Altered Lactylation Myocardial Tissue May Contribute to a More Severe Energy-Deprived State of the Tissue and Left Ventricular Outflow Tract Obstruction in HOCM
Abstract
:1. Introduction
2. Material and Methods
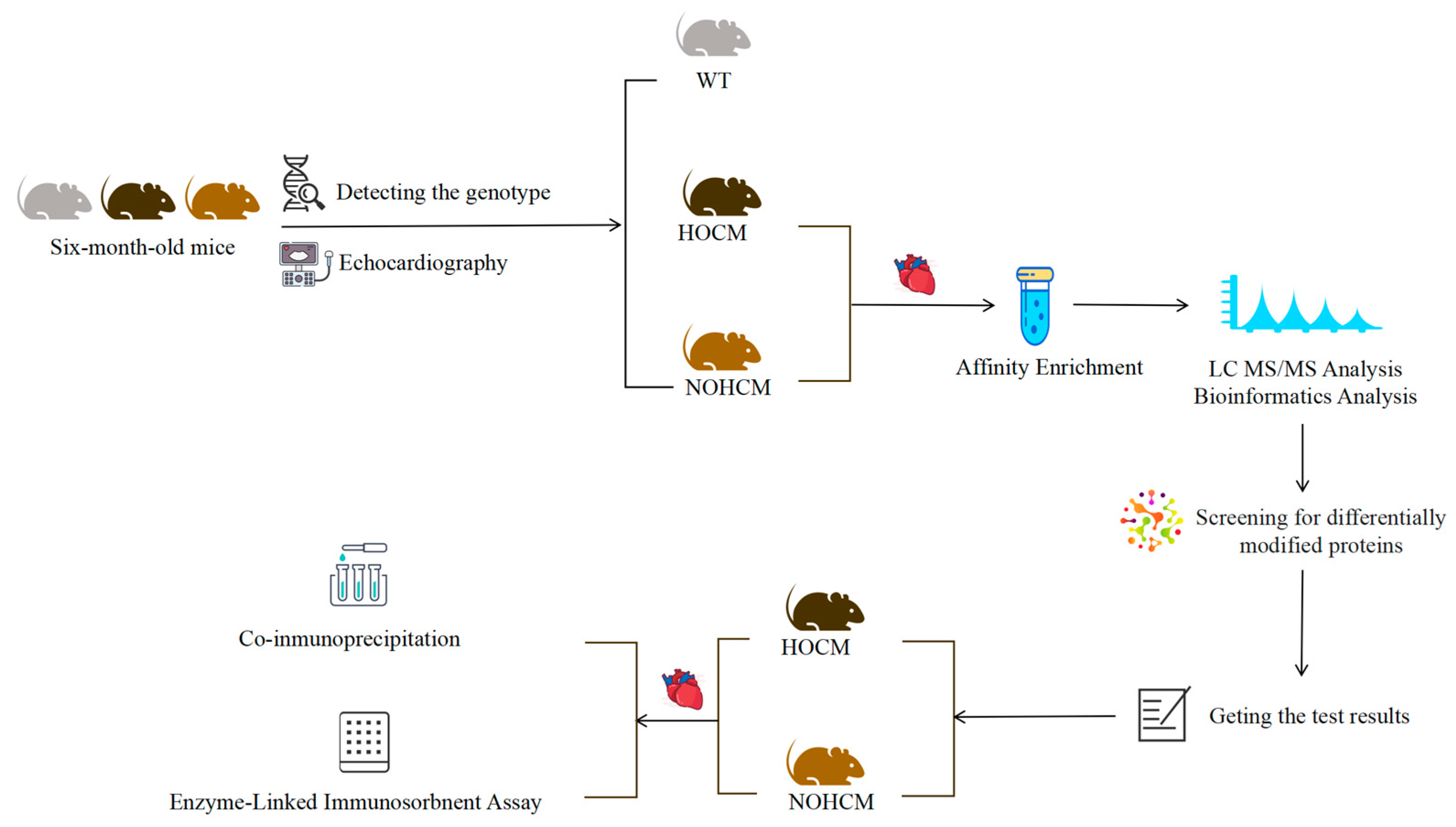
2.1. Mice Screening and Sample Preparation
2.1.1. Genetic Testing of Mice
2.1.2. Basic Information Acquisition and Echocardiography of Mice
2.1.3. Myocardial Tissue Sample Preparation of Mice
2.2. Co-Immunoprecipitation (Co-IP) and Western Blot Analysis
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
2.4.1. Omics Data Analysis
2.4.2. Basic and Experimental Data Analysis
3. Results
3.1. Basic Information and Echocardiography of Mice
3.2. Lactylation of Mouse Myocardial Tissue
3.3. Experimental Verification of Lactylation of Proteins
4. Discussion
4.1. The Mouse Model Used in This Study Carried the Same Mutation Site as HCM Patients Which Can Simulate HCM Patients Well
4.2. Differences in Lactylation of Myocardial Tissue Between HOCM and NOHCM Mice May Relate to Abnormal Energy Metabolism and the Development of LVOTO
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How Hypertrophic Cardiomyopathy Became a Contemporary Treatable Genetic Disease With Low Mortality: Shaped by 50 Years of Clinical Research and Practice. JAMA Cardiol. 2016, 1, 98–105. [Google Scholar]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [PubMed]
- Seitler, S.; De Zoysa Anthony, S.; Obianyo, C.C.C.; Syrris, P.; Patel, V.; Sado, D.M.; Maestrini, V.; Castelletti, S.; Walsh, S.; O’Brien, B.; et al. Systolic anterior motion of the anterior mitral valve leaflet begins in subclinical hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 25, 86–94. [Google Scholar]
- De Gaspari, M.; Mazzucato, M.; Bueno Marinas, M.; Angelini, A.; Calore, C.; Perazzolo Marra, M.; Pilichou, K.; Corrado, D.; Thiene, G.; Rizzo, S.; et al. Is Congenital Muscular Mitral-Aortic Discontinuity Another Feature of Obstructive Hypertrophic Cardiomyopathy? A Pathology Validation Study. Lab. Investig. 2023, 103, 100196. [Google Scholar]
- Ranjbarvaziri, S.; Kooiker, K.B.; Ellenberger, M.; Fajardo, G.; Zhao, M.; Vander Roest, A.S.; Woldeyes, R.A.; Koyano, T.T.; Fong, R.; Ma, N.; et al. Altered Cardiac Energetics and Mitochondrial Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2021, 144, 1714–1731. [Google Scholar] [PubMed]
- Toepfer, C.N.; Garfinkel, A.C.; Venturini, G.; Wakimoto, H.; Repetti, G.; Alamo, L.; Sharma, A.; Agarwal, R.; Ewoldt, J.K.; Cloonan, P.; et al. Myosin Sequestration Regulates Sarcomere Function, Cardiomyocyte Energetics, and Metabolism, Informing the Pathogenesis of Hypertrophic Cardiomyopathy. Circulation 2020, 141, 828–842. [Google Scholar]
