Mechanical Characterization of Porous Bone-like Scaffolds with Complex Microstructures for Bone Regeneration
Abstract
1. Introduction
1.1. Background
1.2. Literature Review
1.3. Gaps and Objectives
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Scaffold Design
2.2.2. Parametric Modeling
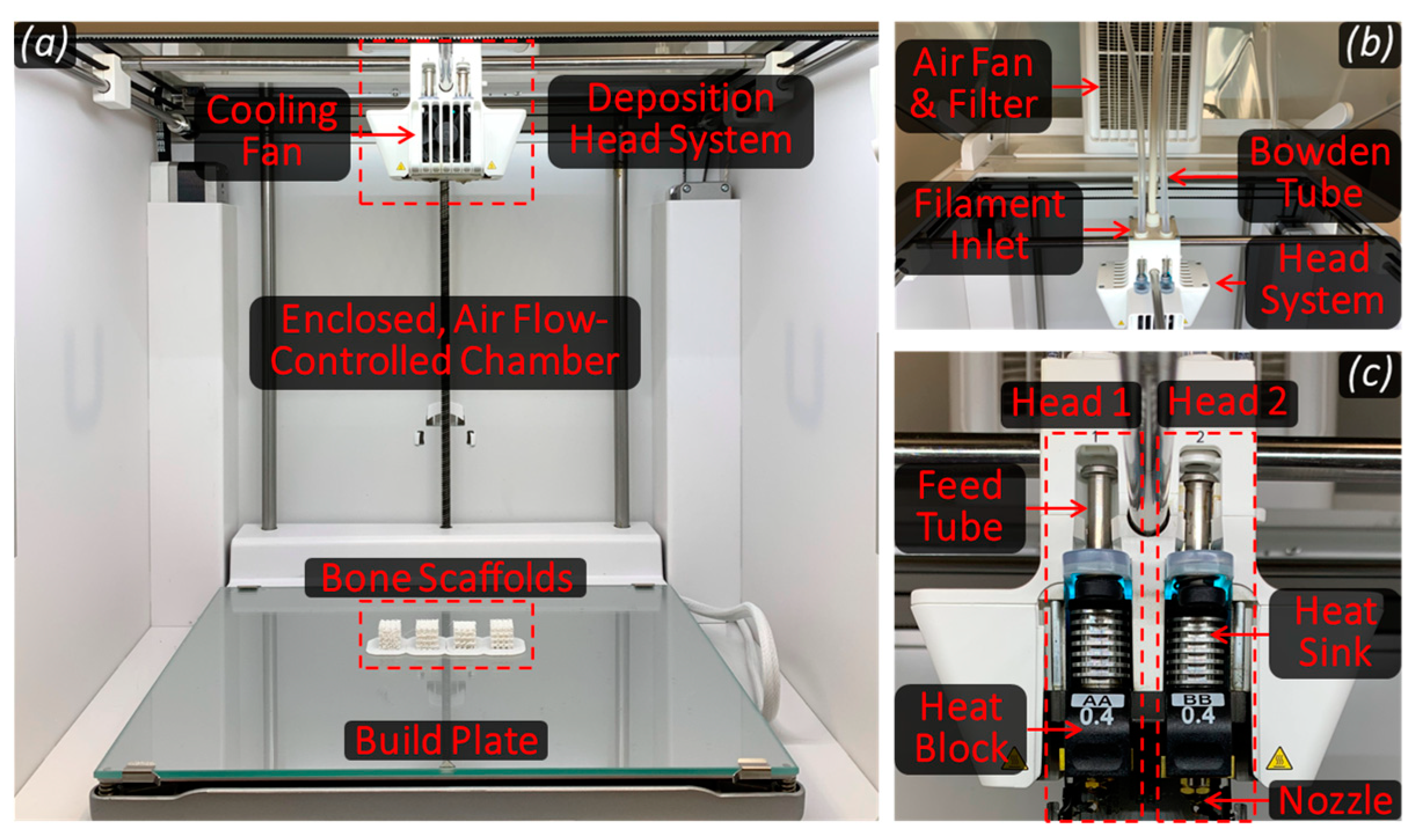
2.2.3. Scaffold Fabrication
2.2.4. Mechanical Characterization of Bone Scaffolds
3. Results and Discussion
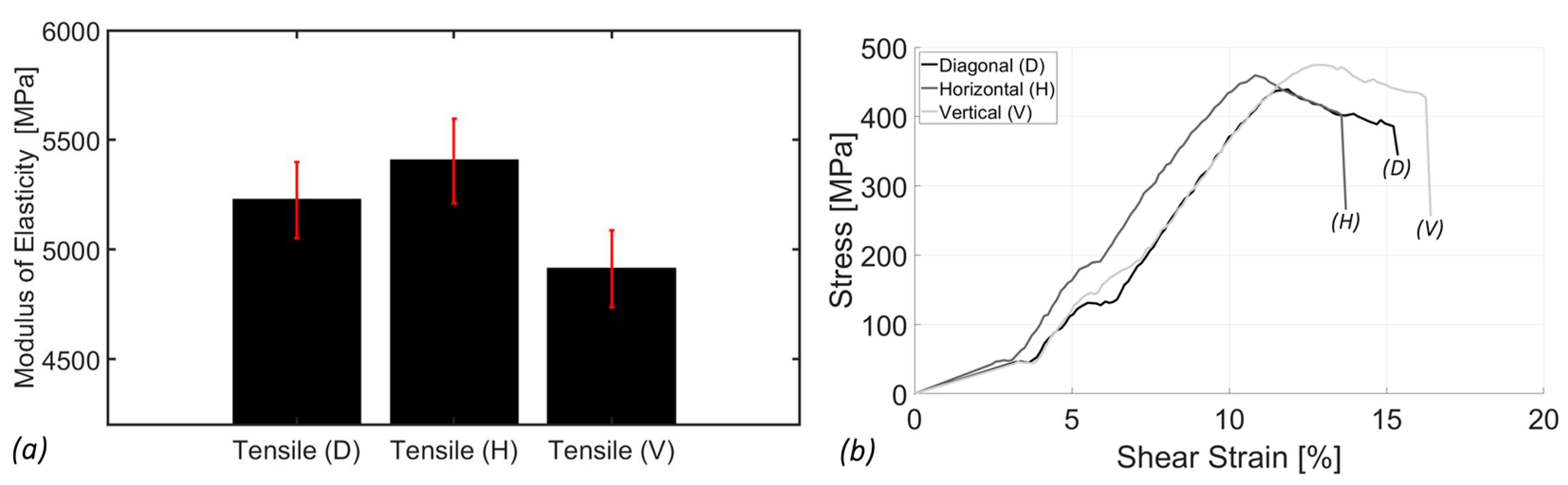
3.1. Tensile Analysis
3.2. Torsion Analysis
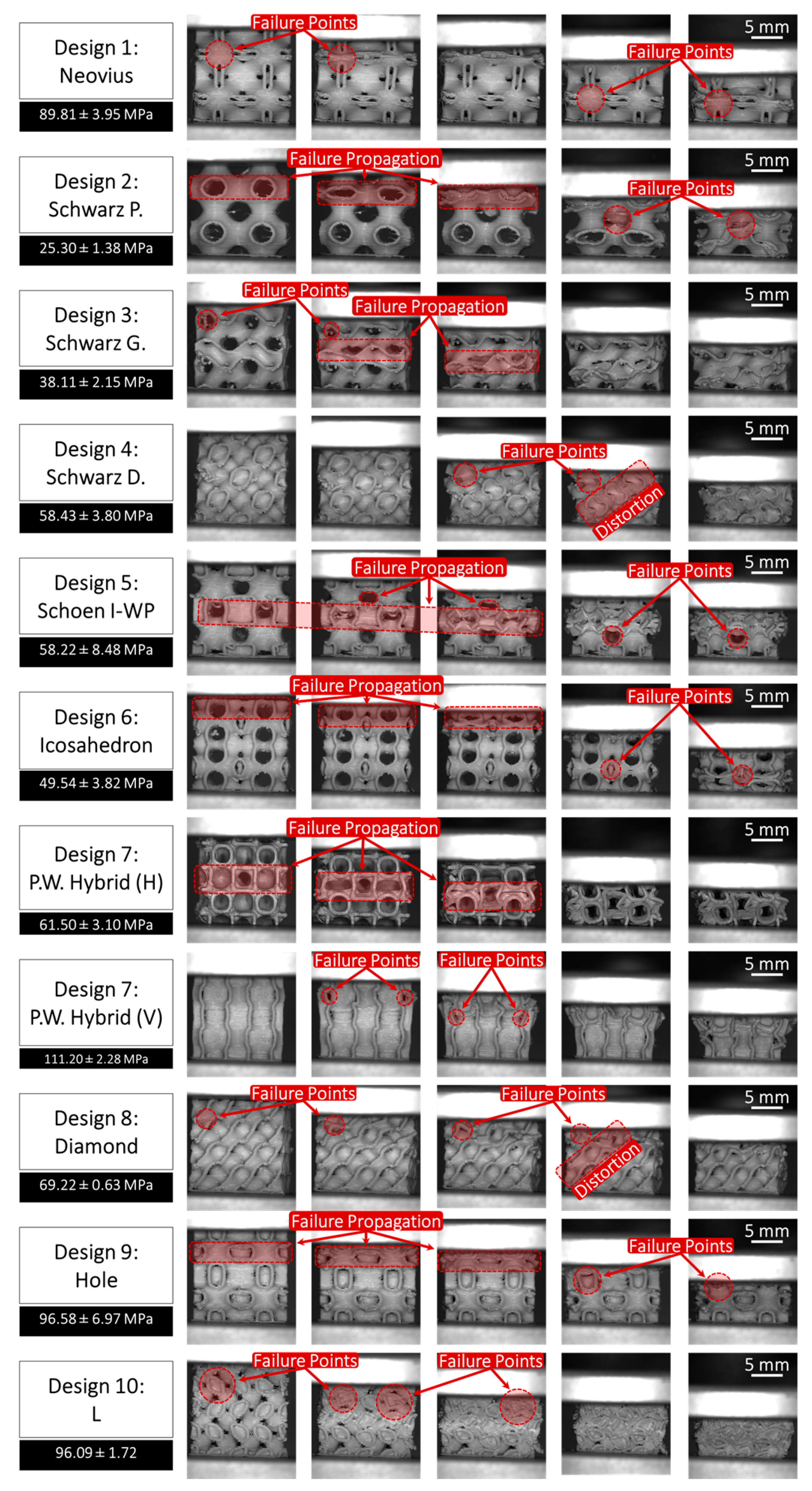
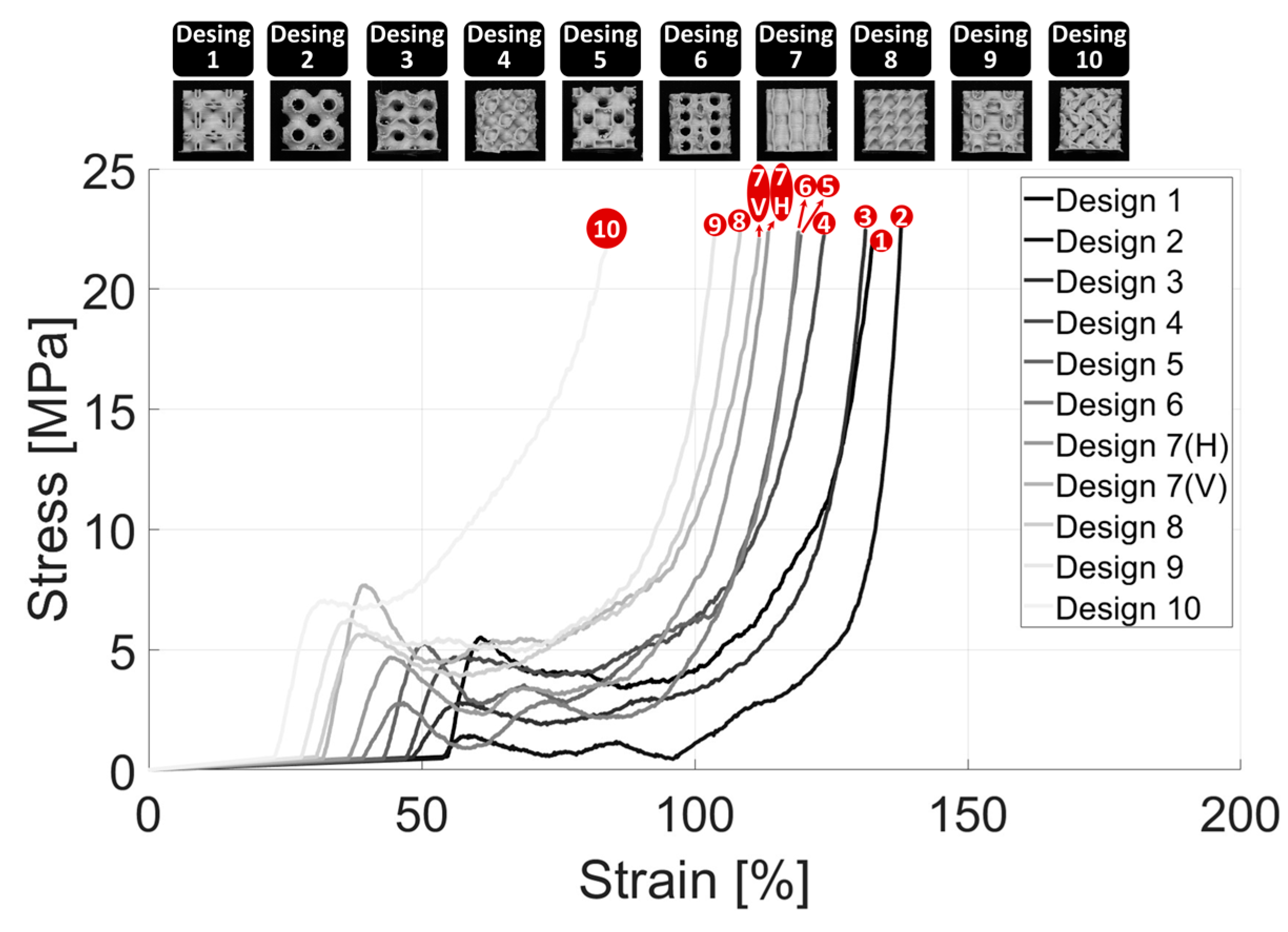
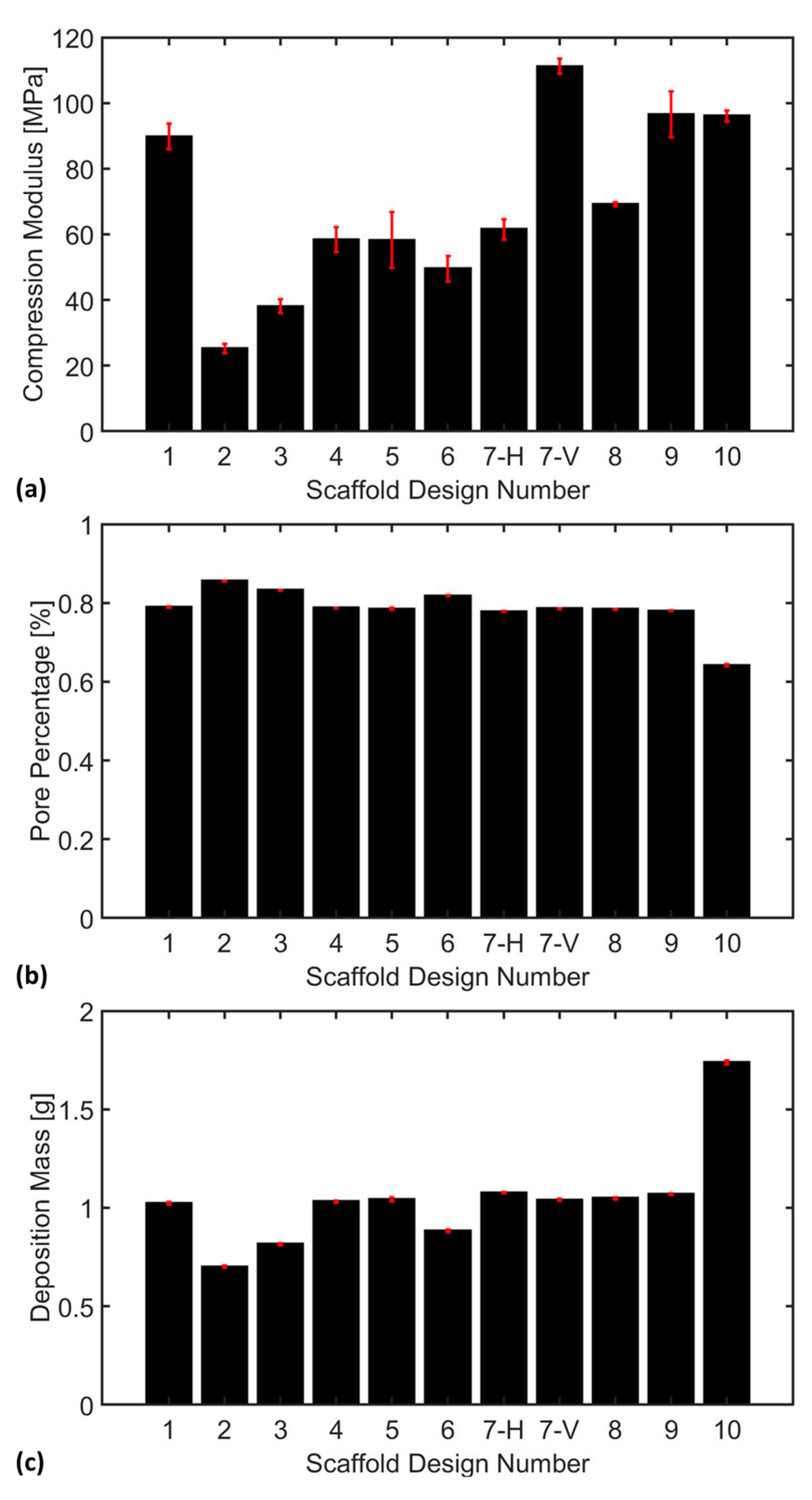
