Review on Current Advancements in Facilitation of Burn Wound Healing
Abstract
1. Introduction
2. Methods
3. Classification of Burn Wound
3.1. Classification Based on Severity by Burn Size
3.2. Classification Based on Depth of Burn
- 2A (Superficial Partial Thickness): These involve injuries to the epidermis and the upper half of the dermis. Blisters and weeping are present, accompanied by pain. Scarring may occur, and victims require dressing and wound care but typically do not need surgery.
- 2B (Deep Partial Thickness): These burns damage the deeper layers of the dermis and are often less painful due to the partial destruction of pain receptors. The wound typically has more blisters, appears drier, and is likely to result in more scarring. Surgical intervention, such as skin grafts, is usually required.
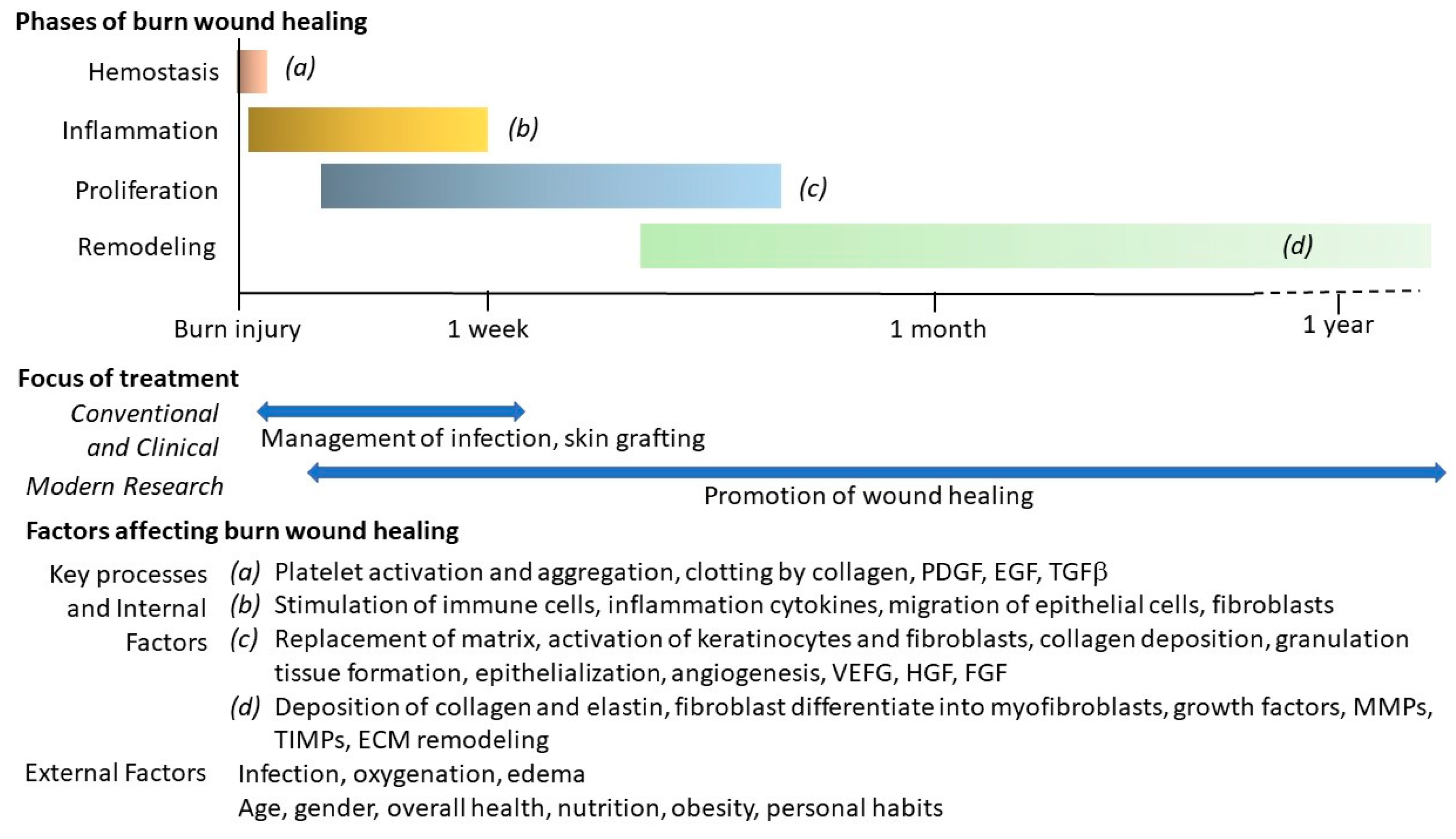
4. Different Phases in Burn Wound Healing
5. Other Factors Affecting Burn Wound Healing
6. Conventional Methods in Burn Wound Management
7. Modern Approaches to Promote Burn Wound Healing
7.1. Modern Wound Dressing
- Interactive Dressings: These include semi-permeable films and foams.