- Deidda, M.; Noto, A.; Pasqualucci, D.; Fattuoni, C.; Barberini, L.; Piras, C.; Bassareo, P.P.; Porcu, M.; Mercuro, G.; Dessalvi, C.C. The Echocardiographic Parameters of Systolic Function Are Associated with Specific Metabolomic Fingerprints in Obstructive and Non-Obstructive Hypertrophic Cardiomyopathy. Metabolites 2021, 11, 787. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, J.; Wang, P.; Wu, B.; Lu, S.; Lu, X.; You, S.; Huang, X.; Li, M.; et al. alpha-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 2023, 33, 679–698. [Google Scholar]
- Cluntun, A.A.; Badolia, R.; Lettlova, S.; Parnell, K.M.; Shankar, T.S.; Diakos, N.A.; Olson, K.A.; Taleb, I.; Tatum, S.M.; Berg, J.A.; et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021, 33, 629–648 e610. [Google Scholar]
- Li, Q.; Li, C.; Elnwasany, A.; Sharma, G.; An, Y.A.; Zhang, G.; Elhelaly, W.M.; Lin, J.; Gong, Y.; Chen, G.; et al. PKM1 Exerts Critical Roles in Cardiac Remodeling Under Pressure Overload in the Heart. Circulation 2021, 144, 712–727. [Google Scholar] [PubMed]
- Tran, D.H.; Wang, Z.V. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J. Am. Heart Assoc. 2019, 8, e012673. [Google Scholar] [PubMed]
- Wang, B.; Guo, R.Q.; Wang, J.; Yang, F.; Zuo, L.; Liu, Y.; Shao, H.; Ju, Y.; Sun, C.; Xu, L.; et al. The Cumulative Effects of the MYH7-V878A and CACNA1C-A1594V Mutations in a Chinese Family with Hypertrophic Cardiomyopathy. Cardiology 2017, 138, 228–237. [Google Scholar] [PubMed]
- Sørensen, L.L.; Bedja, D.; Sysa-Shah, P.; Liu, H.; Maxwell, A.; Yi, X.; Pozios, I.; Olsen, N.T.; Abraham, T.P.; Abraham, R.; et al. Echocardiographic Characterization of a Murine Model of Hypertrophic Obstructive Cardiomyopathy Induced by Cardiac-specific Overexpression of Epidermal Growth Factor Receptor 2. Comp. Med. 2016, 66, 268–277. [Google Scholar]
- Zacchigna, S.; Paldino, A.; Falcao-Pires, I.; Daskalopoulos, E.P.; Dal Ferro, M.; Vodret, S.; Lesizza, P.; Cannata, A.; Miranda-Silva, D.; Lourenco, A.P.; et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: A position paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021, 117, 43–59. [Google Scholar]
- Previs, M.J.; O’Leary, T.S.; Morley, M.P.; Palmer, B.M.; LeWinter, M.; Yob, J.M.; Pagani, F.D.; Petucci, C.; Kim, M.S.; Margulies, K.B.; et al. Defects in the Proteome and Metabolome in Human Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2022, 15, e009521. [Google Scholar]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar]
- Haas, R.; Cucchi, D.; Smith, J.; Pucino, V.; Macdougall, C.E.; Mauro, C. Intermediates of Metabolism: From Bystanders to Signalling Molecules. Trends Biochem. Sci. 2016, 41, 460–471. [Google Scholar]
- Ouyang, J.; Wang, H.; Huang, J. The role of lactate in cardiovascular diseases. Cell Commun. Signal 2023, 21, 317. [Google Scholar]