3.3. Compression Analysis
4. Conclusions and Future Work
4.1. Conclusions
- SimuBone exhibited a shear modulus of rigidity of 714.79 ± 11.97 MPa, indicating its resistance to angular deformation under applied stress. The material’s average yield strength was 44 ± 1.31 MPa, highlighting its ability to withstand mechanical loads before permanent deformation occurred. Additionally, necking and fracture were observed at approximately 1.5% and 1.75% strain, respectively, marking the onset of material instability and failure.
- Scaffold fabrication with the horizontal orientation (0°) exhibited the highest tensile modulus at 5404.20 ± 192.30 MPa, followed by the diagonal orientation (45°) at 5224.42 ± 173.77 MPa, and the vertical orientation (90°) at 4911.84 ± 175.18 MPa. This trend highlighted the anisotropic nature of SimuBone, where the horizontal orientation (0°) provided superior stiffness and structural integrity, making it the preferred configuration for load-bearing applications. Furthermore, SimuBone’s tensile stiffness closely aligned with that of cortical and cancellous bone, reinforcing its potential as a viable material for bone tissue engineering applications.
- Scaffolds with large voids exhibited a tendency to collapse inward under compressive loading, indicating a critical need for enhanced wall thickness to improve structural integrity. Among the scaffold designs, Schwarz Primitive (P) demonstrated the lowest compressive stiffness at 25.30 ± 1.38 MPa, making it the weakest design, whereas P.W. Hybrid (Vertical) emerged as the strongest with a compressive stiffness of 111.20 ± 2.28 MPa, highlighting its superior load-bearing capacity. Additionally, an inverse relationship was observed between pore percentage and deposition mass. However, no clear correlation was found between deposition mass and compression modulus, emphasizing the complex interplay of scaffold architecture, porosity, and mechanical performance.
- P.W. Hybrid demonstrated high stiffness in the vertical orientation but moderate stiffness in the horizontal orientation, highlighting its anisotropic mechanical behavior. Although suitable for load-bearing applications in specific directions, this characteristic may limit its versatility in bone tissue engineering.
- Scaffold #10 (L) exhibited a high compressive stress capacity, making it structurally robust under mechanical loading. However, its low pore percentage could hinder nutrient diffusion and cellular infiltration, potentially impacting biological integration and tissue regeneration.