- Advanced Interactive Dressings: These comprise hydrocolloids and hydrogels, which are highly hydrophilic macromolecular networks.
- Bioactive Dressings: Tissue-engineered skin equivalents fall into this category.
7.2. Platelet-Rich Plasma (PRP)
7.3. Somatic and Stem Cells
7.4. Soluble Factors Derived by MSCs
7.5. Nano-Technology
7.6. Modernized Traditional Medicine
8. Barriers in Translating Innovations for Facilitation of Burn Wound Healing
8.1. Variability in Animal Models
8.2. Complexity of Burn Wounds
8.3. Lack of Standardization
8.4. Limited Clinical Trials
8.5. Interdisciplinary Collaboration
8.6. Others
9. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef]
- Yi, L.; Xie, Z.; Wang, Z.; Guo, G.; Liu, D.; Du, Y.; Chen, S.; Tao, X.; Xie, C.; Dai, X.; et al. Time trends in thermal burns incidence among Brazil, Russia, India, China, and South Africa (BRICS), an age-period-cohort analysis from the GBD 2019. Sci. Rep. 2025, 15, 6877. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.emro.who.int/health-topics/burns/index.html (accessed on 12 March 2025).
- Saavedra, P.A.E.; De Oliveira Leal, J.V.; Areda, C.A.; Galato, D. The Costs of Burn Victim Hospital Care around the World: A Systematic Review. Iran. J. Public Health 2021, 50, 866–878. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Hawkins, H.K.; Lee, J.O.; Cox, R.A.; Kulp, G.A.; Finnerty, C.C.; Chinkes, D.L.; Jeschke, M.G. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care 2009, 13, R183. [Google Scholar] [CrossRef] [PubMed]
- Tasleem, S.; Siddiqui, A.I.; Zuberi, M.A.W.; Tariq, H.; Abdullah, M.; Hameed, A.; Aijaz, A.; Shah, H.H.; Hussain, M.S.; Oduoye, M.O. Mortality patterns and risk factors in burn patients: A cross-sectional study from Pakistan. Burn. Open 2024, 8, 13–18. [Google Scholar] [CrossRef]
- American Burn Association; American College of Surgeons. Guidelines for the operation of burn centers. J. Burn Care Res. 2007, 28, 134–141. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Shih, B.; Garside, E.; McGrouther, D.A.; Bayat, A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010, 18, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds 2022, 21, 18–30. [Google Scholar] [CrossRef]
- American Burn Association. 2024 Annual Burn Injury Summary Report: Analysis of Inpatient Care at Burn Centers 2019–2023. 2024. Available online: https://ameriburn.org/wp-content/uploads/2024/08/2024-bisr-3.pdf (accessed on 12 March 2025).
