In Vitro–In Silico Approach in the Development of Clopidogrel Solid Dispersion Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Clopidogrel Hydrogen Sulfate Solubility
2.2.2. Preparation of Solid Dispersions
2.2.3. Dissolution Studies
2.2.4. Clopidogrel-Specific PBBM Model
| Parameter | Value |
|---|---|
| Molecular weight | 321.82 |
| log D (pH 7.4) | 3.9 a |
| pKa value (base) | 4.55 b |
| Solubility at 37 °C (mg/mL) | see Table 3 c |
| Human effective permeability, Peff (cm/s) | 4.7767 × 10−4 d |
| Diffusion coefficient (cm2/s) | 0.7397 × 10−5 e |
| Drug particle diameter (µm) | 150 (D50); 250 (D90) f |
| Drug dose (mg) | 1, 10, 100, 300 (i.v.) g; 75, 300 (p.o.) h |
| Volume of fluid taken with drug (mL) | 200 h |
| Plasma fraction unbound (%) | 2 i |
| Blood/plasma concentration ratio | 0.72 b |
| First-pass effect in the liver, (FPE %) | 96.5 j |
| Clearance, CL (L/h/kg) | 1.2 k |
| Volume of distribution, Vd (L/kg) | 0.073 k |
| Distribution rate constant, k12 (1/h) | 9.285 k |
| Distribution rate constant, k21 (L1/h) | 2.058 k |
| Distribution rate constant, k13 (1/h) | 1.243 k |
| Distribution rate constant, k31 (1/h) | 0.17 k |
| Elimination half-life, t1/2 (h) | 4.4 l |
| pH Value | Solubility (mg/mL) ± S.D. | Therapeutic Dose (mg) | ||
|---|---|---|---|---|
| 75 | 300 | 600 | ||
| Dose Number | ||||
| 1.2 | 268.750 ± 6.159 | 0.001 | 0.004 | 0.009 |
| 4.5 | 0.055 ± 0.005 | 5.455 | 21.818 | 43.636 |
| 6.8 | 0.016 ± 0.003 | 21.429 | 85.714 | 171.429 |
3. Results and Discussion
3.1. Determination of Clopidogrel Hydrogen Sulfate Solubility
3.2. Dissolution Studies
3.3. PBBM Model Construction and Validation
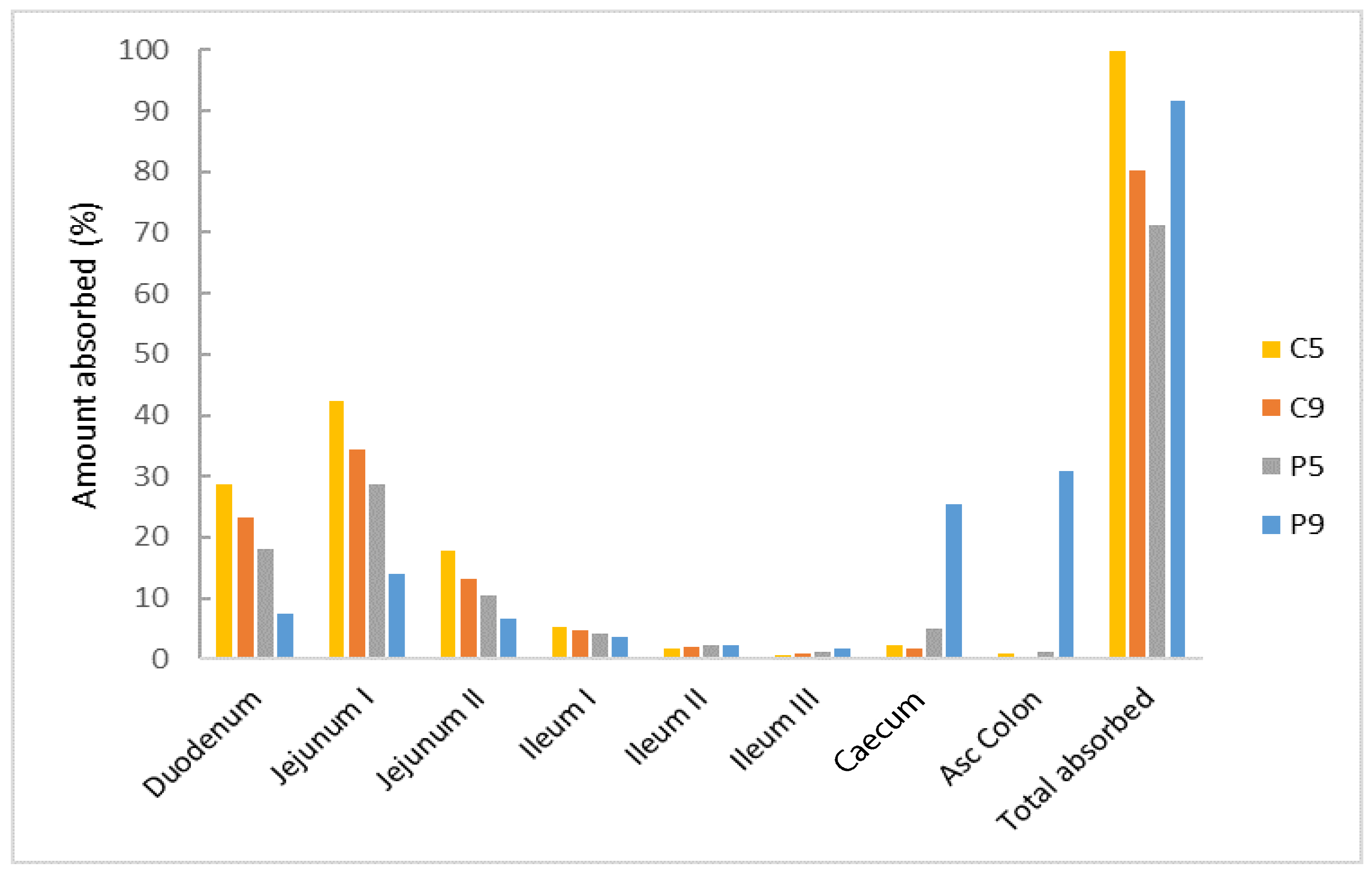
3.4. PBBM Model Exploration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 September 2024).
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Clincalc. The Top 200 Drugs of 2020. 2020. Available online: https://clincalc.com/DrugStats/Top200Drugs.aspx (accessed on 12 September 2024).
- FDA. NDA Dossier 20-389/S-044 for the Innovator Clopidogrel Product (Plavix). 2009. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020839s044lbl.pdf (accessed on 5 September 2024).
- Jourdi, G.; Godier, A.; Lordkipanidzé, M.; Marquis-Gravel, G.; Gaussem, P. Antiplatelet Therapy for Atherothrombotic Disease in 2022—From Population to Patient-Centered Approaches. Front. Cardiovasc. Med. 2022, 9, 805525. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; Samant, S.; Lesko, L.J.; Schmidt, S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin. Pharmacokinet. 2015, 54, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Funck-Brentano, C.; Prats, J.; Salem, J.E.; Hulot, J.S.; Guilloux, E.; Hu, M.; He, K.; Silvain, J.; Gallois, V.; et al. Intravenous Clopidogrel (MDCO-157) Compared with Oral Clopidogrel: The Randomized Cross-Over AMPHORE Study. Am. J. Cardiovasc. Drugs 2016, 16, 43–53. [Google Scholar] [CrossRef]