- Wang, B.; Guo, R.; Zuo, L.; Shao, H.; Liu, Y.; Wang, Y.; Ju, Y.; Sun, C.; Wang, L.; Zhang, Y.; et al. Analysis of genotype and phenotype correlation of MYH7-V878A mutation among ethnic Han Chinese pedigrees affected with hypertrophic cardiomyopathy. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2017, 34, 514–518. [Google Scholar]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003, 107, 2227–2232. [Google Scholar]
- Ingles, J.; Goldstein, J.; Thaxton, C.; Caleshu, C.; Corty, E.W.; Crowley, S.B.; Dougherty, K.; Harrison, S.M.; McGlaughon, J.; Milko, L.V.; et al. Evaluating the Clinical Validity of Hypertrophic Cardiomyopathy Genes. Circ. Genom. Precis. Med. 2019, 12, e002460. [Google Scholar]
- Topriceanu, C.C.; Pereira, A.C.; Moon, J.C.; Captur, G.; Ho, C.Y. Meta-Analysis of Penetrance and Systematic Review on Transition to Disease in Genetic Hypertrophic Cardiomyopathy. Circulation 2024, 149, 107–123. [Google Scholar] [PubMed]
- Yang, Y.; Fang, X.; Yang, R.; Yu, H.; Jiang, P.; Sun, B.; Zhao, Z. MiR-152 Regulates Apoptosis and Triglyceride Production in MECs via Targeting ACAA2 and HSD17B12 Genes. Sci. Rep. 2018, 8, 417. [Google Scholar]
- Piret, S.E.; Attallah, A.A.; Gu, X.; Guo, Y.; Gujarati, N.A.; Henein, J.; Zollman, A.; Hato, T.; Ma’ayan, A.; Revelo, M.P.; et al. Loss of proximal tubular transcription factor Kruppel-like factor 15 exacerbates kidney injury through loss of fatty acid oxidation. Kidney Int. 2021, 100, 1250–1267. [Google Scholar] [PubMed]
- Breilyn, M.S.; Kenny, E.E.; Abul-Husn, N.S. Diverse and unselected adults with clinically relevant ACADS variants lack evidence of metabolic disease. Mol. Genet. Metab. 2023, 138, 106971. [Google Scholar]
- Ritterhoff, J.; Young, S.; Villet, O.; Shao, D.; Neto, F.C.; Bettcher, L.F.; Hsu, Y.A.; Kolwicz, S.C., Jr.; Raftery, D.; Tian, R. Metabolic Remodeling Promotes Cardiac Hypertrophy by Directing Glucose to Aspartate Biosynthesis. Circ. Res. 2020, 126, 182–196. [Google Scholar] [PubMed]
- Li, W.; Long, Q.; Wu, H.; Zhou, Y.; Duan, L.; Yuan, H.; Ding, Y.; Huang, Y.; Wu, Y.; Huang, J.; et al. Nuclear localization of mitochondrial TCA cycle enzymes modulates pluripotency via histone acetylation. Nat. Commun. 2022, 13, 7414. [Google Scholar]
- Zhuang, L.; Jia, K.; Chen, C.; Li, Z.; Zhao, J.; Hu, J.; Zhang, H.; Fan, Q.; Huang, C.; Xie, H.; et al. DYRK1B-STAT3 Drives Cardiac Hypertrophy and Heart Failure by Impairing Mitochondrial Bioenergetics. Circulation 2022, 145, 829–846. [Google Scholar]
- Park, J.B.; Kim, D.H.; Lee, H.; Hwang, I.C.; Yoon, Y.E.; Park, H.E.; Choi, S.Y.; Kim, Y.J.; Cho, G.Y.; Han, K.; et al. Obesity and metabolic health status are determinants for the clinical expression of hypertrophic cardiomyopathy. Eur. J. Prev. Cardiol. 2020, 27, 1849–1857. [Google Scholar]
- Duchnowski, P.; Smigielski, W. Usefulness of myocardial damage biomarkers in predicting cardiogenic shock in patients undergoing heart valve surgery. Kardiol. Pol. 2024, 82, 423–426. [Google Scholar] [PubMed]
