; Bardy, G.H.; Broughton, A.; Critelli, G. Catheter Technique for Closed-Chest Ablation of the Atrioventricular Conduction System. N. Engl. J. Med. 1982, 306, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Haemmerich, D. Biophysics of Radiofrequency Ablation. Crit. Rev. Biomed. Eng. 2010, 38, 53–63. [Google Scholar] [CrossRef]
- Haines, D. The Biophysics of Radiofrequency Catheter Ablation in the Heart: The Importance of Temperature Monitoring. Pacing Clin. Electrophysiol. 1993, 16, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Haines, D. Biophysics of Ablation: Application to Technology. J. Cardiovasc. Electrophysiol. 2004, 15 (Suppl. S10), S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Berger, R.D.; Calkins, H. Radiofrequency Ablation: Technological Trends, Challenges, and Opportunities. Europace 2021, 23, 511–519. [Google Scholar] [CrossRef]
- Andrade, J.G.; Dubuc, M.; Guerra, P.G.; MacLe, L.; Mondésert, B.; Rivard, L.; Roy, D.; Talajic, M.; Thibault, B.; Khairy, P. The Biophysics and Biomechanics of Cryoballoon Ablation. Pacing Clin. Electrophysiol. 2012, 35, 1162–1168. [Google Scholar] [CrossRef]
- Khairy, P.; Dubuc, M. Transcatheter Cryoablation Part I: Preclinical Experience. Pacing Clin. Electrophysiol. 2008, 31, 112–120. [Google Scholar] [CrossRef]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N. Engl. J. Med. 2017, 377, 2325–2336. [Google Scholar] [CrossRef]
- Qian, P.C.; Azpiri, J.R.; Assad, J.; Gonzales Aceves, E.N.; Cardona Ibarra, C.E.; de la Pena, C.; Hinojosa, M.; Wong, D.; Fogarty, T.; Maguire, P.; et al. Noninvasive Stereotactic Radioablation for the Treatment of Atrial Fibrillation: First-in-Man Experience. J. Arrhythm. 2020, 36, 67–74. [Google Scholar] [CrossRef]
- van der Ree, M.H.; Blanck, O.; Limpens, J.; Lee, C.H.; Balgobind, B.V.; Dieleman, E.M.T.; Wilde, A.A.M.; Zei, P.C.; de Groot, J.R.; Slotman, B.J.; et al. Cardiac Radioablation—A Systematic Review. Heart Rhythm 2020, 17, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Kadir, L.A.; Stacey, M.; Barrett-Jolley, R. Emerging Roles of the Membrane Potential: Action beyond the Action Potential. Front. Physiol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 6, 63–91. [Google Scholar] [CrossRef]
- Chun, K.-R.J.; Miklavčič, D.; Vlachos, K.; Bordignon, S.; Scheer, D.; Jais, P.; Schmidt, B. State-of-the-Art Pulsed Field Ablation for Cardiac Arhrythmias: Ongoing Evolution and Future Perspective. Europace 2024, 26, euae134. [Google Scholar] [CrossRef]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of Electroporation: A Review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar]
- Tabaja, C.; Younis, A.; Hussein, A.A.; Taigen, T.L.; Nakagawa, H.; Saliba, W.I.; Sroubek, J.; Santangeli, P.; Wazni, O.M. Catheter-Based Electroporation: A Novel Technique for Catheter Ablation of Cardiac Arrhythmias. JACC Clin. Electrophysiol. 2023, 9, 2008–2023. [Google Scholar] [CrossRef] [PubMed]
- Teissié, J.; Rols, M.P. An Experimental Evaluation of the Critical Potential Difference Inducing Cell Membrane Electropermeabilization. Biophys. J. 1993, 65, 409–413. [Google Scholar] [CrossRef]
- Marszalek, P.; Liu, D.S.; Tsong, T.Y. Schwan Equation and Transmembrane Potential Induced by Alternating Electric Field. Biophys. J. 1990, 58, 1053–1058. [Google Scholar] [CrossRef]
