A Biomechanical Evaluation of a Novel Interspinous Process Device: In Vitro Flexibility Assessment and Finite Element Analysis
Abstract
1. Introduction
2. Methods and Materials
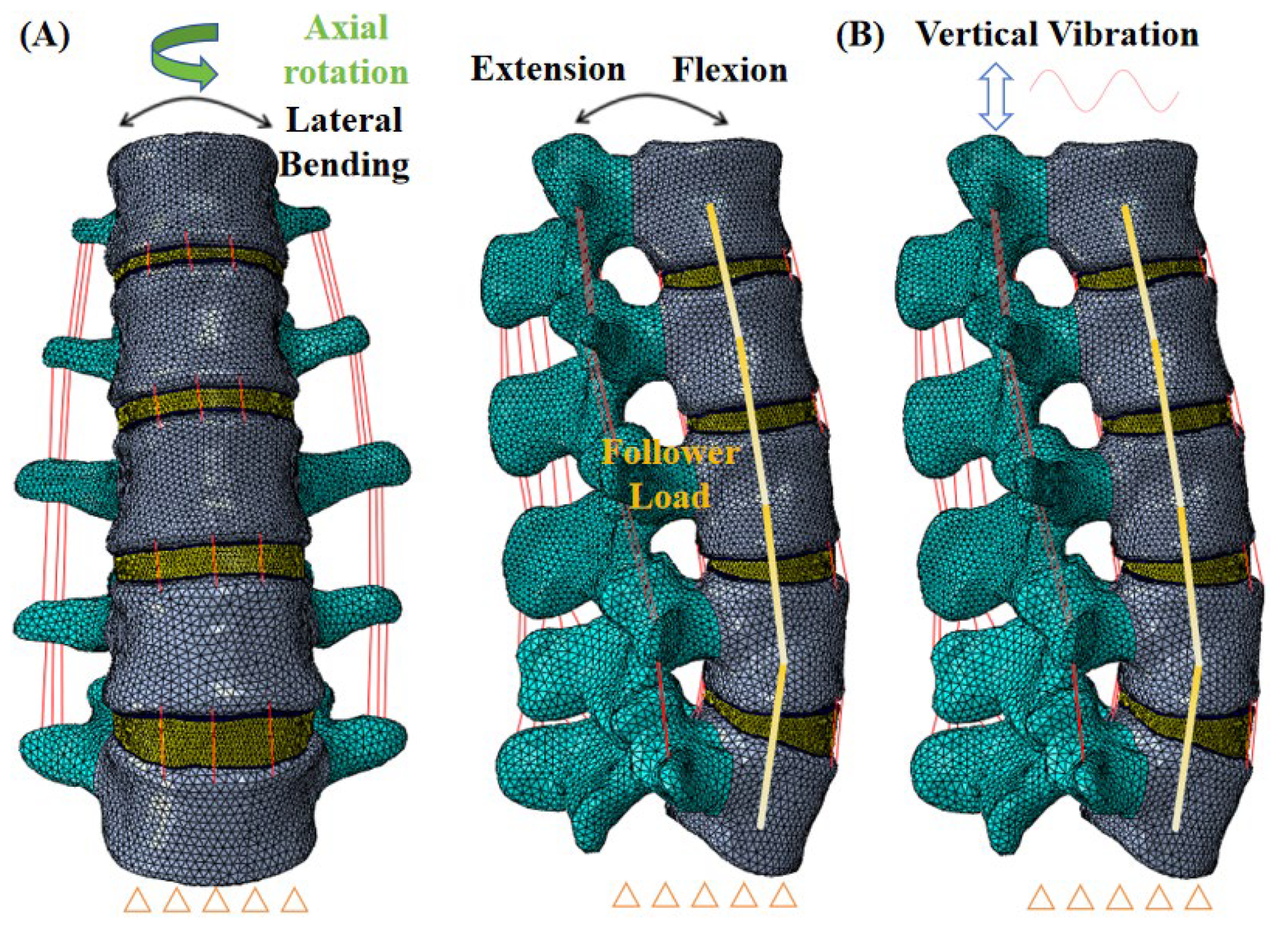
2.1. FE Models of the L1–L5 Lumbar Spine
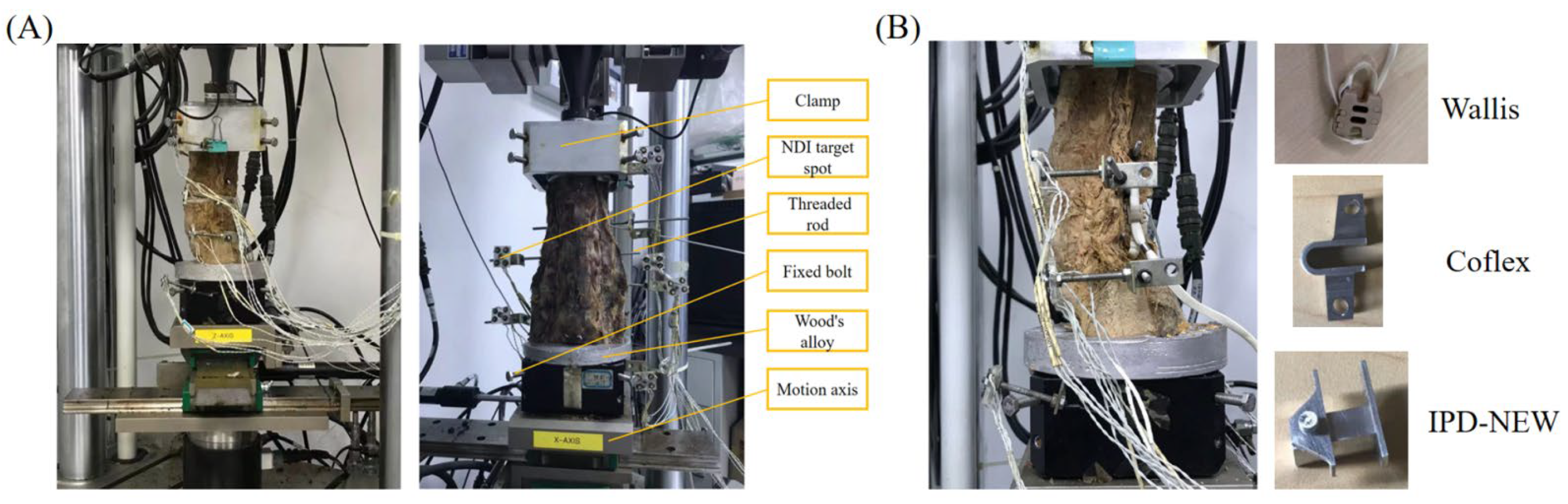
2.2. In Vitro Testing
3. Results
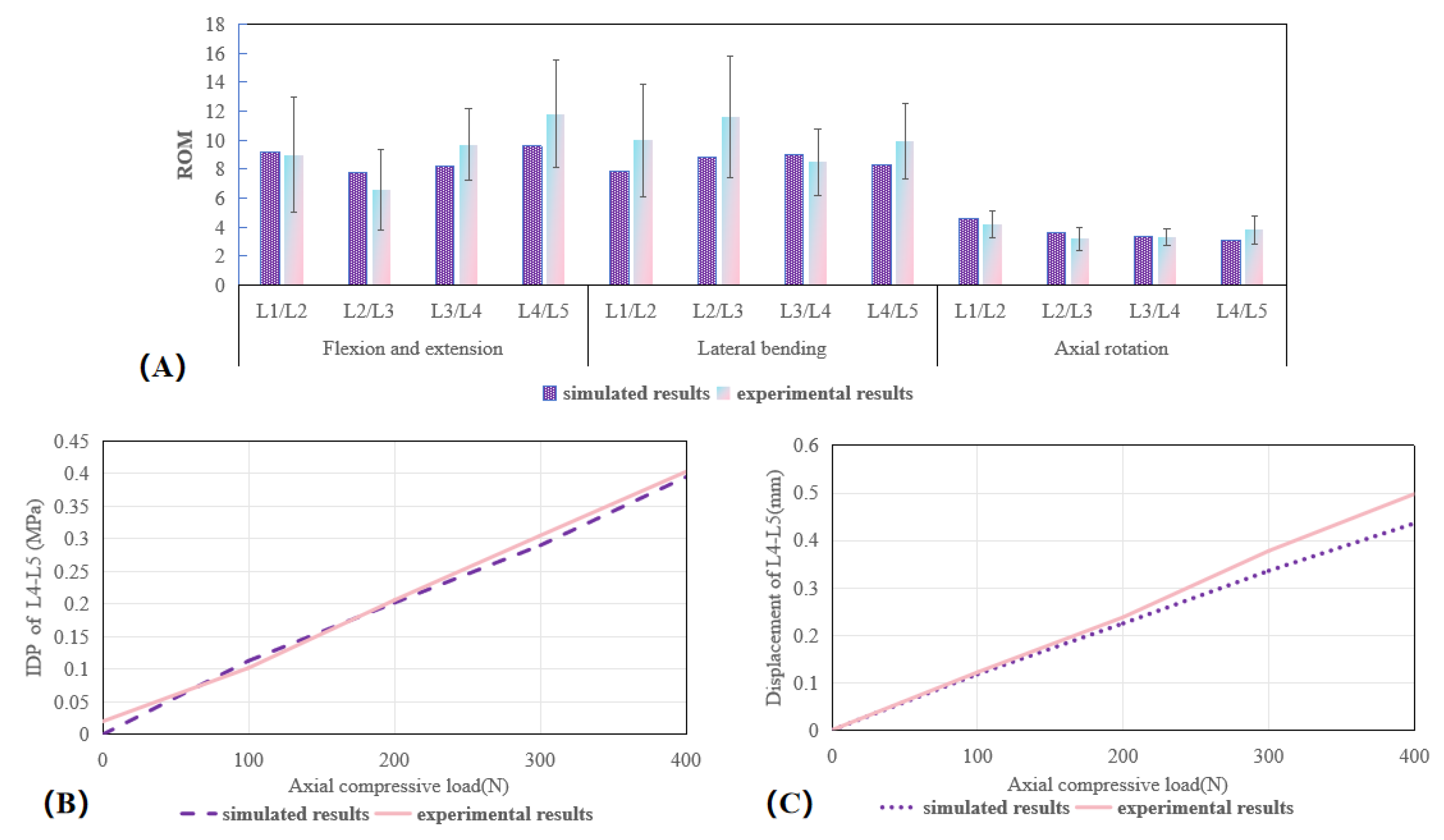
3.1. Validation of the Lumbar Spine FE Model
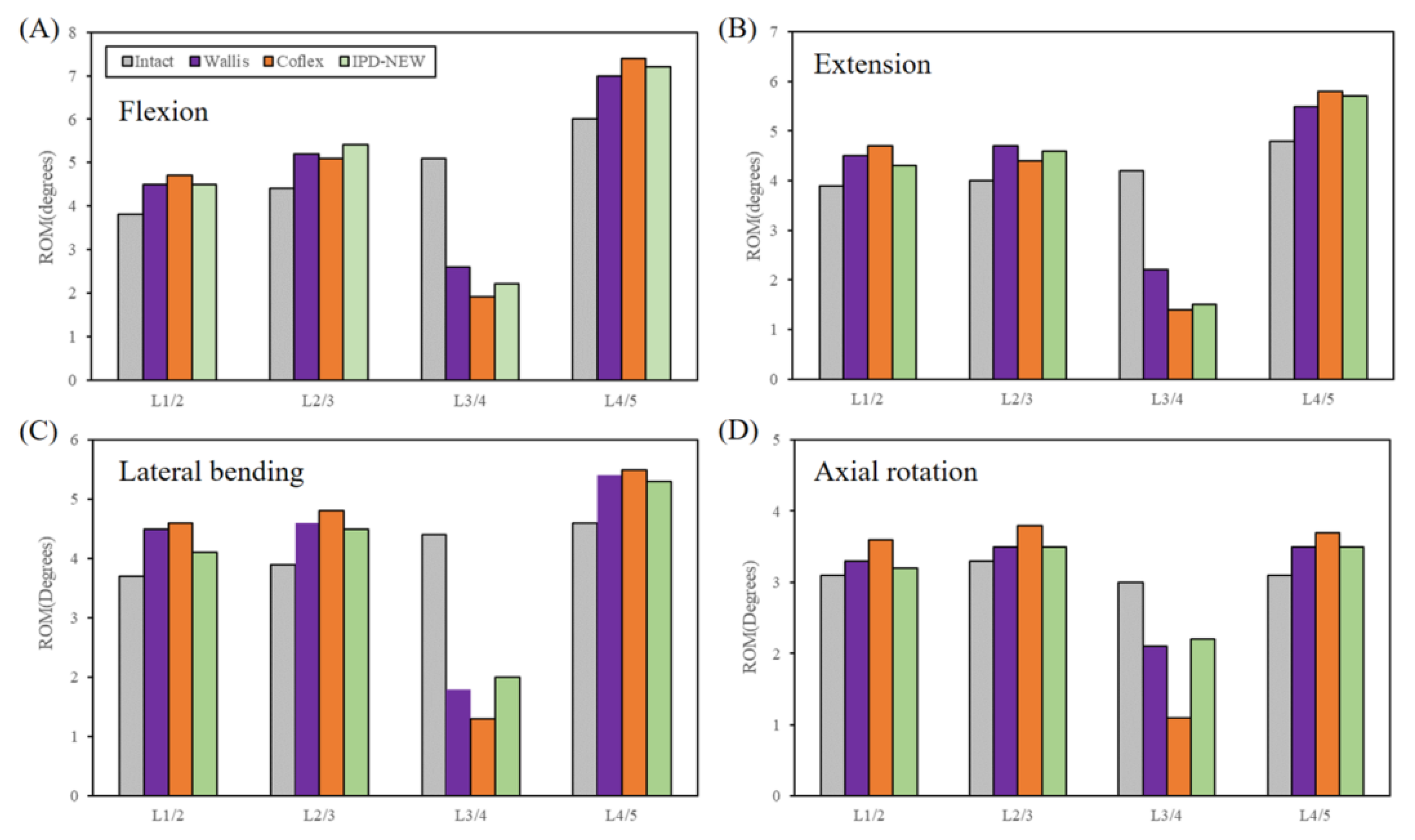
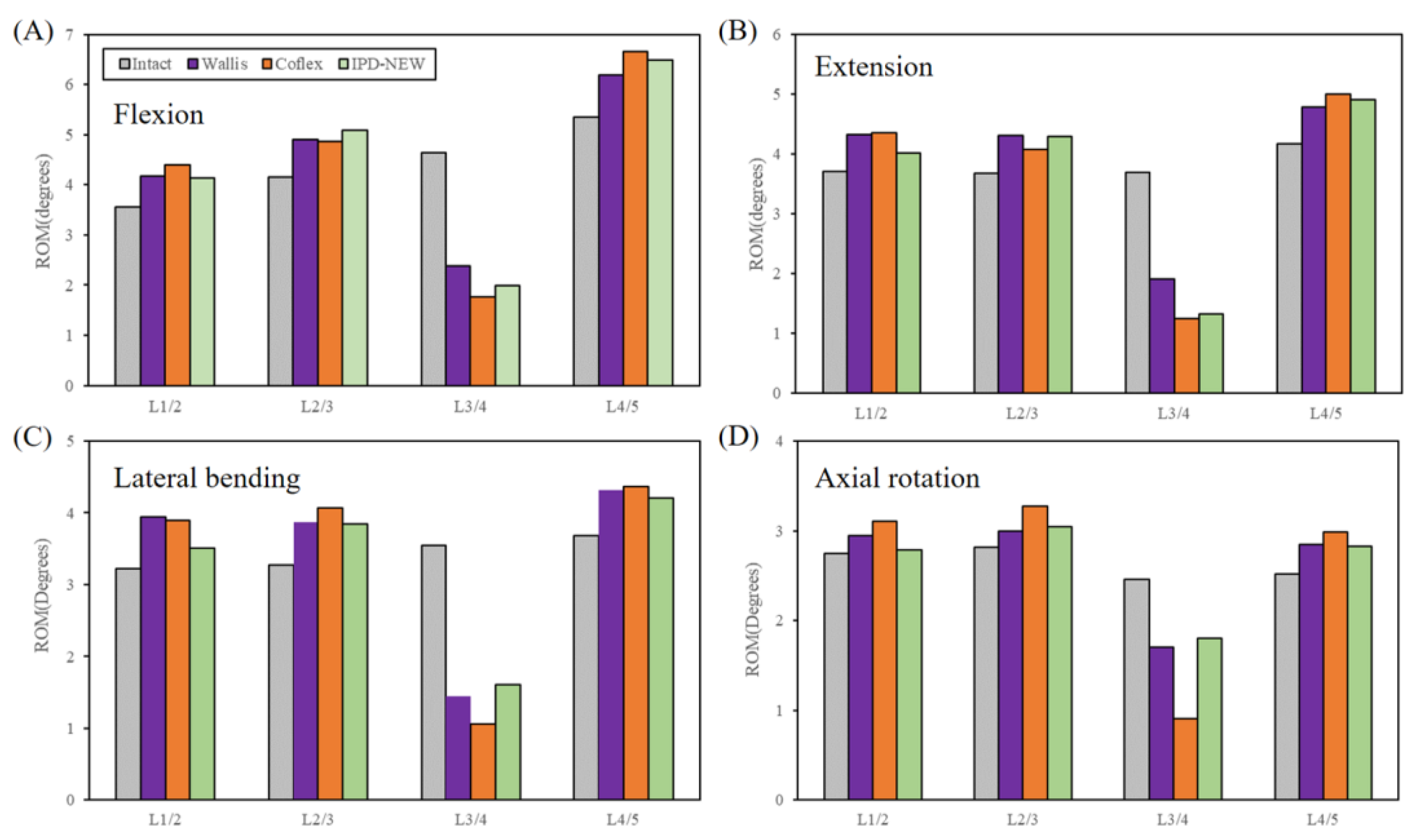
3.2. ROM (In Vitro Results)
3.3. Range of Motion (FE Results)
3.4. Facet Joint Force
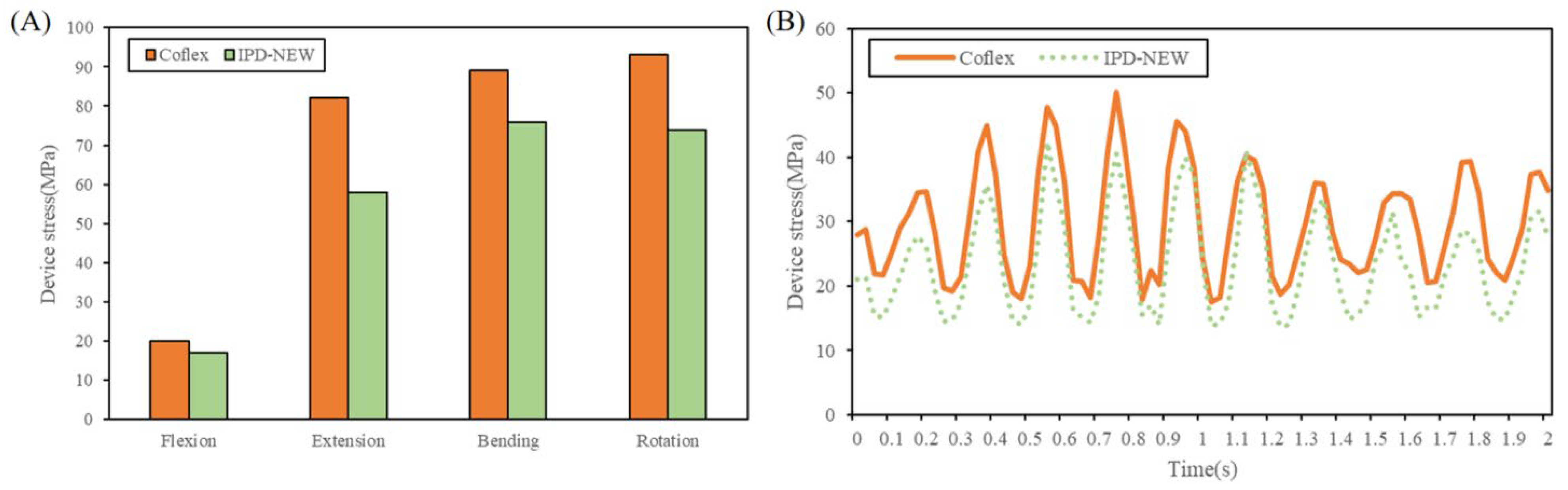
3.5. Device Stress
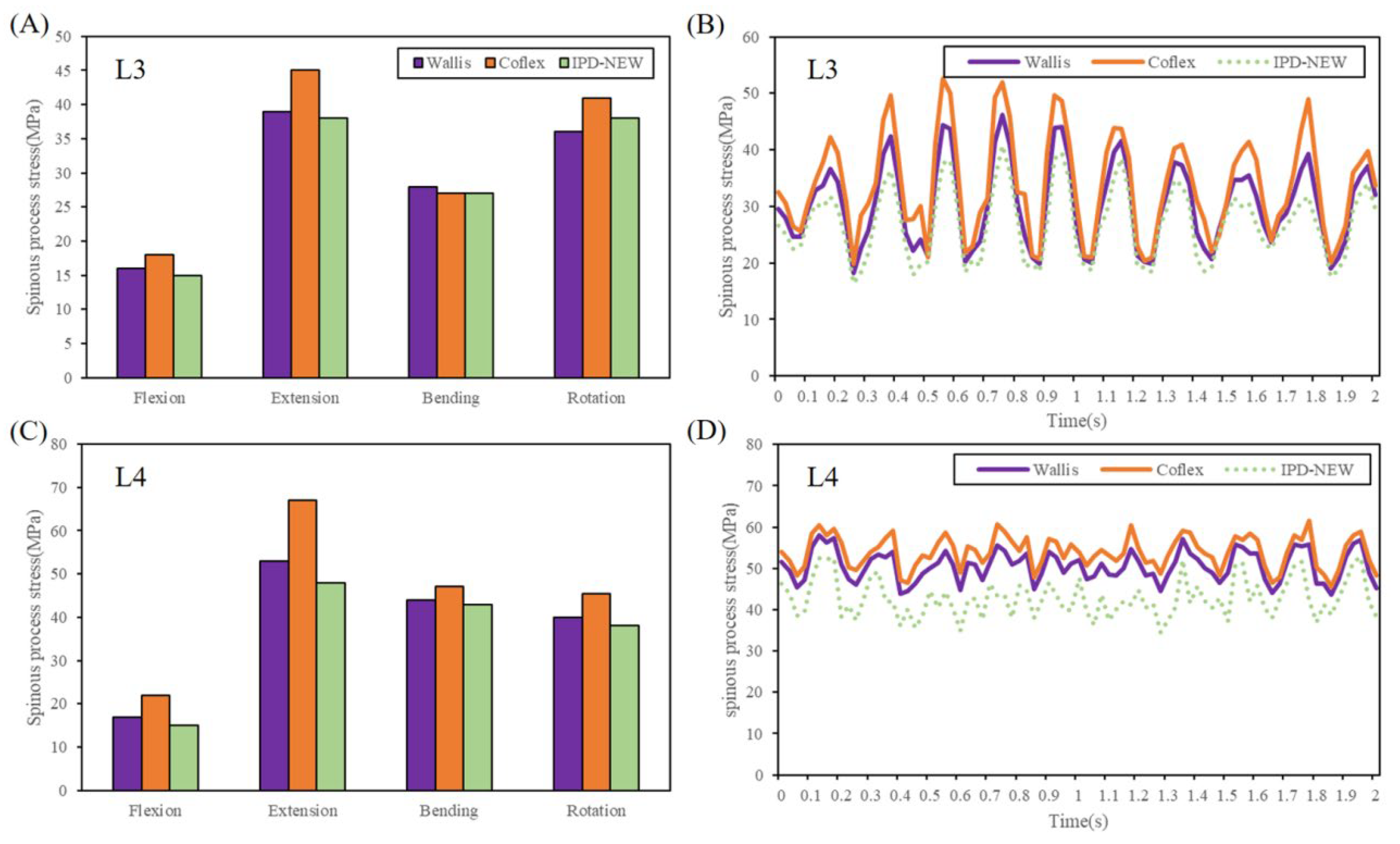
3.6. Spinous Process Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alentado, V.J.; Mobasser, D.; Mobasser, J.P.; Potts, E.A. Midline lumbar interbody fusion: A review of the surgical technique and outcomes. J. Neurosurg. Spine 2023, 39, 462–470. [Google Scholar] [PubMed]
- Phan, K.; Thayaparan, G.K.; Mobbs, R.J. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion-systematic review and meta-analysis. Br. J. Neurosurg. 2019, 29, 705–711. [Google Scholar]
- Wang, B.; Hua, W.; Ke, W.; Lu, S.; Li, X.; Zeng, X.; Yang, C. Biomechanical Evaluation of Transforaminal Lumbar Interbody Fusion and Oblique Lumbar Interbody Fusion on the Adjacent Segment: A Finite Element Analysis. World Neurosurg. 2019, 126, 819–824. [Google Scholar]
- Lee, H.J.; Lee, S.J.; Jung, J.M.; Lee, T.H.; Jeong, C.; Lee, T.J.; Jang, J.E.; Lee, J.W. Biomechanical Evaluation of Lateral Lumbar Interbody Fusion with Various Fixation Options for Adjacent Segment Degeneration: A Finite Element Analysis. World Neurosurg. 2023, 173, 156–167. [Google Scholar]
- Park, P.; Garton, H.J.; Gala, V.C.; Hoff, J.T.; McGillicuddy, J.E. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine 2004, 29, 1938–1944. [Google Scholar]