- Beheshti, A.; Shafigh, Y.; Zangivand, A.A.; Samiee-Rad, F.; Hassanzadeh, G.; Shafigh, N. Comparison of topical sucralfate and silver sulfadiazine cream in second degree burns in rats. Adv. Clin. Exp. Med. 2013, 22, 481–487. [Google Scholar] [PubMed]
- Fong, J.; Wood, F. Nanocrystalline silver dressings in wound management: A review. Int. J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Cappadone, C.; Sallustio, V.; Picone, G.; Rossi, M.; Nicoletta, F.P.; Luppi, B.; Bigucci, F.; Cerchiara, T. Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing: Characterization and Evaluation of Biological Properties. Pharmaceutics 2021, 13, 1192. [Google Scholar] [CrossRef]
- Assis, A.C.L.; Moreira, L.M.C.C.; Rocha, B.P.; Pereira, M.R.B.; de Melo, D.F.; Moura, R.O.; Azevedo, E.P.; Oshiro-Junior, J.A.; Damasceno, B.P.G.L. N-acylhydrazone Derivative-Loaded Cellulose Acetate Films: Thermoanalytical, Spectroscopic, Mechanical and Morphological Characterization. Polymers 2021, 13, 2345. [Google Scholar] [CrossRef]
- Goodwin, N.S.; Spinks, A.; Wasiak, J. The efficacy of hydrogel dressings as a first aid measure for burn wound management in the pre-hospital setting: A systematic review of the literature. Int. Wound J. 2016, 13, 519–525. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Siu, W.S.; Kan, C.W.; Leung, P.C.; Wanxue, C.; Chiou, J.C. Influence of pH-responsive compounds synthesized from chitosan and hyaluronic acid on dual-responsive (pH/temperature) hydrogel drug delivery systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, M.; Peng, Y.; He, S.; Zhu, X.; Hu, C.; Xia, G.; Zuo, T.; Zhang, X.; Yun, Y.; et al. A physicochemical double-cross-linked gelatin hydrogel with enhanced antibacterial and anti-inflammatory capabilities for improving wound healing. J. Nanobiotechnol. 2022, 20, 426, Erratum in J. Nanobiotechnol. 2023, 21, 262. [Google Scholar] [CrossRef]
- Johnson, K.A.; Muzzin, N.; Toufanian, S.; Slick, R.A.; Lawlor, M.W.; Seifried, B.; Moquin, P.; Latulippe, D.; Hoare, T. Drug-impregnated, pressurized gas expanded liquid-processed alginate hydrogel scaffolds for accelerated burn wound healing. Acta Biomater. 2020, 112, 101–111. [Google Scholar] [CrossRef]
- Kushibiki, T.; Mayumi, Y.; Nakayama, E.; Azuma, R.; Ojima, K.; Horiguchi, A.; Ishihara, M. Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor. Sci. Rep. 2021, 11, 23094. [Google Scholar] [CrossRef]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed]
- Andia, I. Platelet-rich plasma biology. In Clinical Indications and Treatment Protocols with Platelet-Rich Plasma in Dermatology; Alves, R., Grimalt, R., Eds.; Ediciones Mayo: Barcelona, Spain, 2016; pp. 3–15. [Google Scholar]
- Villela, D.L.; Santos, V.L. Evidence on the use of platelet-rich plasma for diabetic ulcer: A systematic review. Growth Factors 2010, 28, 111–116. [Google Scholar] [CrossRef]
- Ha, C.W.; Park, Y.B.; Jang, J.W.; Kim, M.; Kim, J.A.; Park, Y.G. Variability of the Composition of Growth Factors and Cytokines in Platelet-Rich Plasma From the Knee With Osteoarthritis. Arthroscopy 2019, 35, 2878–2884.e1. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Viswanath, O.; Galasso, A.C.; Sottosani, E.R.; Mahan, K.M.; Aiudi, C.M.; Kaye, A.D.; Orhurhu, V.J. Platelet-Rich Plasma for the Treatment of Low Back Pain: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Platelet-rich plasma: Underlying biology and clinical correlates. Regen. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef]
