Measurement of Oxygen Transfer Rate and Specific Oxygen Uptake Rate of h-iPSC Aggregates in Vertical Wheel Bioreactors to Predict Maximum Cell Density Before Oxygen Limitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioreactor Set-Up
2.2. kLa Measurement
2.3. Static and Suspension iPSC Culture for sOUR Measurement
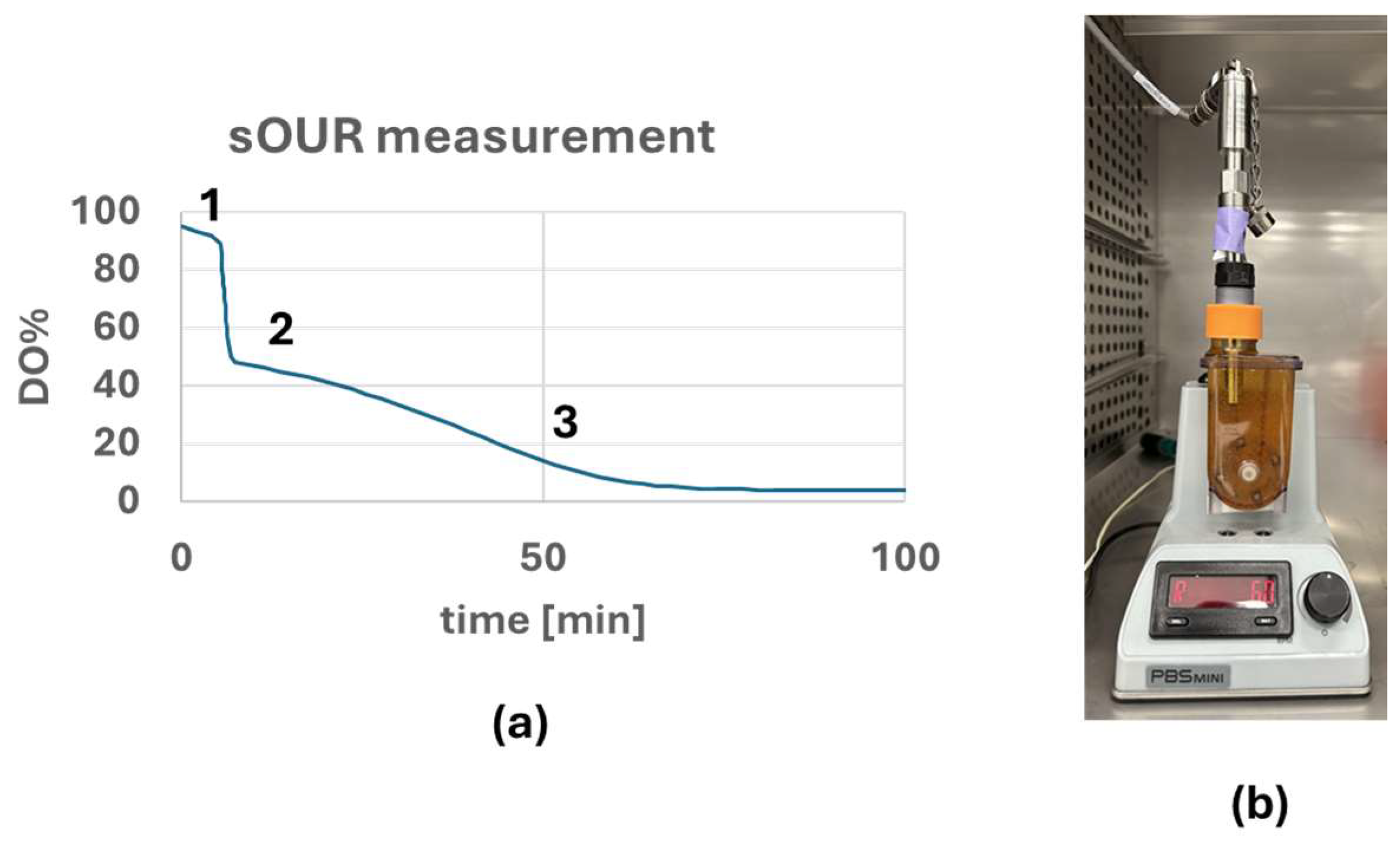
2.4. sOUR Measurement
3. Results
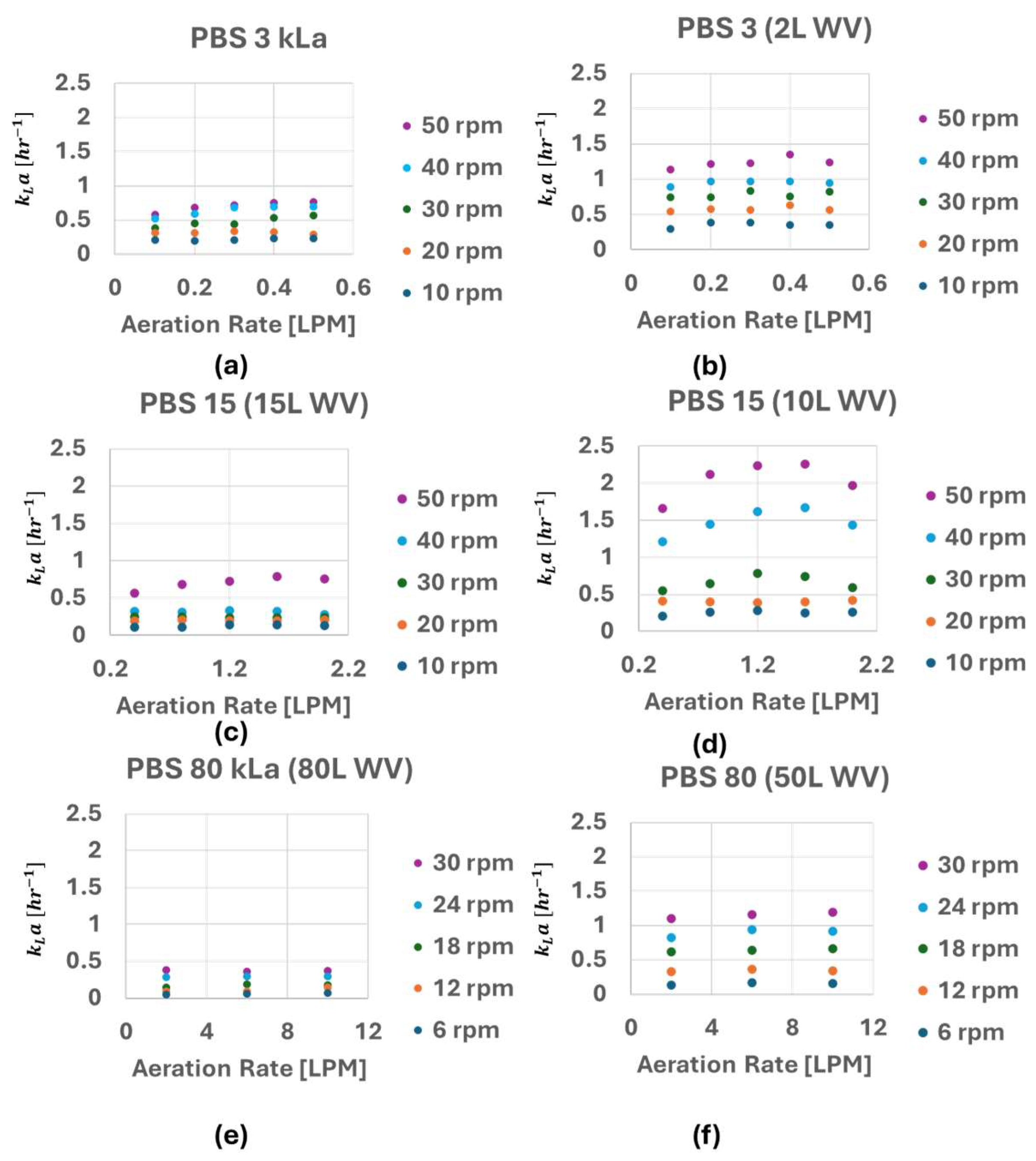
3.1. kLa of PBS 3, 15 and 80 Bioreactor
3.2. sOUR Measurement
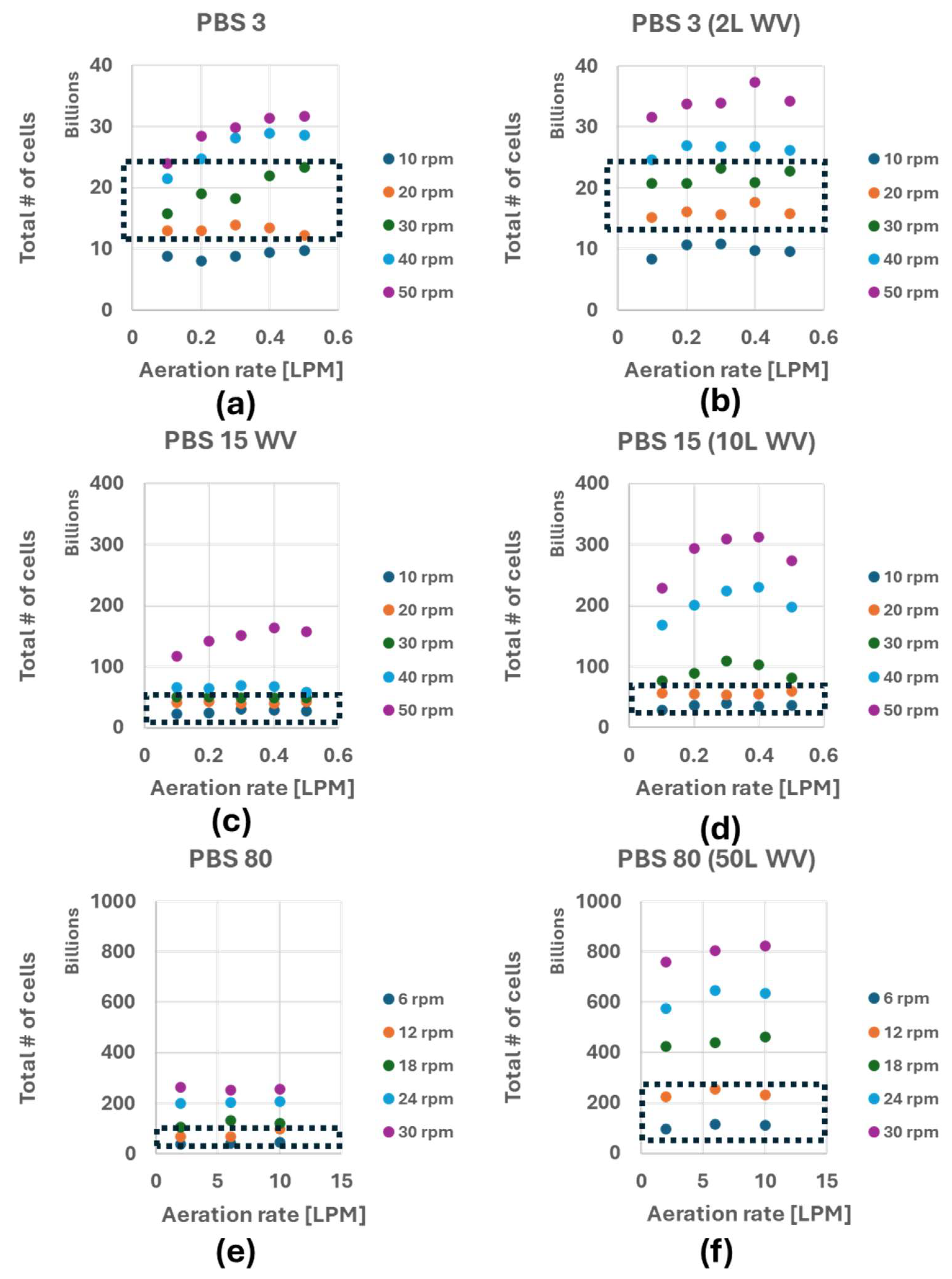
3.3. Maximum Number of Cells Before Oxygen Limitation
4. Discussion
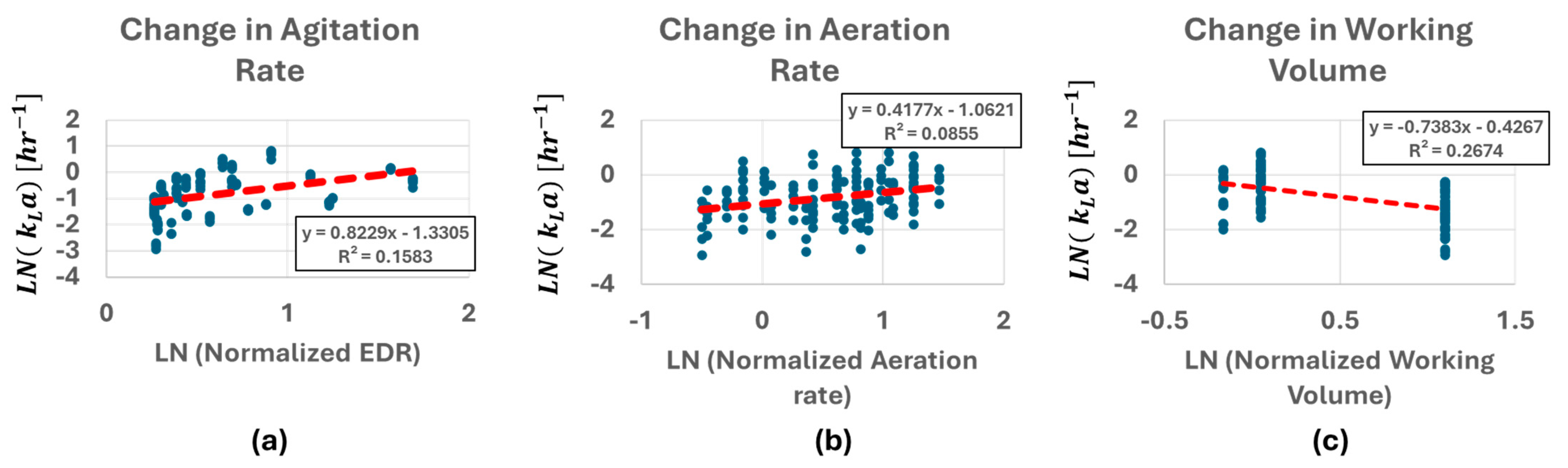
4.1. Impact of Agitation Rate, Main Gals Flow Rate and Working Volume on kLa
4.2. Benefit of Combining the OTR and OUR to Predict Maximum Cell Density Before Oxygen Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuesta-Gomez, N.; Verhoeff, K.; Jasra, I.T.; Pawlick, R.; Dadheech, N.; Shapiro, A.M.J. Characterization of Stem-Cell-Derived Islets During Differentiation and After Implantation; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Ramos, S.A.; Morton, J.J.; Yadav, P.; Reed, B.; Alizadeh, S.I.; Shilleh, A.H.; Perrenoud, L.; Jaggers, J.; Kappler, J.; Jimeno, A.; et al. Generation of functional human thymic cells from induced pluripotent stem cells. J. Allergy Clin. Immunol. 2022, 149, 767–781. [Google Scholar] [CrossRef]