- Guo, L.X.; Yin, J.Y. Finite element analysis and design of an interspinous device using topology optimization. Med. Biol. Eng. Comput. 2019, 57, 89–98. [Google Scholar]
- Merkow, J.; Varhabhatla, N.; Manchikanti, L.; Kaye, A.D.; Urman, R.D.; Yong, R.J. Minimally Invasive Lumbar Decompression and Interspinous Process Device for the Management of Symptomatic Lumbar Spinal Stenosis: A Literature Review. Curr. Pain Headache Rep. 2020, 24, 13. [Google Scholar]
- Skoblar, M.; Hedman, T.; Rogers, A.J.; Jasper, G.P.; Beall, D.P. Instrumented Posterior Arthrodesis of the Lumbar Spine: Prospective Study Evaluating Fusion Outcomes in Patients Receiving an Interspinous Fixation Device for the Treatment of Degenerative Spine Diseases. J. Pain Res. 2023, 16, 2909–2918. [Google Scholar]
- Mo, Z.; Dong, L.; Zhang, R.; Chang, M.; Yang, B.; Tang, S. Comparative effectiveness and safety of posterior lumbar interbody fusion, Coflex, Wallis, and X-stop for lumbar degenerative diseases: A systematic review and network meta-analysis. Clin. Neurol. Neurosurg. 2018, 172, 74–81. [Google Scholar]
- Zhang, Y.; Lu, D.; Ji, W.; He, F.; Chen, A.; Yang, H.; Zhu, X. Which is the most effective treatment for lumbar spinal stenosis: Decompression, fusion, or interspinous process device? A Bayesian network meta-analysis. J. Orthop. Transl. 2021, 26, 45–53. [Google Scholar]
- Wilke, H.J.; Drumm, J.; Hussler, K.; Mack, C.; Steudel, W.L.; Kettler, A. Biomechanical effect of different lumbar interspinous implants on flexibility and intradiscal pressure. Eur. Spine J. 2008, 17, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.K.; Fogel, G.R.; Zhu, J.; Liao, Z.H.; Liu, W.Q. Biomechanical Analysis of Different Lumbar Interspinous Process Devices: A Finite Element Study. World Neurosurg. 2019, 127, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Guo, L.X. Biomechanical analysis of lumbar spine with interbody fusion surgery and U-shaped lumbar interspinous spacers. Comput. Methods Biomech. Biomed. Engin. 