- Scaffold #9 (Holes) and Scaffold #1 (Neovius) achieved a favorable balance between mechanical stiffness and biological functionality, offering both structural integrity and adequate porosity for cell growth and nutrient transport. These scaffolds show strong potential for bone tissue engineering applications, combining mechanical durability with enhanced bioactivity.
4.2. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Z.; Zhao, X. Application of TPMS Structure in Bone Regeneration. Eng. Regen. 2021, 2, 154–162. [Google Scholar] [CrossRef]
- Naghavi, S.A.; Tamaddon, M.; Marghoub, A.; Wang, K.; Babamiri, B.B.; Hazeli, K.; Xu, W.; Lu, X.; Sun, C.; Wang, L. Mechanical Characterisation and Numerical Modelling of TPMS-Based Gyroid and Diamond Ti6Al4V Scaffolds for Bone Implants: An Integrated Approach for Translational Consideration. Bioengineering 2022, 9, 504. [Google Scholar] [CrossRef]
- Du, X.; Ronayne, S.; Lee, S.S.; Hendry, J.; Hoxworth, D.; Bock, R.; Ferguson, S.J. 3D-Printed PEEK/Silicon Nitride Scaffolds with a Triply Periodic Minimal Surface Structure for Spinal Fusion Implants. ACS Appl. Bio Mater. 2023, 6, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Khrapov, D.; Kozadayeva, M.; Manabaev, K.; Panin, A.; Sjöström, W.; Koptyug, A.; Mishurova, T.; Evsevleev, S.; Meinel, D.; Bruno, G. Different Approaches for Manufacturing Ti-6Al-4V Alloy with Triply Periodic Minimal Surface Sheet-Based Structures by Electron Beam Melting. Materials 2021, 14, 4912. [Google Scholar] [CrossRef]
- Mishra, A.K.; Chavan, H.; Kumar, A. Effect of Material Variation on the Uniaxial Compression Behavior of FDM Manufactured Polymeric TPMS Lattice Materials. Mater. Today Proc. 2021, 46, 7752–7759. [Google Scholar] [CrossRef]
- Tilton, M.; Jacobs, E.; Overdorff, R.; Potes, M.A.; Lu, L.; Manogharan, G. Biomechanical Behavior of PMMA 3D Printed Biomimetic Scaffolds: Effects of Physiologically Relevant Environment. J. Mech. Behav. Biomed. Mater. 2023, 138, 105612. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, D.; Maskery, I.; Longhitano, G.; Jardini, A.; Del Conte, E. Investigation of Load Direction on the Compressive Strength of Additively Manufactured Triply Periodic Minimal Surface Scaffolds. Int. J. Adv. Manuf. Technol. 2020, 109, 771–779. [Google Scholar] [CrossRef]
- Hameed, P.; Liu, C.-F.; Ummethala, R.; Singh, N.; Huang, H.-H.; Manivasagam, G.; Prashanth, K.G. Biomorphic Porous Ti6Al4V Gyroid Scaffolds for Bone Implant Applications Fabricated by Selective Laser Melting. Prog. Addit. Manuf. 2021, 6, 455–469. [Google Scholar] [CrossRef]