- Golberg, A.; Yarmush, M.L. Nonthermal Irreversible Electroporation: Fundamentals, Applications, and Challenges. IEEE Trans. Biomed. Eng. 2013, 60, 707–714. [Google Scholar] [CrossRef]
- Maor, E.; Sugrue, A.; Witt, C.; Vaidya, V.R.; DeSimone, C.V.; Asirvatham, S.J.; Kapa, S. Pulsed Electric Fields for Cardiac Ablation and beyond: A State-of-the-Art Review. Heart Rhythm 2019, 16, 1112–1120. [Google Scholar] [CrossRef]
- Rois, M.-P.; Teissié, J. Experimental Evidence for the Involvement of the Cytoskeleton in Mammalian Cell Electropermeabilization. Biochim. Biophys. Acta 1992, 1111, 45–50. [Google Scholar] [CrossRef]
- Zhelev, D.V.; Needham, D. Tension-Stabilized Pores in Giant Vesicles: Determination of Pore Size and Pore Line Tension. Biochim. Biophys. Acta 1993, 1147, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, A.; Rems, L.; Kreutzer, M.T.; Boukany, P.E. Actin Networks Regulate the Cell Membrane Permeability during Electroporation. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183468. [Google Scholar] [CrossRef]
- Graybill, P.M.; Davalos, R.V. Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies. Cancers 2020, 12, 1132. [Google Scholar] [CrossRef]
- Rems, L.; Viano, M.; Kasimova, M.A.; Miklavčič, D.; Tarek, M. The Contribution of Lipid Peroxidation to Membrane Permeability in Electropermeabilization: A Molecular Dynamics Study. Bioelectrochemistry 2019, 125, 46–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, C.; Liu, Y.; Lv, Y.; Chang, T.T.; Rubinsky, B. Molecular and Histological Study on the Effects of Non-Thermal Irreversible Electroporation on the Liver. Biochem. Biophys. Res. Commun. 2018, 500, 665–670. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Polajžer, T.; Miklavčič, D. Cell Death Due to Electroporation—A Review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, A.; Maor, E.; Del-Carpio Munoz, F.; Killu, A.M.; Asirvatham, S.J. Cardiac Ablation with Pulsed Electric Fields: Principles and Biophysics. Europace 2022, 24, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.; Teissié, J. Direct Observation in the Millisecond Time Range of Fluorescent Molecule Asymmetrical Interaction with the Electropermeabilized Cell Membrane. Biophys. J. 1997, 73, 2630–2637. [Google Scholar] [CrossRef]
- Hong, J.; Stewart, M.T.; Cheek, D.S.; Francischelli, D.E.; Kirchhof, N. Cardiac Ablation via Electroporation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 3381–3384. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Rubinsky, B. Intravascular Irreversible Electroporation: Theoretical and Experimental Feasibility Study. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 2051–2054. [Google Scholar] [CrossRef]
- Ivorra, A.; Al-Sakere, B.; Rubinsky, B.; Mir, L.M. In Vivo Electrical Conductivity Measurements during and after Tumor Electroporation: Conductivity Changes Reflect the Treatment Outcome. Phys. Med. Biol. 2009, 54, 5949–5963. [Google Scholar] [CrossRef]
- Ivorra, A.; Villemejane, J.; Mir, L.M. Electrical Modeling of the Influence of Medium Conductivity on Electroporation. Phys. Chem. Chem. Phys. 2010, 12, 10055–10064. [Google Scholar] [CrossRef]
- Hansen, E.L.; Sozer, E.B.; Romeo, S.; Frandsen, S.K.; Vernier, P.T.; Gehl, J. Dose-Dependent ATP Depletion and Cancer Cell Death Following Calcium Electroporation, Relative Effect of Calcium Concentration and Electric Field Strength. PLoS ONE 2015, 10, e0122973. [Google Scholar] [CrossRef]