2021, 24, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Shih, S.L. Biomechanical analysis of a new lumbar interspinous device with optimized topology. Med. Biol. Eng. Comput. 2018, 56, 1333–1341. [Google Scholar] [CrossRef]
- Xu, C.; Ni, W.F.; Tian, N.F.; Hu, X.Q.; Li, F.; Xu, H.Z. Complications in degenerative lumbar disease treated with a dynamic interspinous spacer (Coflex). Int. Orthop. 2013, 37, 2199–2204. [Google Scholar] [CrossRef]
- Lee, N.; Shin, D.A.; Kim, K.N.; Yoon, D.H.; Ha, Y.; Shin, H.C.; Yi, S. Paradoxical Radiographic Changes of Coflex Interspinous Device with Minimum 2-Year Follow-Up in Lumbar Spinal Stenosis. World Neurosurg. 2016, 85, 177–184. [Google Scholar] [CrossRef]
- Tartara, F.; Garbossa, D.; Armocida, D.; Di Perna, G.; Ajello, M.; Marengo, N.; Bozzaro, M.; Petrone, S.; Giorgi, P.D.; Schirò, G.R.; et al. Relationship between lumbar lordosis, pelvic parameters, PI-LL mismatch and outcome after short fusion surgery for lumbar degenerative disease. Literature review, rational and presentation of public study protocol: RELApSE study (registry for evaluation of lumbar arthrodesis sagittal alignEment). World Neurosurg.-X 2023, 18, 100162. [Google Scholar]
- Choi, J.; Shin, D.A.; Kim, S. Finite element analysis of a ball-and-socket artificial disc design to suppress excessive loading on facet joints: A comparative study with ProDisc. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3214. [Google Scholar] [CrossRef]
- Fan, W.; Guo, L.X.; Dan, Z. Posterior Lumbar Interbody Fusion Versus Transforaminal Lumbar Interbody Fusion: Finite Element Analysis of the Vibration Characteristics of Fused Lumbar Spine. World Neurosurg. 2021, 150, 81–88. [Google Scholar] [CrossRef]
- Fan, W.; Guo, L.X. Prediction of the influence of vertical whole-body vibration on biomechanics of spinal segments after lumbar interbody fusion surgery. Clin. Biomech. 2021, 86, 105389. [Google Scholar] [CrossRef]