- Karaźniewicz-Łada, M.; Danielak, D.; Burchardt, P.; Kruszyna, L.; Komosa, A.; Lesiak, M.; Główka, F. Clinical pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases. Clin. Pharmacokinet. 2014, 53, 155–164. [Google Scholar] [CrossRef]
- Frelinger, A.L., 3rd; Bhatt, D.L.; Lee, R.D.; Mulford, D.J.; Wu, J.; Nudurupati, S.; Nudurupati, S.; Nigam, A.; Lampa, M.; Brooks, J.K.; et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J. Am. Coll. Cardiol. 2013, 61, 872–879. [Google Scholar] [CrossRef]
- Furlong, M.T.; Savant, I.; Yuan, M.; Scott, L.; Mylott, W.; Mariannino, T.; Kadiyala, P.; Roongta, V.; Arnold, M.E. A validated HPLC-MS/MS assay for quantifying unstable pharmacologically active metabolites of clopidogrel in human plasma: Application to a clinical pharmacokinetic study. J. Chromatogr. B 2013, 926, 36–41. [Google Scholar] [CrossRef]
- Collette, S.L.; Bokkers, R.P.H.; Dierckx, R.A.J.O.; van der Laan, M.J.; Zeebregts, C.J.; Uyttenboogaart, M. Clinical importance of testing for clopidogrel resistance in patients undergoing carotid artery stenting—A systematic review. Ann. Transl. Med. 2021, 9, 1211. [Google Scholar] [CrossRef]
- Qureshi, M.J.; Phin, F.F.; Patro, S. Enhanced Solubility and Dissolution Rate of Clopidogrel by Nanosuspension: Formulation via High Pressure Homogenization Technique and Optimization Using Box Behnken Design Response Surface Methodology. J. Appl. Pharm. Sci. 2017, 7, 106–113. [Google Scholar] [CrossRef][Green Version]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, H.S.; Jeong, J.W.; Koo, T.S.; Kim, D.K.; Cho, Y.H.; Lee, G.W. The Development and Optimization of Hot-Melt Extruded Amorphous Solid Dispersions Containing Rivaroxaban in Combination with Polymers. Pharmaceutics 2021, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Thakore, S.D.; Sirvi, A.; Joshi, V.C.; Panigrahi, S.S.; Manna, A.; Singh, R.; Sangamwar, A.T.; Bansal, A.K. Biorelevant dissolution testing and physiologically based absorption modeling to predict in vivo performance of supersaturating drug delivery systems. Int. J. Pharm. 2021, 607, 120958. [Google Scholar] [CrossRef]
- Cvijic, S.; Ignjatovic, J.; Parojcic, J.; Djuric, Z. Computer-aided biopharmaceutical characterization: Gastrointestinal absorption simulation. In Computer-Aided Applications in Pharmaceutical Technology, 2nd ed.; Djuris, J., Ed.; Woodhead Publishing: Cambridge, UK, 2024; pp. 199–283. [Google Scholar] [CrossRef]
- Heimbach, T.; Suarez-Sharp, S.; Kakhi, M.; Holmstock, N.; Olivares-Morales, A.; Pepin, X.; Sjögren, E.; Tsakalozou, E.; Seo, P.; Li, M.; et al. Dissolution and Translational Modeling Strategies Toward Establishing an In Vitro-In Vivo Link—A Workshop Summary Report. AAPS J. 2019, 21, 29. [Google Scholar] [CrossRef]
- Wu, D.; Li, M. Current State and Challenges of Physiologically Based Biopharmaceutics Modeling (PBBM) in Oral Drug Product Development. Pharm. Res. 2023, 40, 321–336. [Google Scholar] [CrossRef]