- Goodman, S.; Lyon, K.; Hartwig, K.; Peng, S.; Li, Q.; Murray, D.; Bernareggi, D.; Gonsalves, C.; Schabla, M.; Zhang, G.; et al. Engineering Superaffinity Antibody Dependent Cellular Cytotoxcity Receptors into iPSC-Derived NK Cells As Next-Generation Immunotherapies for Cancer. Blood 2023, 142, 2060. [Google Scholar] [CrossRef]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous Induced Pluripotent Stem Cell–Based Cell Therapies: Promise, Progress, and Challenges. Curr. Protoc. 2021, 1, e88. [Google Scholar] [CrossRef]
- Ghasroldasht, M.M.; Seok, J.; Park, H.S.; Ali, F.B.L.; Al-Hendy, A. Stem Cell Therapy: From Idea to Clinical Practice. Int. J. Mol. Sci. 2022, 23, 2850. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Wan, Z.; Yang, C.; Shen, X.; Wang, R.; Zhang, H.; Yang, R.; Li, J.; Song, Y.; Su, H. Advances and clinical challenges of mesenchymal stem cell therapy. Front. Immunol. 2024, 15, 1421854. [Google Scholar] [CrossRef]
- Lidén, G. Understanding the bioreactor. Bioprocess Biosyst. Eng. 2002, 24, 273–279. [Google Scholar] [CrossRef]