- Margolis, D.J.; Kantor, J.; Santanna, J.; Strom, B.L.; Berlin, J.A. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001, 24, 483–488. [Google Scholar] [CrossRef]
- Ren, Z.Q.; Du, B.; Dong, H.J.; Duan, G.H.; Du, A.C.; Wang, Y.; Zhao, L.X.; Shao, W. Autologous Platelet-Rich Plasma Repairs Burn Wound and Reduces Burn Pain in Rats. J. Burn Care Res. 2022, 43, 263–268. [Google Scholar] [CrossRef]
- Novosad, Y.A.; Shabunin, A.S.; Enukashvily, N.I.; Supilnikova, O.V.; Konkina, A.I.; Semenova, N.Y.; Yatsemirsky, G.S.; Zinoviev, E.V.; Rodionova, K.N.; Kryshen, K.L.; et al. The Wound-Healing Effect of a Novel Fibroblasts-Impregnated Hydroxyethylcellulose Gel in a Rat Full-Thickness Burn Model: A Preclinical Study. Biomedicines 2024, 12, 2215. [Google Scholar] [CrossRef]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018, 123, 18–32. [Google Scholar] [CrossRef]
- Clark, R.A.F.; Ghosh, K.; Tonnesen, M.G. Tissue engineering for cutaneous wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef]
- Ghieh, F.; Jurjus, R.; Ibrahim, A.; Geagea, A.G.; Daouk, H.; El Baba, B.; Chams, S.; Matar, M.; Zein, W.; Jurjus, A. The use of stem cells in burn wound healing: A review. BioMed Res. Int. 2015, 2015, 684084. [Google Scholar] [CrossRef]
- Aliniay-Sharafshadehi, S.; Yousefi, M.H.; Ghodratie, M.; Kashfi, M.; Afkhami, H.; Ghoreyshiamiri, S.M. Exploring the therapeutic potential of different sources of mesenchymal stem cells: A novel approach to combat burn wound infections. Front. Microbiol. 2024, 15, 1495011. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.T.; Pirraco, R.P.; Marques, A.P. Stem cells in skin wound healing: Are we there yet? Adv. Wound Care 2016, 5, 164–175. [Google Scholar] [CrossRef]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Coalson, E.; Bishop, E.; Liu, W.; Feng, Y.; Spezia, M.; Liu, B.; Shen, Y.; Wu, D.; Du, S.; Li, A.J.; et al. Stem cell therapy for chronic skin wounds in the era of personalized medicine: From bench to bedside. Genes. Dis. 2019, 6, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M.; et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int. Wound J. 2017, 14, 112–124. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Moll, G.; Alm, J.J.; Davies, L.C.; von Bahr, L.; Heldring, N.; Stenbeck-Funke, L.; Hamad, O.A.; Hinsch, R.; Ignatowicz, L.; Locke, M.; et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 2014, 32, 2430–2442. [Google Scholar] [CrossRef]
- François, M.; Copland, I.B.; Yuan, S.; Romieu-Mourez, R.; Waller, E.K.; Galipeau, J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 2012, 14, 147–152. [Google Scholar] [CrossRef]
- Hill, E.; Boontheekul, T.; Mooney, D.J. Regulating activation of transplanted cells controls tissue regeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin. Biol. Ther. 2009, 9, 879–887. [Google Scholar] [CrossRef]
- Jiang, D.; Rinkevich, Y. Scars or Regeneration?—Dermal Fibroblasts as Drivers of Diverse Skin Wound Responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef]
- Zagoura, D.; Trohatou, O.; Makridakis, M.; Kollia, A.; Kokla, N.; Mokou, M.; Psaraki, A.; Eliopoulos, A.G.; Vlahou, A.; Roubelakis, M.G. Functional secretome analysis reveals Annexin-A1 as important paracrine factor derived from fetal mesenchymal stem cells in hepatic regeneration. EBioMedicine 2019, 45, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Cheng, Z.; Wu, Y.; Wu, Q.Y.; Liao, X.B.; Zhao, Y.; Li, J.M.; Zhou, X.M.; Fu, X.M. Mesenchymal stem cell-derived conditioned medium attenuate angiotensin II-induced aortic aneurysm growth by modulating macrophage polarization. J. Cell Mol. Med. 2019, 23, 8233–8245. [Google Scholar] [CrossRef]