- Renner, S.M.; Natarajan, R.N.; Patwardhan, A.G.; Havey, R.M.; Voronov, L.I.; Guo, B.Y.; Andersson, G.B.J.; An, H.S. Novel model to analyze the effect of a large compressive follower pre-load on range of motions in a lumbar spine. J. Biomech. 2007, 40, 1326–1332. [Google Scholar] [PubMed]
- Berkson, M.H.; Nachemson, A.; Schultz, A.B. Mechanical-properties of human lumbar spine motion segments. 2. Responses in compression and shear-influence of gross morphology. J Biomech. Eng.—T. ASME 1979, 101, 53–57. [Google Scholar] [CrossRef]
- Brinckmann, P.; Grootenboer, H. Change of disk height, radial disk bulge, and intradiscal pressure from discectomy-an in vitro investigation on human lumbar disks. Spine 1991, 16, 641–646. [Google Scholar] [PubMed]
- Khalaf, K.; Nikkhoo, M. Comparative biomechanical analysis of rigid vs. flexible fixation devices for the lumbar spine: A geometrically patient-specific poroelastic finite element study. Comput. Methods Programs Biomed. 2021, 212, 106481. [Google Scholar]
- Park, W.M.; Choi, D.K.; Kim, Y.H.; Kim, K. Pre-tension effects from tightening the ligature on spinous process fracture risk in interspinous process device implantation. Int. J. Precision Eng. Manuf. 2014, 15, 2597–2604. [Google Scholar]
- Liu, X.; Ma, J.; Park, P.; Huang, X.; Xie, N.; Ye, X. Biomechanical comparison of multilevel lateral interbody fusion with and without supplementary instrumentation: A three-dimensional finite element study. BMC Musculoskelet Disord 2017, 18, 63. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Luo, W.A.; Huang, J.W.; Peng, C.H.; Wang, H.C.; Yuan, C.H.; Chen, G.R.; Zeng, B.R.; Dai, L.Z. Simplification of Hyperelastic Constitutive Model and Finite Element Analysis of Thermoplastic Polyurethane Elastomers. Macromol. Theory Simul. 2020, 29, 2000009. [Google Scholar]