- Dang, T.; Borys, B.S.; Kanwar, S.; Colter, J.; Worden, H.; Blatchford, A.; Croughan, M.S.; Lee, B.; Kallos, M.S.; Jung, S. Computational fluid dynamic characterization of vertical-wheel bioreactors used for effective scale-up of human induced pluripotent stem cell aggregate culture. Can. J. Chem. Eng. 2021, 99, 2536–2553. [Google Scholar] [CrossRef]
- Vallabhaneni, H.; Shah, T.; Shah, P.; Hursh, D.A. Suspension culture on microcarriers and as aggregates enables expansion and differentiation of pluripotent stem cells (PSCs). Cytotherapy 2023, 25, 993–1005. [Google Scholar] [CrossRef]
- Cuesta-Gomez, N.; Verhoeff, K.; Dadheech, N.; Dang, T.; Jasra, I.T.; de Leon, M.B.; Pawlick, R.; Marfil-Garza, B.; Anwar, P.; Razavy, H.; et al. RESEARCH Open Access Suspension culture improves iPSC expansion and pluripotency phenotype. Stem. Cell. Res. Ther. 2023, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Aunins, J.G.; Henzler, H.J. Aeration in Cell Culture Bioreactors. In Biotechnology: Second, Completely Revised Edition; Wiley: Hoboken, NJ, USA, 2008; Volume 3, pp. 219–281. [Google Scholar] [CrossRef]
- Zhu, Y.; Cuenca, J.V.; Zhou, W.; Varma, A. NS0 cell damage by high gas velocity sparging in protein-free and cholesterol-free cultures. Biotechnol. Bioeng. 2008, 101, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, B.; Humphrey, A.E.; Taguchi, H. Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Biotechnol. Bioeng. 1967, 9, 533–544. [Google Scholar] [CrossRef]