- Kaminska, I.; Kotulska, M.; Stecka, A.; Saczko, J.; Drag-Zalesinska, M.; Wysocka, T.; Choromanska, A.; Skolucka, N.; Nowicki, R.; Marczak, J.; et al. Electroporation-Induced Changes in Normal Immature Rat Myoblasts (H9C2). Gen. Physiol. Biophys. 2012, 31, 19–25. [Google Scholar] [CrossRef]
- Hunter, D.W.; Kostecki, G.; Fish, J.M.; Jensen, J.A.; Tandri, H. In Vitro Cell Selectivity of Reversible and Irreversible: Electroporation in Cardiac Tissue. Circ. Arrhythm. Electrophysiol. 2021, 14, 440–448. [Google Scholar] [CrossRef]
- Baena-Montes, J.M.; O’Halloran, T.; Clarke, C.; Donaghey, K.; Dunne, E.; O’Halloran, M.; Quinlan, L.R. Electroporation Parameters for Human Cardiomyocyte Ablation In Vitro. J. Cardiovasc. Dev. Dis. 2022, 9, 240. [Google Scholar] [CrossRef]
- Kos, B.; Mattison, L.; Ramirez, D.; Cindrič, H.; Sigg, D.C.; Iaizzo, P.A.; Stewart, M.T.; Miklavčič, D. Determination of Lethal Electric Field Threshold for Pulsed Field Ablation in Ex Vivo Perfused Porcine and Human Hearts. Front. Cardiovasc. Med. 2023, 10, 1160231. [Google Scholar] [CrossRef]
- Casciola, M.; Keck, D.; Feaster, T.K.; Blinova, K. Human Cardiomyocytes Are More Susceptible to Irreversible Electroporation by Pulsed Electric Field than Human Esophageal Cells. Physiol. Rep. 2022, 10, e15493. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zheng, J.; Fan, L. Nonthermal Irreversible Electroporation to the Esophagus: Evaluation of Acute and Long-Term Pathological Effects in a Rabbit Model. J. Am. Heart Assoc. 2021, 10, e020731. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Rubinski, B. Non Thermal Irreversible Electroporation: Novel Technology for Vascular Smooth Muscle Cells Ablation. PLoS ONE 2009, 4, e4757. [Google Scholar] [CrossRef]
- Venier, S.; Vaxelaire, N.; Jacon, P.; Carabelli, A.; Desbiolles, A.; Garban, F.; Defaye, P. Severe Acute Kidney Injury Related to Haemolysis after Pulsed Field Ablation for Atrial Fibrillation. Europace 2024, 26, euad371. [Google Scholar] [CrossRef]
- Zager, Y.; Kain, D.; Landa, N.; Leor, J.; Maor, E. Optimization of Irreversible Electroporation Protocols for In-Vivo Myocardial Decellularization. PLoS ONE 2016, 11, e0165475. [Google Scholar] [CrossRef]
- Yavin, H.D.; Higuchi, K.; Sroubek, J.; Younis, A.; Zilberman, I.; Anter, E. Pulsed-Field Ablation in Ventricular Myocardium Using a Focal Catheter: The Impact of Application Repetition on Lesion Dimensions. Circ. Arrhythm. Electrophysiol. 2021, 14, 819–828. [Google Scholar] [CrossRef]
- Scuderi, M.; Dermol-Černe, J.; Batista Napotnik, T.; Chaigne, S.; Bernus, O.; Benoist, D.; Sigg, D.C.; Rems, L.; Miklavčič, D. Characterization of Experimentally Observed Complex Interplay between Pulse Duration, Electrical Field Strength, and Cell Orientation on Electroporation Outcome Using a Time-Dependent Nonlinear Numerical Model. Biomolecules 2023, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.K.; Iyengar, S.; Srivathsan, K. The Promise of Pulsed Field Ablation and the Challenges Ahead. Front. Cardiovasc. Med. 2023, 10, 1235317. [Google Scholar] [CrossRef]
- Lavee, J.; Onik, G.; Mikus, P.; Rubinsky, B. A Novel Nonthermal Energy Source for Surgical Epicardial Atrial Ablation: Irreversible Electroporation. Heart Surg. Forum 2007, 10, 96–101. [Google Scholar] [CrossRef]
- Van Driel, V.J.H.M.; Neven, K.G.E.J.; Van Wessel, H.; Du Pré, B.C.; Vink, A.; Doevendans, P.A.F.M.; Wittkampf, F.H.M. Pulmonary Vein Stenosis after Catheter Ablation Electroporation versus Radiofrequency. Circ. Arrhythm. Electrophysiol. 2014, 7, 734–738. [Google Scholar] [CrossRef]