- Krstevska, A.; Đuriš, J.; Ibrić, S.; Cvijić, S. In-Depth Analysis of Physiologically Based Pharmacokinetic (PBPK) Modeling Utilization in Different Application Fields Using Text Mining Tools. Pharmaceutics 2022, 15, 107. [Google Scholar] [CrossRef]
- Osmanović Omerdić, E.; Alagić-Džambić, L.; Krstić, M.; Pašić-Kulenović, M.; Odović, J.; Vasiljević, D. In Vitro Dissolution Study of Acetylsalicylic Acid and Clopidogrel Bisulfate Solid Dispersions: Validation of the RP-HPLC Method for Simultaneous Analysis. Appl. Sci. 2020, 10, 4792. [Google Scholar] [CrossRef]
- Osmanović Omerdić, E.; Alagić-Džambić, L.; Krstić, M.; Pašić-Kulenović, M.; Medarević, Đ.; Ivković, B.; Vasiljević, D. Long-term stability of clopidogrel solid dispersions-Importance of in vitro dissolution test. PLoS ONE 2022, 17, e0266237. [Google Scholar] [CrossRef]
- Wolk, O.; Agbaria, R.; Dahan, A. Provisional in-silico biopharmaceutics classification (BCS) to guide oral drug product development. Drug Des. Dev. Ther. 2014, 8, 1563–1575. [Google Scholar] [CrossRef]
- Agoram, B.; Woltosz, W.S.; Bolger, M.B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 2001, 50, S41–S67. [Google Scholar] [CrossRef] [PubMed]
- Jereb, R.; Opara, J.; Legen, I.; Petek, B.; Bajc, A.l.; Žakelj, S. PBPK Absorption Modeling of Food Effect and Bioequivalence in Fed State for Two Formulations with Crystalline and Amorphous Forms of BCS 2 Class Drug in Generic Drug Development. AAPS PharmSciTech 2019, 20, 59. [Google Scholar] [CrossRef]
- Porat, D.; Dukhno, O.; Vainer, E.; Cvijić, S.; Dahan, A. Antiallergic Treatment of Bariatric Patients: Potentially Hampered Solubility/Dissolution and Bioavailability of Loratadine, but Not Desloratadine, Post-Bariatric Surgery. Mol. Pharm. 2022, 19, 2922–2936. [Google Scholar] [CrossRef]
- Cushing, D.J.; Souney, P.F.; Cooper, W.D.; Mosher, G.L.; Adams, M.P.; Machatha, S.; Zhang, B.; Kowey, P.R. Pharmacokinetics and platelet aggregation inhibitory effects of a novel intravenous formulation of clopidogrel in humans. Clin. Exp. Pharmacol. Physiol. 2012, 39, 3–8. [Google Scholar] [CrossRef]
- Lu, A.T.; Frisella, M.E.; Johnson, K.C. Dissolution modeling: Factors affecting the dissolution rates of polydisperse powders. Pharm. Res. 1993, 10, 1308–1314. [Google Scholar] [CrossRef]
- Djebli, N.; Fabre, D.; Boulenc, X.; Fabre, G.; Sultan, E.; Hurbin, F. Physiologically based pharmacokinetic modeling for sequential metabolism: Effect of CYP2C19 genetic polymorphism on clopidogrel and clopidogrel active metabolite pharmacokinetics. Drug Metab. Dispos. 2015, 43, 510–522. [Google Scholar] [CrossRef]
- Zhang, T.; Heimbach, T.; Lin, W.; Zhang, J.; He, H. Prospective Predictions of Human Pharmacokinetics for Eighteen Compounds. J. Pharm. Sci. 2015, 104, 2795–2806. [Google Scholar] [CrossRef]
- Park, M.H.; Shin, S.H.; Byeon, J.J.; Lee, G.H.; Yu, B.Y.; Shin, Y.G. Prediction of pharmacokinetics and drug-drug interaction potential using physiologically based pharmacokinetic (PBPK) modeling approach: A case study of caffeine and ciprofloxacin. Korean J. Physiol. Pharmacol. 2017, 21, 107–115. [Google Scholar] [CrossRef]