- López, J.L.C.; Porcel, E.M.R.; Alberola, I.O.; Martin, M.M.B.; Pérez, J.A.S.; Sevilla, J.M.F.; Chisti, Y. Simultaneous Determination of Oxygen Consumption Rate and Volumetric Oxygen Transfer Coefficient in Pneumatically Agitated Bioreactors. Ind. Eng. Chem. Res. 2005, 45, 1167–1171. [Google Scholar] [CrossRef]
- Super, A.; Jaccard, N.; Marques, M.P.C.; Macown, R.J.; Griffin, L.D.; Veraitch, F.S.; Szita, N. Real-time monitoring of specific oxygen uptake rates of embryonic stem cells in a microfluidic cell culture device. Biotechnol. J. 2016, 11, 1179–1189. [Google Scholar] [CrossRef]
- Nishimura, K.; Ishiwata, H.; Sakuragi, Y.; Hayashi, Y.; Fukuda, A.; Hisatake, K. Live-cell imaging of subcellular structures for quantitative evaluation of pluripotent stem cells. Sci. Rep. 2019, 9, 1777. [Google Scholar] [CrossRef]
- Mignot, G.; Faure, T.; Ganne, V.; Arbeille, B.; Pavirani, A.; Romet-Lemonne, J.L. Production of recombinant Von Willebrand factor by CHO cells cultured in macroporous microcarriers. Cytotechnology 1990, 4, 163–171. [Google Scholar] [CrossRef]
- Heidemann, R.; Lütkemeyer, D.; Büntemeyer, H.; Lehmann, J. Effects of dissolved oxygen levels and the role of extra- and intracellular amino acid concentrations upon the metabolism of mammalian cell lines during batch and continuous cultures. Cytotechnology 1998, 26, 185. [Google Scholar] [CrossRef]