- Sethi, A.; Muzumdar, A.M.; Ingalhalikar, A.; Vaidya, R. Biomechanical analysis of a novel posterior construct in a transforaminal lumbar interbody fusion model an in vitro study. Spine J. 2011, 11, 863–869. [Google Scholar] [CrossRef]
- Kirnaz, S.; Capadona, C.; Wong, T.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath, L.B.; Härtl, R. Fundamentals of Intervertebral Disc Degeneration. World Neurosurg. 2022, 157, 264–273. [Google Scholar]
- Cho, P.G.; Yoon, S.J.; Shin, D.A.; Chang, M.C. Finite Element Analysis of Stress Distribution and Range of Motion in Discogenic Back Pain. Neurospine 2024, 21, 536–543. [Google Scholar]
- Zhang, X.Y.; Han, Y. Comparison of the biomechanical effects of lumbar disc degeneration on normal patients and osteoporotic patients: A finite element analysis. Med. Eng. Phys. 2023, 112, 103952. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, N.; Hongo, M.; Maekawa, S.; Ishikawa, Y.; Shimada, Y.; Okada, K.; Itoi, E. Factors related to spinal mobility in patients with postmenopausal osteoporosis. Osteoporos. Int. 2005, 16, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Sawa, A.G.U.; Lehrman, J.N.; Crawford, N.R.; Kelly, B.P. Variations Among Human Lumbar Spine Segments and Their Relationships to In Vitro Biomechanics: A Retrospective Analysis of 281 Motion Segments From 85 Cadaveric Spines. Int. J. Spine Surg. 2020, 14, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.G.; Kang, S.W.; Jung, G.H.; Cho, M.G.; Kim, H.; Kim, K.T.; Kim, D.H.; Hwang, J.M. Biomechanical Effect of Disc Height on the Components of the Lumbar Column at the Same Axial Load: A Finite-Element Study. J. Healthc. Eng. 2022, 7069448. [Google Scholar] [CrossRef]