- Sanofi-Aventis. Plavix Product Monograph Including Patient Medication Information. Date of Revision: October 13. 2020. Available online: https://pdf.hres.ca/dpd_pm/00058294.PDF (accessed on 25 September 2024).
- El-Sadek, E.M.; Moustafa, S.M.; Kadi, O.H.; Al-Hakami, A.M.A. Determination of Clopidogrel Carboxylic Acid in Human Plasma by LC-MS/MS. Am. J. Anal. Chem. 2011, 2, 447–455. [Google Scholar] [CrossRef]
- Punčochová, K.; Ewing, A.V.; Gajdošová, M.; Sarvašová, N.; Kazarian, S.G.; Beránek, J.; Štěpánek, F. Identifying the mechanisms of drug release from amorphous solid dispersions using MRI and ATR-FTIR spectroscopic imaging. Int. J. Pharm. 2015, 483, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Maincent, J.; Williams, R.O., 3rd. Sustained-release amorphous solid dispersions. Drug Deliv. Transl. Res. 2018, 8, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosic, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, M.; Gawlak, K.; Paluch, M.; Jachowicz, R. Enhanced dissolution of solid dispersions containing bicalutamide subjected to mechanical stress. Int. J. Pharm. 2018, 542, 18–26. [Google Scholar] [CrossRef]
- Que, C.; Deac, A.; Zemlyanov, D.Y.; Qi, Q.; Indulkar, A.S.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Impact of Drug-Polymer Intermolecular Interactions on Dissolution Performance of Copovidone-Based Amorphous Solid Dispersions. Mol. Pharm. 2021, 18, 3496–3508. [Google Scholar] [CrossRef]
- Que, C.; Lou, X.; Zemlyanov, D.Y.; Mo, H.; Indulkar, A.S.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Insights into the Dissolution Behavior of Ledipasvir-Copovidone Amorphous Solid Dispersions: Role of Drug Loading and Intermolecular Interactions. Mol. Pharm. 2019, 16, 5054–5067. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Deepa, M.K.; Bassim, E.; Rahna, C.S.; Raj, K.R.S. Investigation of kinetic drug release characteristics and in vitro evaluation of sustained-release matrix tablets of a selective COX-2 inhibitor for rheumatic diseases. J. Pharm. Innov. 2021, 16, 551–557. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, J.R.; Lim, K.S.; Shin, H.S.; Yoon, S.H.; Cho, J.Y.; Yang, I.J.; Shin, S.G.; Yu, K.S. Comparative pharmacokinetics/pharmacodynamics of clopidogrel besylate and clopidogrel bisulfate in healthy Korean subjects. Clin. Drug Investig. 2012, 32, 817–826. [Google Scholar] [CrossRef]
- Zaid, A.N.; Al Ramahi, R.; Bustami, R.; Mousa, A.; Khasawneh, S. Comparative fasting bioavailability of two clopidogrel formulations in healthy Mediterranean volunteers: An in vitro-in vivo correlation. Drug Des. Dev. Ther. 2015, 9, 2359–2365. [Google Scholar] [CrossRef]