- Koruth, J.; Kuroki, K.; Iwasawa, J.; Enomoto, Y.; Viswanathan, R.; Brose, R.; Buck, E.D.; Speltz, M.; Dukkipati, S.R.; Reddy, V.Y. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ. Arrhythm. Electrophysiol. 2019, 12, e007781. [Google Scholar] [CrossRef]
- Yavin, H.; Shapira-Daniels, A.; Barkagan, M.; Sroubek, J.; Shim, D.; Melidone, R.; Anter, E. Pulsed Field Ablation Using a Lattice Electrode for Focal Energy Delivery: Biophysical Characterization, Lesion Durability, and Safety Evaluation. Circ. Arrhythm. Electrophysiol. 2020, 13, 529–538. [Google Scholar] [CrossRef]
- Hsu, J.C.; Gibson, D.; Banker, R.; Doshi, S.K.; Gidney, B.; Gomez, T.; Berman, D.; Datta, K.; Govari, A.; Natale, A. In Vivo Porcine Characterization of Atrial Lesion Safety and Efficacy Utilizing a Circular Pulsed-Field Ablation Catheter Including Assessment of Collateral Damage to Adjacent Tissue in Supratherapeutic Ablation Applications. J. Cardiovasc. Electrophysiol. 2022, 33, 1480–1488. [Google Scholar] [CrossRef]
- Wittkampf, F.H.M.; Van Driel, V.J.; Van Wessel, H.; Neven, K.G.E.J.; Gründeman, P.F.; Vink, A.; Loh, P.; Doevendans, P.A. Myocardial Lesion Depth with Circular Electroporation Ablation. Circ. Arrhythm. Electrophysiol. 2012, 5, 581–586. [Google Scholar] [CrossRef]
- Livia, C.; Sugrue, A.; Witt, T.; Polkinghorne, M.D.; Maor, E.; Kapa, S.; Lehmann, H.I.; DeSimone, C.V.; Behfar, A.; Asirvatham, S.J.; et al. Elimination of Purkinje Fibers by Electroporation Reduces Ventricular Fibrillation Vulnerability. J. Am. Heart Assoc. 2018, 7, e009070. [Google Scholar] [CrossRef]
- Im, S.I.; Higuchi, S.; Lee, A.; Stillson, C.; Buck, E.; Morrow, B.; Schenider, K.; Speltz, M.; Gerstenfeld, E.P. Pulsed Field Ablation of Left Ventricular Myocardium in a Swine Infarct Model. JACC Clin. Electrophysiol. 2022, 8, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Zilberman, I.; Krywanczyk, A.; Higuchi, K.; Yavin, H.D.; Sroubek, J.; Anter, E. Effect of Pulsed-Field and Radiofrequency Ablation on Heterogeneous Ventricular Scar in a Swine Model of Healed Myocardial Infarction. Circ. Arrhythm. Electrophysiol. 2022, 15, 683–692. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Koruth, J.; Jais, P.; Petru, J.; Timko, F.; Skalsky, I.; Hebeler, R.; Labrousse, L.; Barandon, L.; Kralovec, S.; et al. Ablation of Atrial Fibrillation With Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin. Electrophysiol. 2018, 4, 987–995. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Anter, E.; Rackauskas, G.; Peichl, P.; Koruth, J.S.; Petru, J.; Funasako, M.; Minami, K.; Natale, A.; Jais, P.; et al. Lattice-Tip Focal Ablation Catheter That Toggles Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation: A First-in-Human Trial. Circ. Arrhythm. Electrophysiol. 2020, 13, 483–495. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Dukkipati, S.R.; Neuzil, P.; Anic, A.; Petru, J.; Funasako, M.; Cochet, H.; Minami, K.; Breskovic, T.; Sikiric, I.; et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin. Electrophysiol. 2021, 7, 614–627. [Google Scholar] [CrossRef]