- Ta, S.; Li, J.; Hsi, D.H.; Hu, R.; Lei, C.; Shan, B.; Li, W.; Wang, J.; Wang, B.; Kang, N.; et al. Percutaneous intramyocardial septal radiofrequency ablation after 5-year follow-up. Heart 2024, 110, 792–799. [Google Scholar] [PubMed]
- Allouba, M.; Walsh, R.; Afify, A.; Hosny, M.; Halawa, S.; Galal, A.; Fathy, M.; Theotokis, P.I.; Boraey, A.; Ellithy, A.; et al. Ethnicity, consanguinity, and genetic architecture of hypertrophic cardiomyopathy. Eur. Heart J. 2023, 44, 5146–5158. [Google Scholar] [PubMed]




| WT Mice (n = 3) | HOCM Mice (n = 3) | NOHCM Mice (n = 3) | p Value | |
|---|---|---|---|---|
| Body Weight (g) | 23.47 ± 0.86 | 23.93 ± 1.65 | 24.23 ± 2.05 | 0.844 |
| Heart Weight (mg) | 69.70 ± 4.06 | 105.27 ± 2.80 ^ | 104.16 ± 0.90 * | <0.05 |
| Tibia Length (mm) | 25.13 ± 0.72 | 25.33 ± 1.00 | 24.60 ± 0.78 | 0.576 |
| Heart Weight/Body Weight | 2.97 ± 0.15 | 4.40 ± 0.23 ^ | 4.32 ± 0.37 * | <0.05 |
| Heart Weight/Tibia Length | 2.77 ± 0.09 | 4.24 ± 0.15 ^ | 4.16 ± 0.10 * | <0.05 |
| IVSd a (mm) | 0.61 ± 0.06 | 1.01 ± 0.05 ^ | 0.82 ± 0.04 *# | <0.05 |
| MLVWT b (mm) | 0.61 ± 0.06 | 1.05 ± 0.06 ^ | 1.11 ± 0.06 * | <0.05 |
| Long Axis/Short Axis c | 1.18 ± 0.12 | 1.03 ± 0.05 | 0.96 ± 0.15 | 0.217 |
| LVEF d (%) | 51.45 ± 5.91 | 56.80 ± 5.21 | 70.33 ± 6.97 *# | <0.05 |
| LVOT-PG e (mmHg) | 1.50 ± 0.63 | 32.63 ± 16.69 ^ | 2.89 ± 1.31 # | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, J.; Zhao, J.; Liu, J.; Qin, Y.; Wang, Y.; Yuan, Y.; Kang, N.; Yao, L.; Yang, F.; et al. Altered Lactylation Myocardial Tissue May Contribute to a More Severe Energy-Deprived State of the Tissue and Left Ventricular Outflow Tract Obstruction in HOCM. Bioengineering 2025, 12, 379. https://doi.org/10.3390/bioengineering12040379
Li R, Wang J, Zhao J, Liu J, Qin Y, Wang Y, Yuan Y, Kang N, Yao L, Yang F, et al. Altered Lactylation Myocardial Tissue May Contribute to a More Severe Energy-Deprived State of the Tissue and Left Ventricular Outflow Tract Obstruction in HOCM. Bioengineering. 2025; 12(4):379. https://doi.org/10.3390/bioengineering12040379
Chicago/Turabian StyleLi, Ruoxuan, Jing Wang, Jia Zhao, Jiao Liu, Yuze Qin, Yue Wang, Yiming Yuan, Nan Kang, Lu Yao, Fan Yang, and et al. 2025. "Altered Lactylation Myocardial Tissue May Contribute to a More Severe Energy-Deprived State of the Tissue and Left Ventricular Outflow Tract Obstruction in HOCM" Bioengineering 12, no. 4: 379. https://doi.org/10.3390/bioengineering12040379
APA StyleLi, R., Wang, J., Zhao, J., Liu, J., Qin, Y., Wang, Y., Yuan, Y., Kang, N., Yao, L., Yang, F., Feng, K., Zhang, L., Ta, S., Wang, B., & Liu, L. (2025). Altered Lactylation Myocardial Tissue May Contribute to a More Severe Energy-Deprived State of the Tissue and Left Ventricular Outflow Tract Obstruction in HOCM. Bioengineering, 12(4), 379. https://doi.org/10.3390/bioengineering12040379