- Ilie, A.R.; Griffin, B.T.; Vertzoni, M.; Kuentz, M.; Kolakovic, R.; Prudic-Paus, A.; Malash, A.; Bohets, H.; Herman, J.; Holm, R. Exploring precipitation inhibitors to improve in vivo absorption of cinnarizine from supersaturated lipid-based drug delivery systems. Eur. J. Pharm. Sci. 2021, 159, 105691. [Google Scholar] [CrossRef]
- Koehl, N.J.; Henze, L.J.; Bennett-Lenane, H.; Faisal, W.; Price, D.J.; Holm, R.; Kuentz, M.; Griffin, B.T. In Silico, In Vitro, and In Vivo Evaluation of Precipitation Inhibitors in Supersaturated Lipid-Based Formulations of Venetoclax. Mol Pharm. 2021, 18, 2174–2188. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Gui, H.; Hunag, Z.; Tu, X. Clopidogrel Hydrogensulfate Enteric-Coated Sustained-Release Tablet and Preparation Thereof. CN Patent 101606918A, 18 June 2008. [Google Scholar]
- Patel, P.R.; Roy, S.B.; Kothari, J.S. Modified Release Clopidogrel Formulation. U.S. patent US20100145053A1, 10 June 2010. [Google Scholar]
- Tan, C.; Degim, İ.T. Development of sustained release formulation of an antithrombotic drug and application of Fuzzy logic. Pharm. Dev. Technol. 2012, 17, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Duong, J.K.; Nand, R.A.; Patel, A.; Della Pasqua, O.; Gross, A.S. A physiologically based pharmacokinetic model of clopidogrel in populations of European and Japanese ancestry: An evaluation of CYP2C19 activity. Pharmacol. Res. Perspect. 2022, 10, e00946. [Google Scholar] [CrossRef]
- Jiang, X.-L.; Samant, S.; Lewis, J.P.; Horenstein, R.B.; Shuldiner, A.R.; Yerges-Armstrong, L.M.; Peletier, L.A.; Lesko, L.J.; Schmidt, S. Development of a physiology-directed population pharmacokinetic and pharmacodynamic model for characterizing the impact of genetic and demographic factors on clopidogrel response in healthy adults. Eur. J. Pharm. Sci. 2016, 82, 64–78. [Google Scholar] [CrossRef]
- Loer, H.L.H.; Türk, D.; Gómez-Mantilla, J.D.; Selzer, D.; Lehr, T. Physiologically Based Pharmacokinetic (PBPK) Modeling of Clopidogrel and Its Four Relevant Metabolites for CYP2B6, CYP2C8, CYP2C19, and CYP3A4 Drug-Drug-Gene Interaction Predictions. Pharmaceutics 2022, 14, 915. [Google Scholar] [CrossRef]
- Shebley, M.; Fu, W.; Badri, P.; Bow, D.; Fischer, V. Physiologically Based Pharmacokinetic Modeling Suggests Limited Drug–Drug Interaction Between Clopidogrel and Dasabuvir. Clin. Pharmacol. Ther. 2017, 102, 679–687. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, H.; Liu, Y.; Lai, X.; Gong, Y.; Liu, X.; Li, Y.G.; He, Y.; Zhang, E.Y.; Yan, X. Semi-mechanistic population pharmacokinetics analysis reveals distinct CYP2C19 dependency in the bioactivation of vicagrel and clopidogrel to active metabolite M15-2. Eur. J. Pharm. Sci. 2022, 177, 106264. [Google Scholar] [CrossRef]



| Row | I | II | III | IV | V |
|---|---|---|---|---|---|
| GIT region | stomach | duodenum | proximal jejunum | distal jejunum | distal ileum |
| pH value | pH 1.2 | pH 6.0 | pH 6.4 | pH 6.9 | pH 7.4 |
| Residence time (min) | 15 | 15 | 90 | 60 | 150 |
| Volume of dissolution medium (mL) | 240 | ||||
| Hold dip time (s) | 30 | ||||