- Loh, P.; Van Es, R.; Groen, M.H.A.; Neven, K.; Kassenberg, W.; Wittkampf, F.H.M.; Doevendans, P.A. Pulmonary Vein Isolation With Single Pulse Irreversible Electroporation: A First in Human Study in 10 Patients With Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, 1083–1091. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Anic, A.; Koruth, J.; Petru, J.; Funasako, M.; Minami, K.; Breskovic, T.; Sikiric, I.; Dukkipati, S.R.; Kawamura, I.; et al. Pulsed Field Ablation in Patients With Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1068–1080. [Google Scholar] [CrossRef]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 1422–1432. [Google Scholar] [CrossRef]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes From the MANIFEST-PF Registry. Circulation 2023, 148, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Peichl, P.; Anter, E.; Rackauskas, G.; Petru, J.; Funasako, M.; Minami, K.; Koruth, J.S.; Natale, A.; Jais, P.; et al. A Focal Ablation Catheter Toggling Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 1786–1801. [Google Scholar] [CrossRef]
- Duytschaever, M.; De Potter, T.; Grimaldi, M.; Anic, A.; Vijgen, J.; Neuzil, P.; Van Herendael, H.; Verma, A.; Skanes, A.; Scherr, D.; et al. Paroxysmal Atrial Fibrillation Ablation Using a Novel Variable-Loop Biphasic Pulsed Field Ablation Catheter Integrated With a 3-Dimensional Mapping System: 1-Year Outcomes of the Multicenter InspIRE Study. Circ. Arrhythm. Electrophysiol. 2023, 16, 119–128. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Calkins, H.; Mansour, M.; Wazni, O.M.; Di Biase, L.; Bahu, M.M.; Newton, D.W.; Liu, C.F.; Sauer, W.H.; Goyal, S.; et al. Long-Term Safety and Effectiveness after Paroxysmal Atrial Fibrillation Pulsed Field Ablation from the U.S. Multicenter AdmIRE Study. In Heart Rhythm; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1197–1198. [Google Scholar] [CrossRef]
- Urbanek, L.; Bordignon, S.; Schaack, D.; Chen, S.; Efe, T.H.; Ebrahimi, R.; Pansera, F.; Hirokami, J.; Plank, K.; Koch, A.; et al. Pulsed Field Versus Cryoballoon Pulmonary Vein Isolation for Atrial Fibrillation: Efficacy, Safety, and Long-term Follow-up in a 400-Patient Cohort. Circ. Arrhythm. Electrophysiol. 2023, 16, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Schipper, J.H.; Steven, D.; Lüker, J.; Wörmann, J.; van den Bruck, J.H.; Filipovic, K.; Dittrich, S.; Scheurlen, C.; Erlhöfer, S.; Pavel, F.; et al. Comparison of Pulsed Field Ablation and Cryoballoon Ablation for Pulmonary Vein Isolation. J. Cardiovasc. Electrophysiol. 2023, 34, 2019–2026. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef]
- Anter, E.; Mansour, M.; Nair, D.G.; Sharma, D.; Taigen, T.L.; Neuzil, P.; Kiehl, E.L.; Kautzner, J.; Osorio, J.; Mountantonakis, S.; et al. Dual-Energy Lattice-Tip Ablation System for Persistent Atrial Fibrillation: A Randomized Trial. Nat. Med. 2024, 30, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Neuzil, P.; Petru, J.; Funasako, M.; Koruth, J.S.; Skoda, J.; Kralovec, S.; Reddy, V.Y. AF Ablation Using a Novel “Single-Shot” Map-and-Ablate Spherical Array Pulsed Field Ablation Catheter: 1-Year Outcomes of the First-in-Human PULSE-EU Trial. Heart Rhythm 2024, 21, 1218–1226. [Google Scholar] [CrossRef]
- Anter, E.; Mansour, M.; Nair, D.G.; Sharma, D.; Taigen, T.L.; Neuzil, P.; Kiehl, E.L.; Kautzner, J.; Osorio, J.; Mountantonakis, S.; et al. Real World Data Collection in Subjects Treated with the Farapulse Pulsed Field Ablation System (FARADISE). In Heart Rhythm; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1196–1198. [Google Scholar] [CrossRef]