- Machino, M.; Nakashima, H.; Ito, K.; Katayama, Y.; Matsumoto, T.; Tsushima, M.; Ando, K.; Kobayashi, K.; Imagama, S. Age-related degenerative changes and sex-specific differences in osseous anatomy and intervertebral disc height of the thoracolumbar spine. J. Clin. Neurosci. 2021, 90, 317–324. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, C.; Zhang, D.X.; Guo, L.X.; Zhang, M. Biomechanical responses of the human lumbar spine to vertical whole-body vibration in normal and osteoporotic conditions. Clin. Biomech. 2023, 102, 105872. [Google Scholar]
- Zhang, C.; Guo, L.X. Prediction of the biomechanical behaviour of the lumbar spine under multi-axis whole-body vibration using a whole-body finite element model. Int. J. Numer. Methods Biomed. Eng. 2023, 39, e3764. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, C.; Zhang, D.X.; Guo, L.X.; Zhang, M. Biomechanical analysis of lumbar nonfusion dynamic stabilization using a pedicle screw-based dynamic stabilizer or an interspinous process spacer. Int. J. Numer. Methods Biomed. Eng. 2022, 38, e3645. [Google Scholar] [CrossRef]
- Fan, W.; Guo, L.X.; Zhao, D. Stress analysis of the implants in transforaminal lumbar interbody fusion under static and vibration loadings: A comparison between pedicle screw fixation system with rigid and flexible rods. Journal of materials science. Mater. Med. 2019, 30, 118. [Google Scholar] [CrossRef]
- Bae, H.W.; Davis, R.J.; Lauryssen, C.; Leary, S.; Maislin, G.; Musacchio, M.J. Three-year follow-up of the prospective, randomized, controlled trial of coflex interlaminar stabilization vs instrumented fusion in patients with lumbar stenosis. Neurosurg. 2016, 79, 169–180. [Google Scholar]
- Alfieri, A.; Gazzeri, R.; Prell, J.; Scheller, C.; Rachinger, J.; Strauss, C.; Schwarz, A. Role of lumbar interspinous distraction on the neural elements. Neurosurg. Rev. 2012, 35, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.K.; Fogel, G.R.; Zhu, J.; Liao, Z.H.; Liu, W.Q. Biomechanical analysis of lumbar fusion with proximal interspinous process device implantation. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3498. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.J.; Li, C.H.; Sun, J.Y.; Xie, H.X.; Qi, Y.; Niu, W.X.; Zhang, M. The trunk segmental motion complexity and balance performance in challenging seated perturbation among individuals with spinal cord injury. J. Neuroeng. Rehabil. 2025, 22, 4. [Google Scholar] [CrossRef]
- Chabarova, O.; Selivonec, J.; Menendez Hurtado, A. Investigation of the Role of Osteoporotic Vertebra Degeneration on the Stability of the Lumbar Spine: In Silico Modelling under Compressive Loading. Bioengineering 2024, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.B.; Liu, S.; Nie, M.D.; Yuan, J.B.; Lin, X.M.; Chu, X.R.; Shi, Z.C. Treatment of Lumbar Degenerative Disease with a Novel Interlaminar Screw Elastic Spacer Technique: A Finite Element Analysis. Bioengineering 2023, 10, 1204. [Google Scholar] [CrossRef]



| Components of the Model | Young’s Modulus (MPa) | Poisson Ratio | Sectional Area (mm2) | Density (kg/mm3) | References |
|---|---|---|---|---|---|
| Cortical bone | 12,000 | 0.3 | 1.7 × 10−6 | Zhang et al. [18] | |
| Cancellous bone | 100 | 0.2 | 1.1 × 10−6 | ||
| Posterior bone | 3500 | 0.25 | 1.4 × 10−6 | ||
| Endplate | 24 | 0.25 | 1.2 × 10−6 | Liu et al. [26] | |
| Nucleus pulposus | 1 | 0.49 | 1.02 × 10−6 | Zhang et al. [18] | |
| Annulus fibrosus | 4.2 | 0.45 | 1.05 × 10−6 | ||
| Anterior longitudinal ligament | 20 | 0.3 | 63.7 | 1 × 10−6 | |
| Posterior longitudinal ligament | 20 | 0.3 | 20 | 1 × 10−6 | |
| Ligament flava | 19.5 | 0.3 | 40 | 1 × 10−6 | |
| Interspinal ligament | 11.6 | 0.3 | 40 | 1 × 10−6 | |
| Supraspinal ligament | 15 | 0.3 | 30 | 1 × 10−6 | |
| Intertransverse ligament | 58.7 | 0.3 | 3.6 | 1 × 10−6 | |
| Capsular ligament | 32.9 | 0.3 | 60 | 1 × 10−6 | |
| Coflex (Ti6Al4V) | 110,000 | 0.3 | 4.5 × 10−6 | Guo et al. [6] | |
| Wallis (PEEK) | 3500 | 0.4 | 1.32 × 10−6 | Park et al. [25] | |
