Enhanced External Counterpulsation Intervention Induces the Variation of Physiological Parameters and Shear Stress Metrics in the Carotid Artery
Abstract
:1. Introduction
2. Animal Experiment
2.1. Animal Model
2.2. EECP Intervention
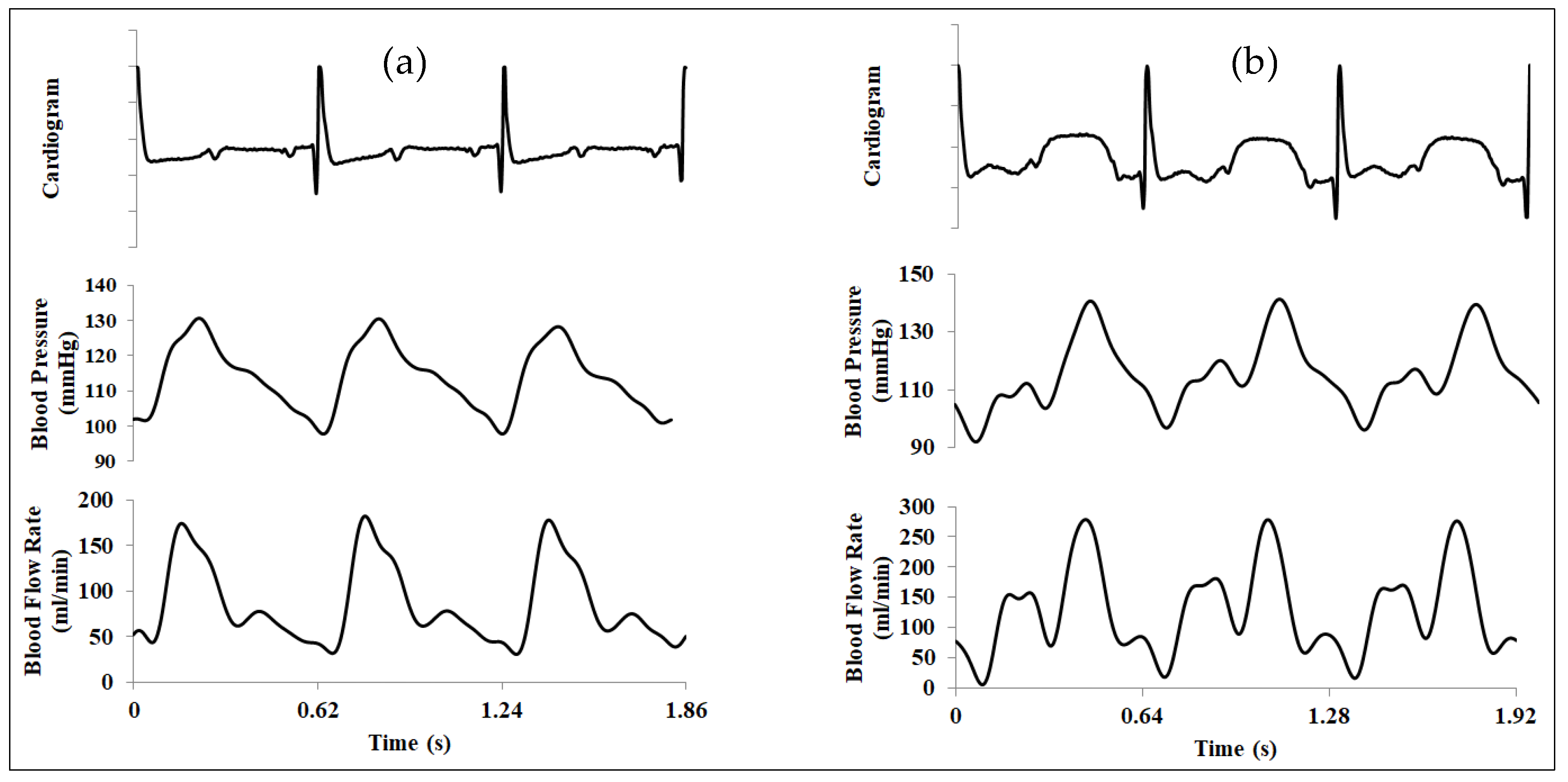
2.3. Invasive Physiological Measurement
3. Numerical Simulation Procedure
3.1. Geometry Reconstructions and Boundary Condition Settings
3.2. Mesh Generation and Mesh Independence Analysis
3.3. Governing Equations and the Solver
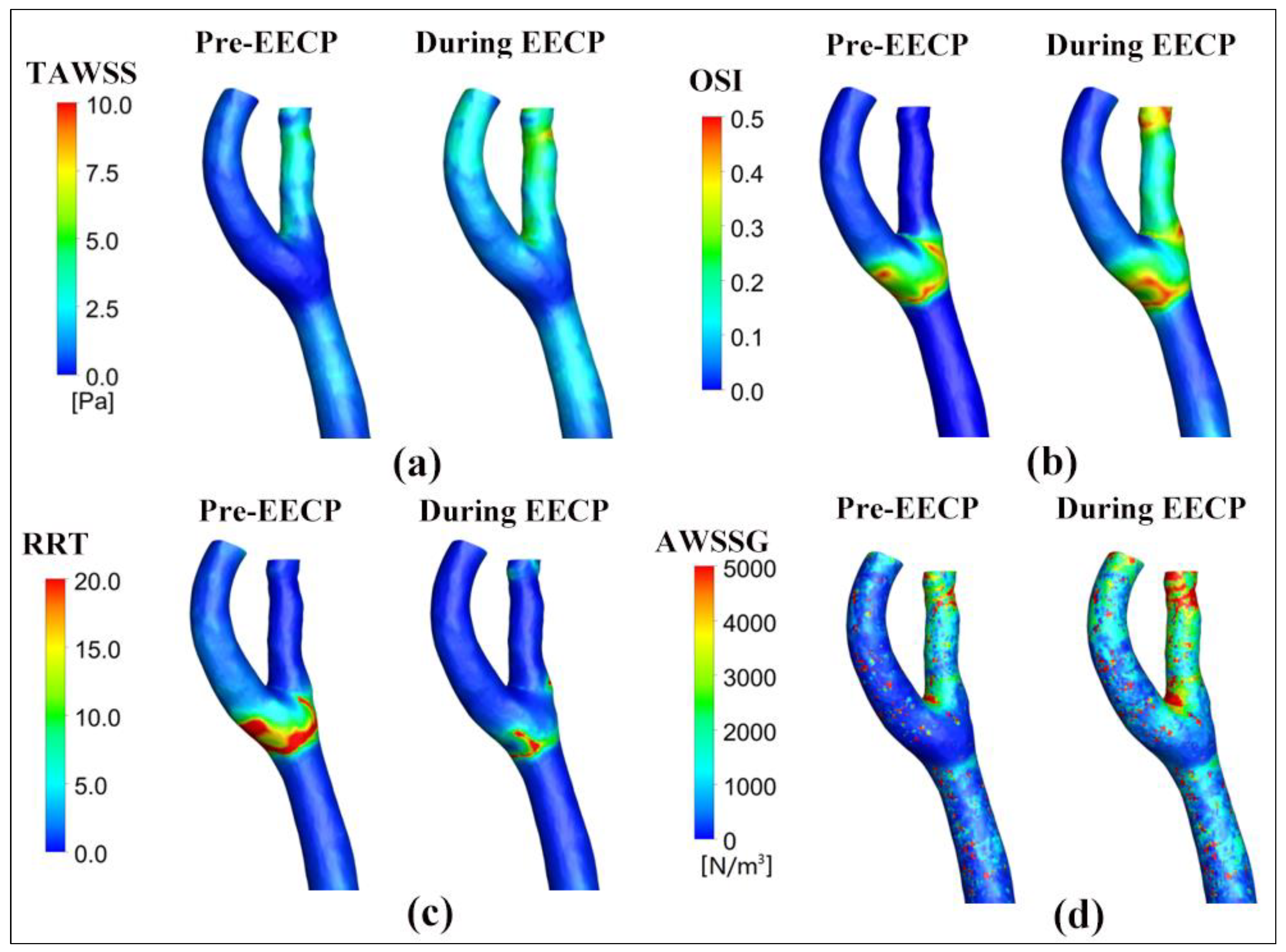
3.4. Wall Shear-Stress-Derived Hemodynamic Metrics
4. Computational Results and Discussion
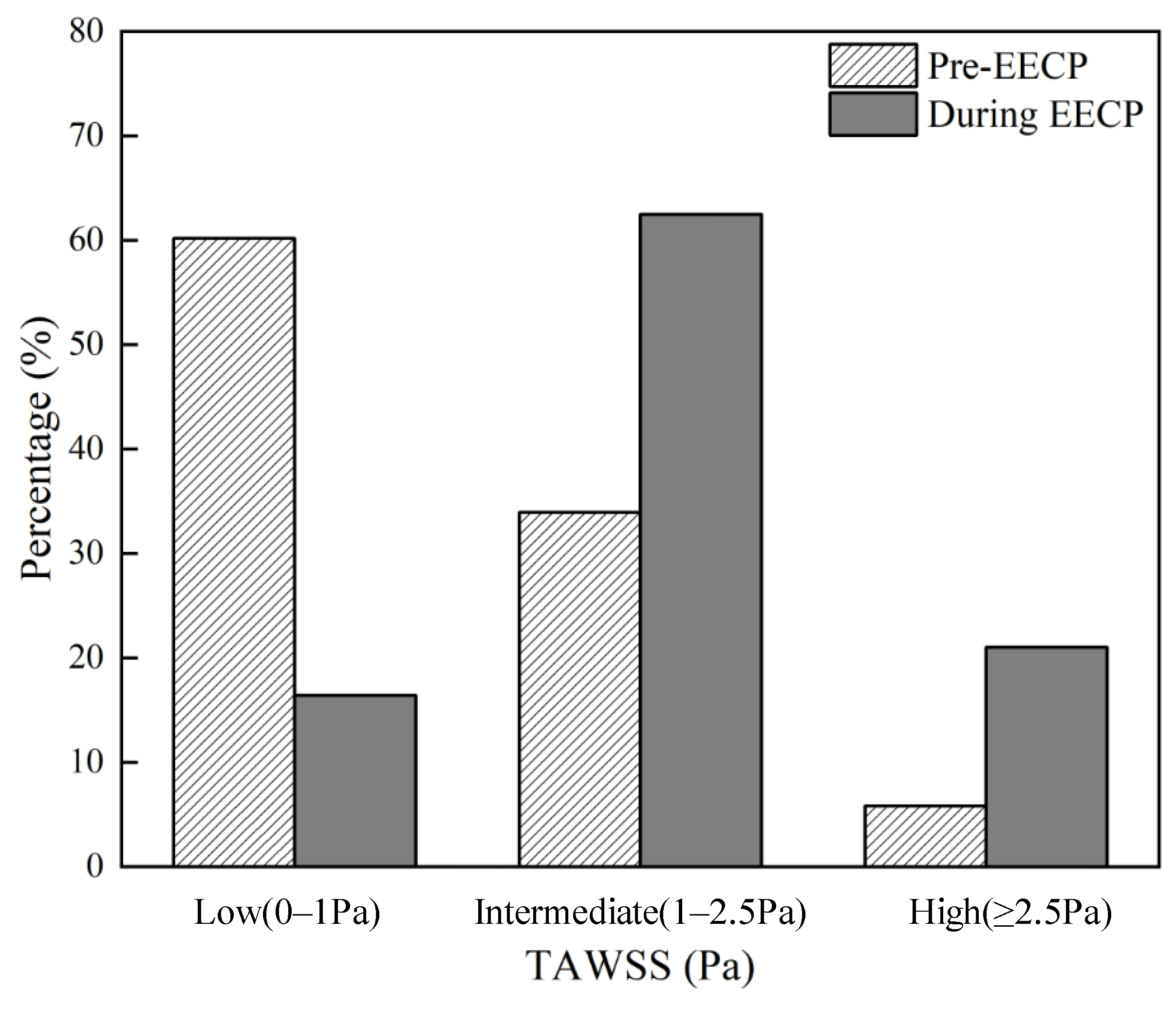
4.1. The Influence of EECP on WSS Distribution
4.2. The Effects of EECP Stimulus on WSS Metrics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Z.; Yu, L.; Cai, S.; Kambic, H.; Li, T.; Ma, H.; Chen, P.; Huang, B.; Nosé, Y. New Sequential External Counterpulsation for the Treatment of Acute Myocardial Infarction. Artif. Organs 1984, 8, 470–477. [Google Scholar] [PubMed]
- Werner, D.; Schneider, M.; Weise, M.; Nonnast-Daniel, B.; Daniel, W.G. Pneumatic External Counterpulsation: A New Noninvasive Method to Improve Organ Perfusion. Am. J. Cardiol. 1999, 84, 950–952. [Google Scholar] [PubMed]
- Michaels, A.D.; Accad, M.; Ports, T.A.; Grossman, W. Left Ventricular Systolic Unloading and Augmentation of Intracoronary Pressure and Doppler Flow during Enhanced External Counterpulsation. Circulation 2002, 106, 1237–1242. [Google Scholar] [PubMed]
- Braith, R.W.; Conti, C.R.; Nichols, W.W.; Choi, C.Y.; Khuddus, M.A.; Beck, D.T.; Casey, D.P. Enhanced External Counterpulsation Improves Peripheral Artery Flow-Mediated Dilation in Patients with Chronic Angina: A Randomized Sham-Controlled Study. Circulation 2010, 122, 1612–1620. [Google Scholar]