- Ekanem, E.; Neuzil, P.; Reichlin, T.; Kautzner, J.; van der Voort, P.; Jais, P.; Chierchia, G.B.; Bulava, A.; Blaauw, Y.; Skala, T.; et al. Safety of Pulsed Field Ablation in More Than 17,000 Patients With Atrial Fibrillation in the MANIFEST-17K Study. Nat. Med. 2024, 30, 2020–2029. [Google Scholar] [CrossRef]
- Musikantow, D.R.; Neuzil, P.; Anic, A.; Balin, P.; Petru, J.; Funasako, M.; Lisica, L.; Jurisic, Z.; Jais, P.; Reddy, V.Y. Long-Term Clinical Outcomes of Pulsed Field Ablation in the Treatment of Paroxysmal Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 2001–2003. [Google Scholar] [CrossRef]
- Turagam, M.K.; Neuzil, P.; Petru, J.; Funasako, M.; Koruth, J.S.; Reinders, D.; Skoda, J.; Kralovec, S.; Reddy, V.Y. PV Isolation Using a Spherical Array PFA Catheter: Application Repetition and Lesion Durability (PULSE-EU Study). JACC Clin. Electrophysiol. 2023, 9, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Im, S.I.; Stillson, C.; Buck, E.D.; Jerrell, S.; Schneider, C.W.; Speltz, M.; Gerstenfeld, E.P. Effect of Epicardial Pulsed Field Ablation Directly on Coronary Arteries. JACC Clin. Electrophysiol. 2022, 8, 1486–1496. [Google Scholar] [CrossRef]
- Kuroki, K.; Whang, W.; Eggert, C.; Lam, J.; Leavitt, J.; Kawamura, I.; Reddy, A.; Morrow, B.; Schneider, C.; Petru, J.; et al. Ostial Dimensional Changes after Pulmonary Vein Isolation: Pulsed Field Ablation vs Radiofrequency Ablation. Heart Rhythm 2020, 17, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.; Kollias, G.; Pürerfellner, H.; Narasimhan, C.; Osorio, J.; Lip, G.Y.H.; Gupta, D. Silent Cerebral Lesions Following Catheter Ablation for Atrial Fibrillation: A State-of-the-Art Review. Europace 2023, 25, euad151. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Li, W.; Li, F.; Tong, Y.; Cao, Y.; Zeng, R. Safety and Efficacy of Pulse Field Ablation in the Treatment of Atrial Fibrillation and Its Comparison with Traditional Thermal Ablation: A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2024, 25, 415. [Google Scholar] [CrossRef]
- Ouss, A.; van Stratum, L.; van der Voort, P.; Dekker, L. First in Human Pulsed Field Ablation to Treat Scar-Related Ventricular Tachycardia in Ischemic Heart Disease: A Case Report. J. Interv. Card. Electrophysiol. 2023, 66, 509–510. [Google Scholar] [CrossRef]
- Lozano-Granero, C.; Hirokami, J.; Franco, E.; Tohoku, S.; Matía-Francés, R.; Schmidt, B.; Hernández-Madrid, A.; Zamorano Gómez, J.L.; Moreno, J.; Chun, J. Case Series of Ventricular Tachycardia Ablation With Pulsed-Field Ablation: Pushing Technology Further (Into the Ventricle). JACC Clin. Electrophysiol. 2023, 9, 1990–1994. [Google Scholar] [CrossRef]
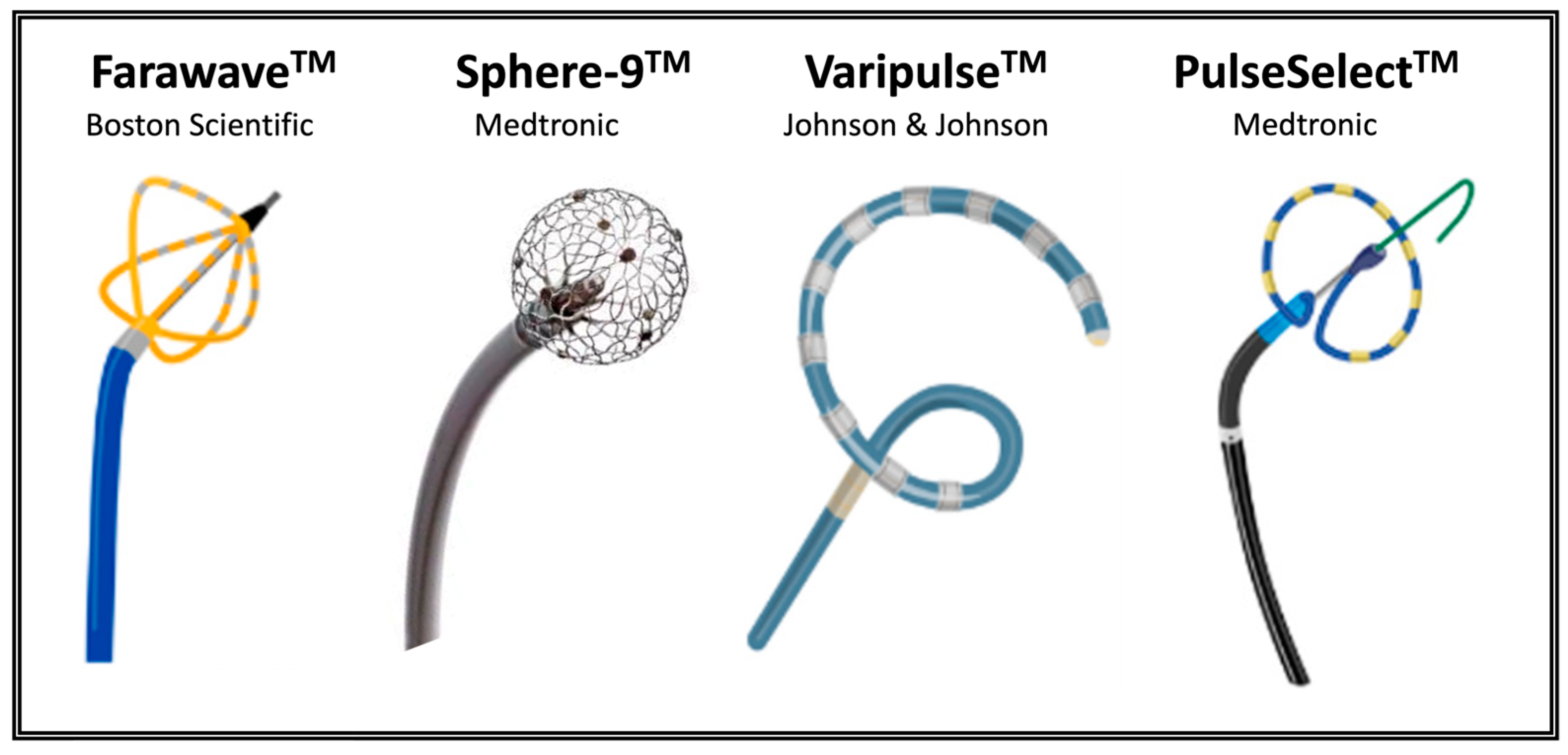
| Study, Year | Catheter (Company) | Methods | Efficacy Outcome | Safety Outcome |
| Reddy et al., 2018 [56] | Endocardial: Basket/Flower Pentaspline, 12F (Iowa Approach Inc., Menlo Park, CA, USA) Epicardial: Linear cinching (Iowa Approach Inc.) | NRT n = 22 (15 endocardial, 7 epicardial) PAF Acute F/U | 100% acute PVI (endocardial) 86% acute PVI (epicardial) | No adverse events |
| Reddy et al., 2020 [57] | Lattice-tip, 7.5F (Sphere-9, Affera Inc., Watertown, MA, USA) | NRT n = 76 PAF or PersAF 12-month F/U | 100% acute PVI | No adverse events |