- Maevskaia, E.; Guerrero, J.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Triply Periodic Minimal Surface-Based Scaffolds for Bone Tissue Engineering: A Mechanical, In Vitro and In Vivo Study. Tissue Eng. Part A 2023, 29, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Ruben, R.; Gonçalves, S.; Pinheiro, J.; Guedes, J.; Fernandes, P. Numerical and Experimental Evaluation of TPMS Gyroid Scaffolds for Bone Tissue Engineering. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 567–573. [Google Scholar] [CrossRef]
- Valainis, D.; Dondl, P.; Foehr, P.; Burgkart, R.; Kalkhof, S.; Duda, G.N.; Van Griensven, M.; Poh, P.S. Integrated Additive Design and Manufacturing Approach for the Bioengineering of Bone Scaffolds for Favorable Mechanical and Biological Properties. Biomed. Mater. 2019, 14, 065002. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Z.; Hu, X.; Kuang, H.; Zhai, J. The Effect of Porosity on the Mechanical Properties of 3D-Printed Triply Periodic Minimal Surface (TPMS) Bioscaffold. Bio Des. Manuf. 2019, 2, 242–255. [Google Scholar] [CrossRef]
- Yang, L.; Yan, C.; Cao, W.; Liu, Z.; Song, B.; Wen, S.; Zhang, C.; Shi, Y.; Yang, S. Compression–Compression Fatigue Behaviour of Gyroid-Type Triply Periodic Minimal Surface Porous Structures Fabricated by Selective Laser Melting. Acta Mater. 2019, 181, 49–66. [Google Scholar] [CrossRef]
- Gabrieli, R.; Wenger, R.; Mazza, M.; Verné, E.; Baino, F. Design, Stereolithographic 3D Printing, and Characterization of TPMS Scaffolds. Materials 2024, 17, 654. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Kuan, L.Y.; Lu, W.F. 3D-Printed Ceramic Triply Periodic Minimal Surface Structures for Design of Functionally Graded Bone Implants. Mater. Des. 2020, 191, 108602. [Google Scholar] [CrossRef]
- Piper, S. Maths Models: Triply Periodic Minimal Surface Structures Mega Pack. 2018. Available online: https://www.myminifactory.com/object/3d-print-maths-models-triply-periodic-minimal-surface-structures-mega-pack-73944 (accessed on 31 March 2025).
- Feng, J.; Fu, J.; Yao, X.; He, Y. Triply Periodic Minimal Surface (TPMS) Porous Structures: From Multi-Scale Design, Precise Additive Manufacturing to Multidisciplinary Applications. Int. J. Extreme Manuf. 2022, 4, 022001. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- TecQuipment. SM1001 Torsion Testing Machine (30 Nm). Available online: https://www.tecquipment.com/torsion-testing-machine-30-nm (accessed on 31 March 2025).