- Lin, W.; Xiong, L.; Han, J.; Leung, T.W.H.; Soo, Y.O.Y.; Chen, X.; Wong, K.S.L. External Counterpulsation Augments Blood Pressure and Cerebral Flow Velocities in Ischemic Stroke Patients with Cerebral Intracranial Large Artery Occlusive Disease. Stroke 2012, 43, 3007–3011. [Google Scholar] [CrossRef]
- Qin, X.; Deng, Y.; Wu, D.; Yu, L.; Huang, R. Does Enhanced External Counterpulsation (EECP) Significantly Affect Myocardial Perfusion?: A Systematic Review & Meta-Analysis. PLoS ONE 2016, 11, e0151822. [Google Scholar]
- Du, J.; Peng, J.; Shen, X.; Li, X.; Zhong, H.; Gao, Z.; Chen, M.; Qi, L.; Xie, Q. Enhanced External Counterpulsation Treatment Regulates Blood Flow and Wall Shear Stress Metrics in Femoral Artery: An in Vivo Study in Healthy Subjects. J. Biomech. 2023, 159, 111797. [Google Scholar]
- Tian, S.; Pan, W.; Peng, J.; Wang, H.; Deng, B.; Liang, Y.; Li, X.; Liu, H.; Wang, Y.; Luo, B.; et al. Hemodynamic Responses in Carotid Bifurcation Induced by Enhanced External Counterpulsation Stimulation in Healthy Controls and Patients with Neurological Disorders. Front. Physiol. 2021, 12, 717080. [Google Scholar]
- Zhang, Y.; He, X.; Chen, X.; Ma, H.; Liu, D.; Luo, J.; Du, Z.; Jin, Y.; Xiong, Y.; He, J.; et al. Enhanced External Counterpulsation Inhibits Intimal Hyperplasia by Modifying Shear Stress–Responsive Gene Expression in Hypercholesterolemic Pigs. Circulation 2007, 116, 526–534. [Google Scholar]
- Bonetti, P.O.; Barsness, G.W.; Keelan, P.C.; Schnell, T.I.; Pumper, G.M.; Kuvin, J.T.; Schnall, R.P.; Holmes, D.R.; Higano, S.T.; Lerman, A. Enhanced External Counterpulsation Improves Endothelial Function in Patients with Symptomatic Coronary Artery Disease. J. Am. Coll. Cardiol. 2003, 41, 1761–1768. [Google Scholar]