| IMPULSE, 2020 [58,59] | Basket/Flower Pentaspline, 12F (Farawave, Farapulse Inc., Newton, MA, USA) | NRT n = 40 PAF Acute and 12-month F/U | Combined analysis (total n = 121): 100% acute PVI 96% PV durability 85% free of atrial arrhythmia at 12-months | Combined analysis (total n = 121): 1 cardiac tamponade 1 pericardial effusion 1 vascular hematoma 1 TIA |
| PEFCAT, 2020 [58,59] | Basket/Flower Pentaspline, 12F (Farawave, Farapulse Inc.) | NRT n = 71 PAF Acute and 12-month F/U | ||
| PEFCAT II, 2020 [59] | PVI: Basket/Flower Pentaspline, 12F (Farawave, Farapulse Inc.) CTI: Deflectable tetraspline, 12F (Faraflex, Farapulse Inc.) | NRT n = 10 PAF Acute and 12-month F/U | ||
| Loh et al., 2020 [60] | Circular 14-polar, 8F (not specified) | NRT n = 10 PAF or PersAF Acute F/U | 100% acute PVI | No adverse events |
| PersAFOne, 2020 [61] | PVI/LAPW: Basket/Flower Pentaspline, 12F (Farawave, Farapulse Inc.) CTI: Deflectable tetraspline, 12F (Faraflex, Farapulse Inc.) | NRT n = 25 PersAF Acute and 2–3 month F/U | 100% acute PVI and LAPW ablation. 100% successful CTI (n = 13) Durability at 2–3 months: 96% PVI and 100% LAPW | No adverse events |
| PULSED AF, 2022 [62] | Circular lasso-type 9-electrode, 9F (PulseSelect, Medtronic, Inc., St. Brampton, ON, USA) | NRT n = 300 PAF or PersAF Acute and 12-month F/U | 100% acute PVI Freedom from atrial arrhythmia at 12 months: 69.5% (paroxysmal), 62.3% (persistent) | 1 stroke 1 cardiac tamponade 2 non-procedure related deaths during the F/U period |
| MANIFEST-PF, 2022 [63] | Basket/Flower Pentaspline, 12F (Farawave, Farapulse Inc.) | Retrospective registry n = 1568 PAF or PersAF Acute and 12-month F/U | 99.2% acute PVI Freedom from atrial arrhythmia at 12 months: 81.6% (paroxysmal), 71.5% (persistent) | Procedure-related adverse events: 1.9% (n = 30) 1 death, 18 tamponade, 6 stroke, 2 vascular complications, 2 coronary artery spasm, 1 persistent phrenic nerve palsy |
| Reddy et al., 2023 [64] | Lattice-tip, bidirectional deflectable, 8F (Sphere-9, Medtronic, Inc.) | NRT n = 178 PAF or PersAF Acute and 12-month F/U | 100% acute PVI, LA roof, mitral, and CTI. Freedom from atrial arrhythmia at 12 months: 78.3% (paroxysmal), 77.9% (persistent) | 1 pericardial effusion |
| inspIRE, 2023 [65] | Variable-loop circular 10-electrode, 8.5F (Varipulse, Biosense Webster, Inc.) | NRT n = 226 PAF Acute and 12-month F/U | 100% acute PVI Freedom from atrial arrhythmia at 12 months: 78.9% | No adverse events 8 SCLs. |
| admIRE, 2023 [66] | Variable-loop circular 10-electrode, 8.5F (Varipulse, Biosense Webster, Inc.) | NRT n = 277 PAF Acute and 12-month F/U | 100% acute PVI Freedom from atrial arrhythmia at 12 months: 76.2% | Procedure-related adverse events: 2.9% (n = 8) including 3 cardiac tamponade |
| Urbanek et al., 2023 [67] | PFA: Basket/Flower Pentaspline, 12F (Farawave, Boston Scientific Inc.) CB: 28-mm (Arctic Front Advance, Medtronic, Inc.) | NRT n = 400 (200 PFA, 200 CB) PAF or PersAF Acute and 12-month F/U | PFA: 100% acute PVI, freedom from atrial arrhythmia at 12 months: 74.5% CB: 98% acute PVI, freedom from atrial arrhythmia at 12 months: 78.1% | PFA: 5 vascular access complication, 1 tamponade. CB: 7 vascular access complication, 3 persistent phrenic nerve palsy, 1 stroke/TIA, 1 esophageal injury, 1 hemoptysis |
| Schipper et al., 2023 [68] | PFA: Basket/Flower Pentaspline, 12F (Farawave, Boston Scientific Inc., Marlborough, MA, USA) CB: 31 mm (POLARx, Boston Scientific Inc.) | NRT n = 108 (54 PFA, 54 CB) PAF or PersAF Acute and 273 ± 129 day F/U | PFA: 100% acute PVI, 74% arrhythmia free survival CB: 99.5% acute PVI, 72% arrhythmia free survival | PFA: 2 tamponade CB: 2 vascular access complication, 1 TIA, 2 phrenic nerve injury |