| IPD-NEW (Ti6Al4V) | 110,000 | 0.3 | 4.5 × 10−6 | Guo et al. [6] | |
| Ligatures | 2400 | 0.4 | 1.0 × 10−6 | Park et al. [25] | |
| TPU cushion | Hyperelastic, Mooney–Rivlin C01 = 17.4, C10 = −11.11, C02 = 3.134 | 0.8 × 10−6 | Wang et al. [27] | ||
| Wallis | Coflex | IPD-NEW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dynamic Responses | Max | Min | VA | Max | Min | VA | Max | Min | VA |
| FJF (N) | |||||||||
| L3–L4 FJF | 96.5 | 83.1 | 13.4 | 79.8 | 63.0 | 16.8 | 54.7 | 46.8 | 7.9 |
| Device stress (N) | |||||||||
| IPD stress | - | - | - | 50.1 | 17.5 | 32.6 | 42.4 | 13.7 | 28.7 |
| Interspinous process stress (MPa) | |||||||||
| L3 stress | 46.2 | 18.2 | 28.0 | 52.6 | 19.8 | 32.8 | 40.8 | 16.4 | 24.4 |
| L4 stress | 58.1 | 43.6 | 14.5 | 61.6 | 45.6 | 16.0 | 52.7 | 34.6 | 18.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Ju, C.; Gao, T.; Zhu, J.; Liu, W. A Biomechanical Evaluation of a Novel Interspinous Process Device: In Vitro Flexibility Assessment and Finite Element Analysis. Bioengineering 2025, 12, 384. https://doi.org/10.3390/bioengineering12040384
Shen H, Ju C, Gao T, Zhu J, Liu W. A Biomechanical Evaluation of a Novel Interspinous Process Device: In Vitro Flexibility Assessment and Finite Element Analysis. Bioengineering. 2025; 12(4):384. https://doi.org/10.3390/bioengineering12040384
Chicago/Turabian StyleShen, Hangkai, Chuanguang Ju, Tao Gao, Jia Zhu, and Weiqiang Liu. 2025. "A Biomechanical Evaluation of a Novel Interspinous Process Device: In Vitro Flexibility Assessment and Finite Element Analysis" Bioengineering 12, no. 4: 384. https://doi.org/10.3390/bioengineering12040384
APA StyleShen, H., Ju, C., Gao, T., Zhu, J., & Liu, W. (2025). A Biomechanical Evaluation of a Novel Interspinous Process Device: In Vitro Flexibility Assessment and Finite Element Analysis. Bioengineering, 12(4), 384. https://doi.org/10.3390/bioengineering12040384