- Tao, J.; Tu, C.; Yang, Z.; Zhang, Y.; Chung, X.-L.; Ma, H.; Zhen, Z.-S. Enhanced External Counterpulsation Improves Endothelium-Dependent Vasorelaxation in the Carotid Arteries of Hypercholesterolemic Pigs. Int. J. Cardiol. 2006, 112, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.P.; Beck, D.T.; Nichols, W.W.; Conti, C.R.; Choi, C.Y.; Khuddus, M.A.; Braith, R.W. Effects of Enhanced External Counterpulsation on Arterial Stiffness and Myocardial Oxygen Demand in Patients with Chronic Angina Pectoris. Am. J. Cardiol. 2011, 107, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Steinberg, K.; Tartaglia, J.; Frishman, W.H.; Gupta, T. Enhanced External Counterpulsation Therapy: Past, Present, and Future. Cardiol. Rev. 2017, 25, 59–67. [Google Scholar] [CrossRef]
- Brown, A.J.; Teng, Z.; Evans, P.C.; Gillard, J.H.; Samady, H.; Bennett, M.R. Role of Biomechanical Forces in the Natural History of Coronary Atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 210–220. [Google Scholar] [CrossRef]
- Mohamied, Y.; Rowland, E.M.; Bailey, E.L.; Sherwin, S.J.; Schwartz, M.A.; Weinberg, P.D. Change of Direction in the Biomechanics of Atherosclerosis. Ann. Biomed. Eng. 2015, 43, 16–25. [Google Scholar] [CrossRef]
- Thondapu, V.; Bourantas, C.V.; Foin, N.; Jang, I.-K.; Serruys, P.W.; Barlis, P. Biomechanical Stress in Coronary Atherosclerosis: Emerging Insights from Computational Modelling. Eur. Heart J. 2017, 38, 81–92. [Google Scholar] [CrossRef]
- Buchanan, J.R., Jr.; Kleinstreuer, C.; Truskey, G.A.; Lei, M. Relation between Non-Uniform Hemodynamics and Sites of Altered Permeability and Lesion Growth at the Rabbit Aorto-Celiac Junction. Atherosclerosis 1999, 143, 27–40. [Google Scholar] [CrossRef]