| ADVENT, 2023 [69] | Basket/Flower Pentaspline, 12F (Farawave, Boston Scientific Inc.) | RCT, non-inferiority n = 706, 1:1 randomization to PFA vs. conventional thermal ablation (RF or cryoballoon) Acute and 12-month F/U | 99.6% acute PVI (PFA) and 99.8% acute PVI (Thermal) Primary outcome at 12-months (freedom from composite of PVI procedural failure, recurrent atrial arrhythmia, AAD use and repeat ablation): PFA 73.3% vs. Thermal 71.3%, posterior probability of non-inferiority >0.999 | Serious adverse events: PFA 2.1% (n = 6) vs. thermal 1.5% (n = 4), posterior probability of non-inferiority >0.999 PFA: 1 death, 2 tamponade or perforation, 1 TIA, 1 pericarditis, 1 pulmonary edema, 1 vascular access complication Thermal: 1 stroke, 1 pulmonary edema, 2 vascular access complication |
| SPHERE Per-AF, 2024 [70] | Lattice-tip, bidirectional deflectable, 8F (Sphere-9, Medtronic, Inc.) | RCT, non-inferiority n = 420, 1:1 randomization PFA/RF vs. RF alone 12-month F/U | Freedom from atrial arrhythmia at 12 months: 76.7% (PFA/RF) vs. 72.8% (RF), p < 0.0001 for non-inferiority | Serious adverse events: PFA/RF 1.4% (n = 3) vs. RF 1.0% (n = 2), p < 0.0001 for non-inferiority. PFA/RF: 1 pulmonary edema, 1 COPD exacerbation, 1 hemoptysis RF: 2 pulmonary edema |
| PULSE-EU, 2024 [71] | 30 mm spherical array 122-electrode, 16F (Globe, Kardium Inc., Burnaby, BC, Canada) | NRT n = 48 PAF or PersAF Acute and 12-month F/U | 100% acute PVI and posterior wall, 91% mitral isthmus 93.5% PVI durability at 3 months. Freedom from atrial arrhythmia at 12 months: 84.2% (paroxysmal), 80.0% (persistent) | 1 pericarditis |
| FARADISE, 2024 [72] | Basket/Flower Pentaspline, 12F (Farawave, Boston Scientific Inc.) | Prospective global registry n = 986 PAF or PersAF Acute F/U | 98.6% acute PVI | Procedure-related adverse events: 3.07% (n = 32) 1 vascular complication, 1 air embolism, 1 stroke, 1 hemolysis, 2 pericarditis, 2 pericardial effusion, 1 tamponade |
| MANIFEST-17K, 2024 [73] | Basket/Flower Pentaspline, 12F (Farawave, Boston Scientific Inc.) | Retrospective observational n = 17,642 PAF or PersAF Safety outcomes | Efficacy data not reported | Major adverse events 173 (0.98%), death 0.03%, stroke 0.12%, esophageal fistula 0%, PV stenosis 0%, phrenic nerve injury 0%, tamponade 0.36%, vascular complication with intervention 0.30%, coronary artery spasm 0.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sullivan, A.P.; Aguilar, M.; Laksman, Z. Pulsed Field Ablation: A Review of Preclinical and Clinical Studies. Bioengineering 2025, 12, 329. https://doi.org/10.3390/bioengineering12040329
Sullivan AP, Aguilar M, Laksman Z. Pulsed Field Ablation: A Review of Preclinical and Clinical Studies. Bioengineering. 2025; 12(4):329. https://doi.org/10.3390/bioengineering12040329
Chicago/Turabian StyleSullivan, Andrew P., Martin Aguilar, and Zachary Laksman. 2025. "Pulsed Field Ablation: A Review of Preclinical and Clinical Studies" Bioengineering 12, no. 4: 329. https://doi.org/10.3390/bioengineering12040329
APA StyleSullivan, A. P., Aguilar, M., & Laksman, Z. (2025). Pulsed Field Ablation: A Review of Preclinical and Clinical Studies. Bioengineering, 12(4), 329. https://doi.org/10.3390/bioengineering12040329