- Ma, H.; Siu, W.S.; Leung, P.C. The Potential of MSC-Based Cell-Free Therapy in Wound Healing-A Thorough Literature Review. Int. J. Mol. Sci. 2023, 24, 9356. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lam, P.K.; Siu, W.S.; Tong, C.S.W.; Lo, K.K.Y.; Koon, C.M.; Wu, X.X.; Li, X.; Cheng, W.; Shum, W.T.; et al. Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs) and ADMSC-Derived Secretome Expedited Wound Healing in a Rodent Model—A Preliminary Study. Clin. Cosmet. Investig. Dermatol. 2021, 14, 753–764. [Google Scholar] [CrossRef]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef]
- Gangadaran, P.; Oh, E.J.; Rajendran, R.L.; Oh, J.M.; Kim, H.M.; Kwak, S.; Chung, H.Y.; Lee, J.; Ahn, B.C.; Hong, C.M. Three-dimensional culture conditioned bone marrow MSC secretome accelerates wound healing in a burn injury mouse model. Biochem. Biophys. Res. Commun. 2023, 673, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Zhu, K.; He, W.; Guo, X.; Wang, T.; Gong, S.; Zhu, Z. Exosomes from mesenchymal stem cells: Potential applications in wound healing. Life Sci. 2024, 357, 123066. [Google Scholar] [CrossRef]
- Kang, D.; Wang, X.; Chen, W.; Mao, L.; Zhang, W.; Shi, Y.; Xie, J.; Yang, R. Epidermal stem cell-derived exosomes improve wound healing by promoting the proliferation and migration of human skin fibroblasts. Burn. Trauma 2024, 12, tkae047. [Google Scholar] [CrossRef]
- Bo, Y.; Yang, L.; Liu, B.; Tian, G.; Li, C.; Zhang, L.; Yan, Y. Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing through miR-762 mediated promotion of keratinocytes and endothelial cells migration. J. Nanobiotechnol. 2022, 20, 291. [Google Scholar] [CrossRef]
- Palani, N.; Vijayakumar, P.; Monisha, P.; Ayyadurai, S.; Rajadesingu, S. Electrospun nanofibers synthesized from polymers incorporated with bioactive compounds for wound healing. J. Nanobiotechnol. 2024, 22, 211. [Google Scholar] [CrossRef]
- Ali, N.; Arshad, R.; Kousar, S.; Aman, W.; Ahmad, W.; Azeem, M.; Malik, A.; Shafique, M. Advanced wound healing: The synergy of nature and nanotechnology. J. Drug Deliv. Sci. Technol. 2025, 105, 106579. [Google Scholar] [CrossRef]
- Sellappan, L.K.; Manoharan, S. Fabrication of bioinspired keratin/sodium alginate based biopolymeric mat loaded with herbal drug and green synthesized zinc oxide nanoparticles as a dual drug antimicrobial wound dressing. Int. J. Biol. Macromol. 2024, 259 Pt 1, 129162. [Google Scholar] [CrossRef]
- Stojko, M.; Włodarczyk, J.; Sobota, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Dobrzyński, P.; Starczynowska, G.; Orchel, A.; Stojko, J.; et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics 2020, 12, 883. [Google Scholar] [CrossRef]
- Anisiei, A.; Andreica, B.I.; Mititelu-Tartau, L.; Coman, C.G.; Bilyy, R.; Bila, G.; Rosca, I.; Sandu, A.I.; Amler, E.; Marin, L. Biodegradable trimethyl chitosan nanofiber mats by electrospinning as bioabsorbable dressings for wound closure and healing. Int. J. Biol. Macromol. 2023, 249, 126056. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy 2002–2005. 2002. Available online: https://www.who.int/publications/i/item/WHO-EDM-TRM-2002.1 (accessed on 12 March 2025).
- World Health Organization. The Regional Strategy for Traditional Medicine in the Western Pacific (2011–2020). 2012. Available online: https://www.who.int/publications/i/item/9789290615590 (accessed on 12 March 2025).