- Rikhtegar, F.; Knight, J.A.; Olgac, U.; Saur, S.C.; Poulikakos, D.; Marshall, W., Jr.; Cattin, P.C.; Alkadhi, H.; Kurtcuoglu, V. Choosing the Optimal Wall Shear Parameter for the Prediction of Plaque Location—A Patient-Specific Computational Study in Human Left Coronary Arteries. Atherosclerosis 2012, 221, 432–437. [Google Scholar] [CrossRef]
- Murphy, J.; Boyle, F. Predicting Neointimal Hyperplasia in Stented Arteries Using Time-Dependant Computational Fluid Dynamics: A Review. Comput. Biol. Med. 2010, 40, 408–418. [Google Scholar] [CrossRef]
- Iasiello, M.; Vafai, K.; Andreozzi, A.; Bianco, N. Analysis of Non-Newtonian Effects within an Aorta-Iliac Bifurcation Region. J. Biomech. 2017, 64, 153–163. [Google Scholar] [CrossRef]
- Samady, H.; Eshtehardi, P.; McDaniel, M.C.; Suo, J.; Dhawan, S.S.; Maynard, C.; Timmins, L.H.; Quyyumi, A.A.; Giddens, D.P. Coronary Artery Wall Shear Stress Is Associated with Progression and Transformation of Atherosclerotic Plaque and Arterial Remodeling in Patients with Coronary Artery Disease. Circulation 2011, 124, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Ku, D.N.; Giddens, D.P.; Zarins, C.K.; Glagov, S. Pulsatile Flow and Atherosclerosis in the Human Carotid Bifurcation. Positive Correlation between Plaque Location and Low Oscillating Shear Stress. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1985, 5, 293–302. [Google Scholar]
- Hoi, Y.; Zhou, Y.-Q.; Zhang, X.; Henkelman, R.M.; Steinman, D.A. Correlation between Local Hemodynamics and Lesion Distribution in a Novel Aortic Regurgitation Murine Model of Atherosclerosis. Ann. Biomed. Eng. 2011, 39, 1414–1422. [Google Scholar] [CrossRef]