- Guarino, R.D.; Dike, L.E.; Haq, T.A.; Rowley, J.A.; Pitner, J.B.; Timmins, M.R. Method for determining oxygen consumption rates of static cultures from microplate measurements of pericellular dissolved oxygen concentration. Biotechnol. Bioeng. 2004, 86, 775–787. [Google Scholar] [CrossRef]
- Aunins, J.G.; Goldstein, J.M.; Croughan, M.S.; Wang, D.I.C. Engineering Developments in the Homogenoeous Culture of Animal Cells: Oxygenation of Reactors and Scale-Up. Biotechnol. Bioeng. Symp. 1986, 17, 699–723. [Google Scholar]
- Fuchs, R.; Ryu, D.D.Y.; Humphrey, A.E. Effect of Surface aeration on Scale-Up procedures for fermentation processes. Ind. Eng. Chem. Process Des. Dev. 1971, 10, 190–196. [Google Scholar]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Croughan, M.S.; Giroux, D.; Agbojo, O.M.; McCain, E.; Starkweather, N.; Guerra, S.; Hashimura, Y.; Lee, B.; Jung, S. Initial power measurements for a family of novel vertical-wheel bioreactors. Can. J. Chem. Eng. 2023, 101, 1235–1246. [Google Scholar] [CrossRef]
- Oxygen-Solubility in Fresh and Sea Water vs. Temperature. Available online: https://www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html (accessed on 18 February 2025).