- Maenthaisong, R.; Chaiyakunapruk, N.; Niruntraporn, S.; Kongkaew, C. The efficacy of aloe vera used for burn wound healing: A systematic review. Burns 2007, 33, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Chen, K.C.; Wang, J.H.; Lin, Y.K. Effects of Aloe vera on Burn Injuries: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Burn Care Res. 2024, 45, 1536–1545. [Google Scholar] [CrossRef]
- Subrahmanyam, M. Topical application of honey for burn wound treatment—An overview. Ann. Burn. Fire Disasters 2007, 20, 137–139. [Google Scholar]
- Moniruzzaman, M.; Khan, A.R.; Haq, M.A.; Naznin, R.A.; Haque, M. Pediatric First-Degree Burn Management With Honey and 1% Silver Sulfadiazine (Ag-SD): Comparison and Contrast. Cureus 2022, 14, e32842. [Google Scholar] [CrossRef] [PubMed]
- Dorai, A.A. Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg. 2012, 45, 418–424. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef]
- Pathak, D.; Mazumder, A. A critical overview of challenging roles of medicinal plants in improvement of wound healing technology. Daru 2024, 32, 379–419. [Google Scholar] [CrossRef] [PubMed]
- Akhoondinasab, M.R.; Khodarahmi, A.; Akhoondinasab, M.; Saberi, M.; Iranpour, M. Assessing effect of three herbal medicines in second and third degree burns in rats and comparison with silver sulfadiazine ointment. Burns 2015, 41, 125–131. [Google Scholar] [CrossRef]
- Demyashkin, G.; Sataieva, T.; Shevkoplyas, L.; Kuevda, T.; Ahrameeva, M.; Parshenkov, M.; Mimuni, A.; Pimkin, G.; Atiakshin, D.; Shchekin, V.; et al. Burn Wound Healing Activity of Hydroxyethylcellulose Gels with Different Water Extracts Obtained from Various Medicinal Plants in Pseudomonas aeruginosa-Infected Rabbits. Int. J. Mol. Sci. 2024, 25, 8990. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; de Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1938–1949. [Google Scholar] [CrossRef]
- Kopp, J.; Wang, G.Y.; Horch, R.E.; Pallua, N.; Ge, S.D. Ancient traditional Chinese medicine in burn treatment: A historical review. Burns 2003, 29, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.X. Burns Regenerative Medicine and Therapy; Sun, X., Weeks, B.S., Eds.; Karger: Basel, Switzerland, 2004. [Google Scholar]
- Alshehabat, M.; Hananeh, W.; Ismail, Z.B.; Rmilah, S.A.; Abeeleh, M.A. Wound healing in immunocompromised dogs: A comparison between the healing effects of moist exposed burn ointment and honey. Vet. World 2020, 13, 2793–2797. [Google Scholar] [CrossRef]
- Gong, Y.; Jiang, Y.; Huang, J.; He, Z.; Tang, Q. Moist exposed burn ointment accelerates diabetes-related wound healing by promoting re-epithelialization. Front. Med. 2023, 9, 1042015. [Google Scholar] [CrossRef]
- Ioannovich, J.D.; Magliacani, G.; Costagliola, M.; Atiyeh, B.; Dahm, R.; Berger, A.; Masselis, M.; Gravvanis, A. Moist exposed therapy of partial-thickness burn wounds. A multicenter study. Eur. J. Plast. Surg. 2003, 26, 338–345. [Google Scholar] [CrossRef]
- Hirsch, T.; Ashkar, W.; Schumacher, O.; Steinstraesser, L.; Ingianni, G.; Cedidi, C.C. Moist Exposed Burn Ointment (MEBO) in partial thickness burns—A randomized, comparative open mono-center study on the efficacy of dermaheal (MEBO) ointment on thermal 2nd degree burns compared to conventional therapy. Eur. J. Med. Res. 2008, 13, 505–510. [Google Scholar]
- Carayanni, V.J.; Tsati, E.G.; Spyropoulou, G.C.; Antonopoulou, F.N.; Ioannovich, J.D. Comparing oil based ointment versus standard practice for the treatment of moderate burns in Greece: A trial based cost effectiveness evaluation. BMC Complement. Altern. Med. 2011, 11, 122. [Google Scholar] [CrossRef]