- LaDisa, J.F.; Olson, L.E.; Douglas, H.A.; Warltier, D.C.; Kersten, J.R.; Pagel, P.S. Alterations in Regional Vascular Geometry Produced by Theoretical Stent Implantation Influence Distributions of Wall Shear Stress: Analysis of a Curved Coronary Artery Using 3D Computational Fluid Dynamics Modeling. Biomed. Eng. Online 2006, 5, 40. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef]



| Mesh Quantity | 444,236 | 800,064 | 1,728,230 | 2,996,975 | |
| TAWSS (Pa) | Pre EECP | 8.549 | 8.267 | 8.023 | 8.070 |
| During EEECP | 8.650 | 8.504 | 8.425 | 8.490 | |
| Enhancement (-) | 1.012 | 1.029 | 1.050 | 1.052 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Wu, Z.; Wang, Y.; Jakhongirkhon, I.; Zhou, Q.; Du, J. Enhanced External Counterpulsation Intervention Induces the Variation of Physiological Parameters and Shear Stress Metrics in the Carotid Artery. Bioengineering 2025, 12, 386. https://doi.org/10.3390/bioengineering12040386
Ren Z, Wu Z, Wang Y, Jakhongirkhon I, Zhou Q, Du J. Enhanced External Counterpulsation Intervention Induces the Variation of Physiological Parameters and Shear Stress Metrics in the Carotid Artery. Bioengineering. 2025; 12(4):386. https://doi.org/10.3390/bioengineering12040386
Chicago/Turabian StyleRen, Zhenfeng, Zi’an Wu, Yanjing Wang, Israilov Jakhongirkhon, Qianxiang Zhou, and Jianhang Du. 2025. "Enhanced External Counterpulsation Intervention Induces the Variation of Physiological Parameters and Shear Stress Metrics in the Carotid Artery" Bioengineering 12, no. 4: 386. https://doi.org/10.3390/bioengineering12040386
APA StyleRen, Z., Wu, Z., Wang, Y., Jakhongirkhon, I., Zhou, Q., & Du, J. (2025). Enhanced External Counterpulsation Intervention Induces the Variation of Physiological Parameters and Shear Stress Metrics in the Carotid Artery. Bioengineering, 12(4), 386. https://doi.org/10.3390/bioengineering12040386