- Neto, P.M.; Hashimura, Y.; Jung, S.; Pedras, B.; Berberan-Santos, M.N.; Palmeira, T.; Lee, B.; Cabral, J.M.S.; Geralder, V.; Rodrigues, C.A.V.; et al. Characterization of the Aeration and Hydrodynamics in Vertical-WheelTM Bioreactors. Bioengineering 2022, 9, 386. [Google Scholar] [CrossRef]
- Hu, W.; Berdugo, C.; Chalmers, J.J. The potential of hydrodynamic damage to animal cells of industrial relevance: Current understanding. Cytotechnology 2011, 63, 445–460. [Google Scholar] [CrossRef]
- Borys, B.S.; Le, A.; Roberts, E.L.; Dang, T.; Rohani, L.; Hsu, C.Y.-M.; Wyma, A.A.; Rancourt, D.E.; Gates, I.D.; Kallos, M.S. Using computational fluid dynamics (CFD) modeling to understand murine embryonic stem cell aggregate size and pluripotency distributions in stirred suspension bioreactors. J. Biotechnol. 2019, 304, 16–27. [Google Scholar] [CrossRef]
- Seidel, S.; Maschke, R.W.; Mozaffari, F.; Eibl-Schindler, R.; Eibl, D. Improvement of HEK293 Cell Growth by Adapting Hydrodynamic Stress and Predicting Cell Aggregate Size Distribution. Bioengineering 2023, 10, 478. [Google Scholar] [CrossRef]
- Hewitt, C.J.; Lee, K.; Nienow, A.W.; Thomas, R.J.; Smith, M.; Thomas, C.R. Expansion of human mesenchymal stem cells on microcarriers. Biotechnol. Lett. 2011, 33, 2325–2335. [Google Scholar] [CrossRef]

| PBS 3 2 L WV | PBS 3 3 L WV | PBS 15 10 L WV | PBS 15 15 L WV | PBS 80 50 L WV | PBS 80 80 L WV | |
|---|---|---|---|---|---|---|
| X at lower rpm * [cells/mL] | 6.64 × 10−6 | 4.32 × 10−6 | 2.91 × 10−6 | 1.49 × 10−6 | 1.91 × 10−6 | 7.47 × 10−5 |
| X at higher rpm * [cells/mL] | 1.14 × 10−7 | 7.81 × 10−6 | 5.90 × 10−6 | 2.82 × 10−6 | 5.07 × 10−6 | 1.99 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Agbojo, O.; Jung, S.; Croughan, M. Measurement of Oxygen Transfer Rate and Specific Oxygen Uptake Rate of h-iPSC Aggregates in Vertical Wheel Bioreactors to Predict Maximum Cell Density Before Oxygen Limitation. Bioengineering 2025, 12, 332. https://doi.org/10.3390/bioengineering12040332
Kim J, Agbojo O, Jung S, Croughan M. Measurement of Oxygen Transfer Rate and Specific Oxygen Uptake Rate of h-iPSC Aggregates in Vertical Wheel Bioreactors to Predict Maximum Cell Density Before Oxygen Limitation. Bioengineering. 2025; 12(4):332. https://doi.org/10.3390/bioengineering12040332
Chicago/Turabian StyleKim, James, Omokhowa Agbojo, Sunghoon Jung, and Matt Croughan. 2025. "Measurement of Oxygen Transfer Rate and Specific Oxygen Uptake Rate of h-iPSC Aggregates in Vertical Wheel Bioreactors to Predict Maximum Cell Density Before Oxygen Limitation" Bioengineering 12, no. 4: 332. https://doi.org/10.3390/bioengineering12040332
APA StyleKim, J., Agbojo, O., Jung, S., & Croughan, M. (2025). Measurement of Oxygen Transfer Rate and Specific Oxygen Uptake Rate of h-iPSC Aggregates in Vertical Wheel Bioreactors to Predict Maximum Cell Density Before Oxygen Limitation. Bioengineering, 12(4), 332. https://doi.org/10.3390/bioengineering12040332