| Drain time (s) | 60 | ||||
| SDs | Zero- Order | First- Order | Korsmeyer–Peppas | Higuchi | Hixon– Crowell |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | |
| P5 | 0.7549 | 0.8307 | 0.9742 | 0.9769 | 0.8060 |
| C5 | 0.7141 | 0.8787 | 0.9595 | 0.9734 | 0.8422 |
| P9 | 0.9304 | 0.9409 | 0.9937 | 0.7395 | 0.9376 |
| C9 | 0.6603 | 0.7728 | 0.9901 | 0.9984 | 0.7328 |
| Parameter | AUC0→∞ (ng h/mL) | AUC0→t (ng h/mL) | |
|---|---|---|---|
| 1 mg i.v. injection | Predicted | 11.45 | 11.31 |
| In vivo mean | 10.80 | 10.71 | |
| Fold error | 1.06 | 1.06 | |
| 10 mg i.v. injection | Predicted | 114.45 | 113.08 |
| In vivo mean | 121.41 | 121.25 | |
| Fold error | 0.94 | 0.93 | |
| 100 mg i.v. infusion | Predicted | 1144.50 | 1142.40 |
| In vivo mean | 1137.60 | 1135.40 | |
| Fold error | 1.01 | 1.01 | |
| 300 mg i.v. infusion | Predicted | 3433.50 | 3427.20 |
| In vivo mean | 2406.50 | 2393.30 | |
| Fold error | 1.43 | 1.43 |
| Parameter | Cmax(ng/mL) | tmax (h) | AUC0→∞ (ng h/mL) | AUC0→t (ng h/mL) | |
|---|---|---|---|---|---|
| 75 mg IR tablet | Predicted | 1.60 | 0.94 | 9.43 | 5.76 |
| In vivo mean | 1.81 | 0.50 | 10.87 | 6.50 | |
| Fold error | 0.88 | 1.88 | 0.87 | 0.89 | |
| 300 mg IR tablet | Predicted | 5.03 | 0.92 | 21.32 | 17.69 |
| In vivo mean | 5.47 | 0.50 | 17.70 | 15.95 | |
| Fold error | 0.92 | 1.84 | 1.20 | 1.11 |
| Parameter | IR Tablet 1 | P5 2 | C5 2 | P9 2 | C9 2 |
|---|---|---|---|---|---|
| Fa (%) | 45.385 | 71.330 | 99.883 | 91.637 | 80.126 |
| Fb (%) | 1.585 | 2.497 | 3.496 | 3.207 | 2.804 |
| Cmax (ng/mL) | 2.00 | 12.00 | 19.00 | 6.00 | 15.00 |
| tmax (h) | 0.96 | 0.80 | 0.56 | 0.80 | 0.50 |
| AUC0→∞ (ng h/mL) | 18.00 | 22.00 | 31.00 | 29.00 | 25.00 |
| AUC0→t (ng h/mL) | 14.00 | 22.00 | 31.00 | 28.00 | 25.00 |
| Cmax liver (ng/mL) | 5.00 | 35.00 | 55.00 | 18.00 | 42.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmanović Omerdić, E.; Cvijić, S.; Ignjatović, J.; Ivković, B.; Vasiljević, D. In Vitro–In Silico Approach in the Development of Clopidogrel Solid Dispersion Formulations. Bioengineering 2025, 12, 357. https://doi.org/10.3390/bioengineering12040357
Osmanović Omerdić E, Cvijić S, Ignjatović J, Ivković B, Vasiljević D. In Vitro–In Silico Approach in the Development of Clopidogrel Solid Dispersion Formulations. Bioengineering. 2025; 12(4):357. https://doi.org/10.3390/bioengineering12040357
Chicago/Turabian StyleOsmanović Omerdić, Ehlimana, Sandra Cvijić, Jelisaveta Ignjatović, Branka Ivković, and Dragana Vasiljević. 2025. "In Vitro–In Silico Approach in the Development of Clopidogrel Solid Dispersion Formulations" Bioengineering 12, no. 4: 357. https://doi.org/10.3390/bioengineering12040357
APA StyleOsmanović Omerdić, E., Cvijić, S., Ignjatović, J., Ivković, B., & Vasiljević, D. (2025). In Vitro–In Silico Approach in the Development of Clopidogrel Solid Dispersion Formulations. Bioengineering, 12(4), 357. https://doi.org/10.3390/bioengineering12040357