- Li, F.L.; Wang, G.C.; Wu, B.Q. Clinical application of traditional Chinese medicine powder in the treatment of acute and chronic wounds. Int. Wound J. 2023, 20, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.D.; Pan, Z.H.; Pan, L.Q. Danggui Buxue Extract-Loaded Liposomes in Thermosensitive Gel Enhance In Vivo Dermal Wound Healing via Activation of the VEGF/PI3K/Akt and TGF-β/Smads Signaling Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8407249. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Han, X.; Liu, S.; Gao, X.; Guo, C.; Wu, X. Aramid Nanofibers-Reinforced Rhein Fibrous Hydrogels as Antibacterial and Anti-Inflammatory Burn Wound Dressings. ACS Appl. Mater. Interfaces 2022, 14, 45167–45177. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Shan, Y.; Xiong, J.; Hu, Z.; Zhang, Y.; Gao, J. In vivo study of silk fibroin/gelatin electrospun nanofiber dressing loaded with astragaloside IV on the effect of promoting wound healing and relieving scar. J. Drug Deliv. Sci. Technol. 2019, 52, 272–281. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, G.; Wang, L.; Wang, H.; Che, X. Pharmacodynamic study: Astragaloside IV/chitosan/polylactic acid composite electrospinning scaffold for wound healing in diabetic rats. J. Drug Deliv. Sci. Technol. 2024, 96, 105632. [Google Scholar] [CrossRef]
- Hoogewerf, C.J.; Hop, M.J.; Nieuwenhuis, M.K.; Oen, I.M.; Middelkoop, E.; Van Baar, M.E. Topical treatment for facial burns. Cochrane Database Syst. Rev. 2020, 7, CD008058. [Google Scholar] [CrossRef] [PubMed]
| Burn Area | Burn Depth |
|---|---|
| Minor: <10% of TBSA in adults <5% in children and the elderly Primarily consist of superficial burns <2% classified as full-thickness burns | First-Degree Burns (Superficial Thickness): Affect only the uppermost layer The skin appears red, congested, and dry Mild pain |
| Major: >20% of TBSA in adults >10% in children and the elderly >5% as full-thickness burns Significant burns to critical areas such as the face, eyes, ears, joints, or genitalia | Second-Degree Burns (Partial Thickness): Partial damage to the dermis 2A: (Superficial Partial Thickness) Affect epidermis and the upper half of the dermis Blisters and weeping, painful Require dressing and wound care but typically do not need surgery 2B (Deep Partial Thickness) Affect deeper layers of the dermis More blisters, drier, result in more scarring, less painful Surgical intervention required |
| Third-Degree Burns (Full Thickness): Damage the entire dermis, not painful Require protection against infection and surgical management | |
| Fourth-Degree Burns: Affect deeper tissues, including muscle and bone Burned areas blackened Significant loss of tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siu, W.S.; Ma, H.; Leung, P.C. Review on Current Advancements in Facilitation of Burn Wound Healing. Bioengineering 2025, 12, 428. https://doi.org/10.3390/bioengineering12040428
Siu WS, Ma H, Leung PC. Review on Current Advancements in Facilitation of Burn Wound Healing. Bioengineering. 2025; 12(4):428. https://doi.org/10.3390/bioengineering12040428
Chicago/Turabian StyleSiu, Wing Sum, Hui Ma, and Ping Chung Leung. 2025. "Review on Current Advancements in Facilitation of Burn Wound Healing" Bioengineering 12, no. 4: 428. https://doi.org/10.3390/bioengineering12040428
APA StyleSiu, W. S., Ma, H., & Leung, P. C. (2025). Review on Current Advancements in Facilitation of Burn Wound Healing. Bioengineering, 12(4), 428. https://doi.org/10.3390/bioengineering12040428