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical Properties and the Hierarchical Structure of Bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Morgan, E.F.; Niebur, G.L.; Yeh, O.C. Biomechanics of Trabecular Bone. Annu. Rev. Biomed. Eng. 2001, 3, 307–333. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
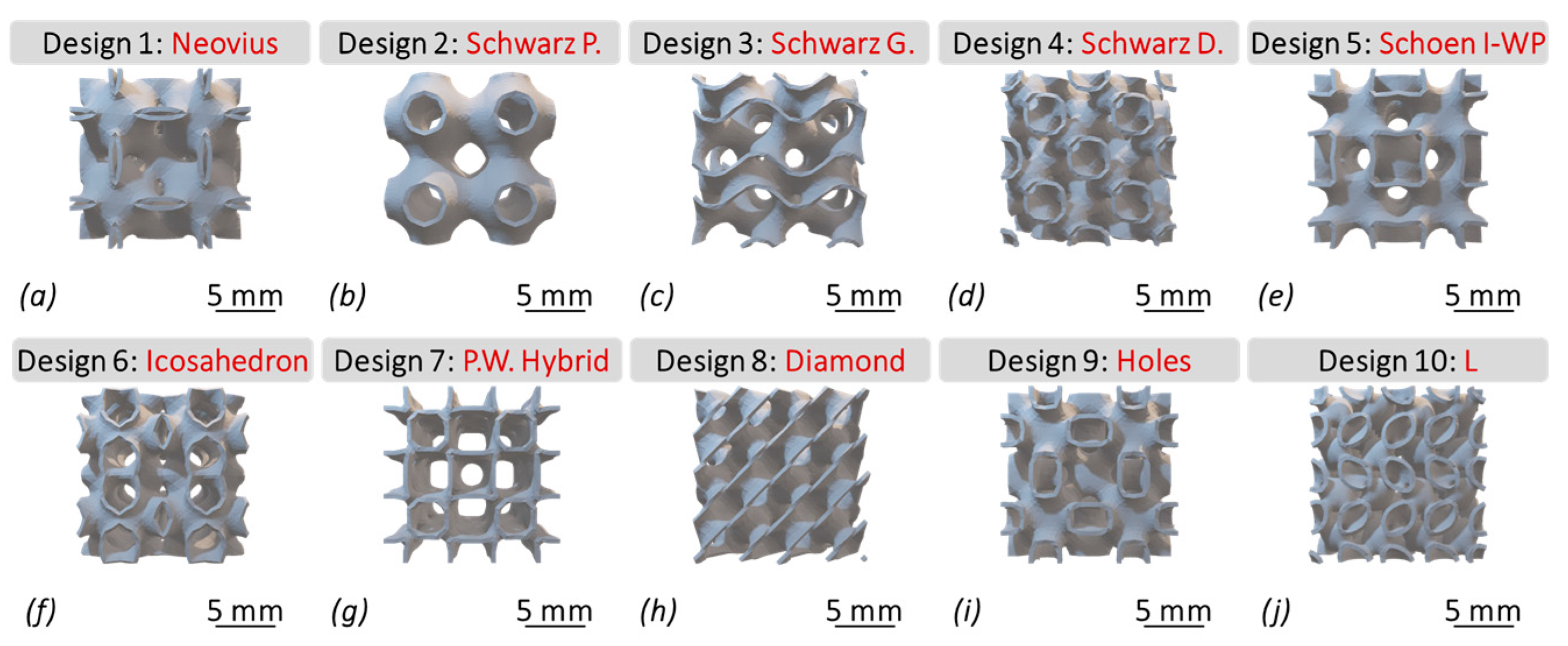



| Equations | TPMS Design |
|---|---|
| (1) | |
| (2) | |
| (3) | |
| (4) | |
| (5) | |
| (6) | |
| (7) | |
| (8) | |
| (9) | |
| (10) |
| Parameters | Values |
|---|---|
| Model Dimensions (mm3) | 6 × 6 |
| Iteration Step Size for Designs (b) through (i) Iteration Step Size for Designs (a) and (j) | 7.710 18.710 |
| Merged Toggle | True |
| IsoValue | −0.269 |
| ArrBox (x,y,z) Count | 2 |
| Level | 1 |
| WBThickness Distance (mm) | 0.15 |
| Parameter | Type | Level [Unit] |
| Medical Composite | Material | SimuBone (Medical Grade) |
| Scaffold Dimensions | Design | 15 × 15 × 15 [mm] |
| Layer Height (Thickness) | Design | 200 [µm] |
| Layer (Line) Width | Design | 300 [µm] |
| Infill Density | Design | 100 [%] |
| Nozzle Size | Machine | 400 [µm] |
| Bed Temperature | Machine | 60 [°C] |
| Print Speed | Machine | 10 [mm/s] |
| Deposition Head Temperature | Machine | 240 [°C] |
| Flow (Feed) Rate | Machine | 120 [%] |
| Build Plate Adhesion Type | Machine | Brim |
| Parameters | Torsion Bar | Tensile Bar |
| Type | SM 1001 | ASTM D638−14 (Type IV) |
| Width narrow section (mm) | 6 | 6 |
| Length narrow section (mm) | 76.2 | 33 |
| Width Overall (mm) | - | 19 |
| Length Overall (mm) | 143 | 115 |
| Gage Length (mm) | - | 25 |
| Distance Between Grips (mm) | - | 65 |
| Radius of Fillets (mm) | - | 14 |
| Outer Radius (mm) | - | 25 |
| Outer Area (mm2) | 12 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coburn, B.; Salary, R.R. Mechanical Characterization of Porous Bone-like Scaffolds with Complex Microstructures for Bone Regeneration. Bioengineering 2025, 12, 416. https://doi.org/10.3390/bioengineering12040416
Coburn B, Salary RR. Mechanical Characterization of Porous Bone-like Scaffolds with Complex Microstructures for Bone Regeneration. Bioengineering. 2025; 12(4):416. https://doi.org/10.3390/bioengineering12040416
Chicago/Turabian StyleCoburn, Brandon, and Roozbeh Ross Salary. 2025. "Mechanical Characterization of Porous Bone-like Scaffolds with Complex Microstructures for Bone Regeneration" Bioengineering 12, no. 4: 416. https://doi.org/10.3390/bioengineering12040416
APA StyleCoburn, B., & Salary, R. R. (2025). Mechanical Characterization of Porous Bone-like Scaffolds with Complex Microstructures for Bone Regeneration. Bioengineering, 12(4), 416. https://doi.org/10.3390/bioengineering12040416








